Method Article
In Vivo Leaf Inoculation: An Alternative Method to Assess the Disease Resistance of Hybrid Clones in Poplar Breeding of Stem Canker Disease
* Wspomniani autorzy wnieśli do projektu równy wkład.
W tym Artykule
Podsumowanie
We provide a step-by-step protocol for assessing poplar resistance to stem canker pathogens using an in vivo leaf inoculation method. This method is especially suitable for large-scale assessment of Cytospora chrysosperma and Botryosphaeria dothidea canker disease resistance in poplar breeding progeny in China.
Streszczenie
Stem canker diseases caused by the pathogen Cytospora chrysosperma (Pers.) Fr.) and Botryosphaeria dothidea (Moug. ex Fr.) Ces. & de Not. are the two major forest diseases in the poplar plantations in China, sometimes which can destroy all the poplar seedlings or severely damage mature poplar forests. Hybrid breeding is the most direct and efficient method of controlling and managing tree diseases. However, assessing disease resistance or selecting disease-resistance clones based on In vitro stem inoculation is inefficient, time-consuming, and expensive, limiting the development of hybrid breeding of poplar stem canker disease. In this study, we proposed an alternative method to assess disease resistance to stem canker pathogens through in vivo leaf inoculation. The test materials used in this method can be on 1-year-old poplar saplings or the annual branches of perennial poplars in the greenhouse or the field. The critical step of this alternative method is the selection of inoculating leaves: the 5-7th newly matured leaves might be the most suitable. The second critical step of the leaf inoculation method is to make wounds on plant leaves through needle pierces, providing sufficient lesions to measure disease severity. For the adequate number of leaves produced in the early stage of poplar breeding, this in vivo leaf inoculation contributes to the rapid, accurate, and large-scale screening of the disease-resistance poplar clones to stem canker pathogens. Moreover, this leaf inoculation method will also serve as an efficient method for screening pathotypes of stem canker disease pathogen C. chrysosperma, B.dothidea, or other poplar stem canker pathogens.
Wprowadzenie
Poplar stem canker diseases, mainly caused by two necrotrophic pathogens, Cytospora chrysosperma (Pers.) Fr. and Botryosphaeria dothidea (Moug. ex Fr.) Ces. & de Not., severely threaten the development and survival of poplar plantations in the Northeast, North, and Northwest (Three-north) of China. Hybrid breeding is the most direct and efficient method to control and manage tree diseases; however, compared with the progress in breeding for high-yield, fast-growing, or other poplar hybrid clones, research on resistance breeding for poplar canker disease is scarce. Only limited studies of disease-resistance hybrid breeding have been reported1,2,3, and no canker-resistance hybrid clone has been cultivated in poplar afforestation.
The crucial step of regular hybrid breeding is the clone selection based on the phenotype acquisition of hybrid progeny. However, acquiring the acquisition of pathological phenotypes (pathotypes) is time-consuming, laborious, tiring, expensive, and experts-depending process. For woody plants, it is more challenging due to the time-, labor-, and economy-consuming nature caused by their long lifecycle, slow growth, and massive body. For example, in poplar, the canker resistance screening of hybrid progeny was conducted 5-7 years after hybridization through conventional in vitro stem inoculation methods1,2,3. Moreover, limited by this low-efficiency and high-consuming disease-resistant screening method, researchers re-selected the canker-resistant clones from a small subgroup (for example, the selected high-yield or fast-growing poplar clones), not from all hybrid progeny. Therefore, using the regular stem inoculation methods, it is not sure to identify the authentic resistant clones and can not reveal the disease-resistant diversity of the breeding progeny, thus limiting the exploration of disease-resistance-related genes or gene modules. Compared to the rapid development of poplar fast-growing/high-yield breeding, those breeding programs can obtain hybrids through phenotypic selection or even genomic selection in the first two years of breeding4, the poplar canker-resistance breeding developed slowly. The disease-resistant screening (or detection) of hybrid progeny, or the pathogen inoculation method, has become the crucial speed-limiting step in poplar canker disease-resistance breeding.
In this protocol, we introduce a novel inoculation method for poplar stem canker pathogens, in vivo leaf inoculation method. Using this method, we can quickly and efficiently test the canker disease resistance of dozens of poplar species (cultivars or clones) within 5-7 days. The validation assay illustrated that the in vivo leaf inoculation is consistent with the traditional in vitro stem inoculation on the resistance detection of stem canker disease, suggesting that the leaf inoculation method is suitable for large-scale resistance screening of poplar canker diseases, such as the whole genotype selection of resistance progeny in the breeding of stem canker disease. This method solves the pathological problem of selecting resistant offspring in the hybrid breeding of poplar canker diseases.
Protokół
1. Fungal culture for canker pathogen
- Prepare the potato dextrose agar (PDA; potato extraction 6.0 g, dextrose 20.0 g, agar 20.0 g) culture medium for fungal strains; dissolve the above materials in water up to 1000 mL, completely dissolve the medium at 100 °C for 10 min.
- Pour PDA medium into 25 mL tubes (each containing 15.0 mL); sterilize all tubes at 121.1 °C for 30 min. Pour the medium into culture plates (9.0 cm in diameter) and cool the plates at room temperature (RT).
- Cut the mycelium of the fungal pathogen, which is cultured in PDA medium at 28 °C for 7 days, into ~0.5 cm square cubes; inoculate the fungal cubes at the center of PDA plates (the mycelium sides face the medium).
- Culture the fungal pathogens in a thermostatic incubator (28 °C, in dark) for 7-10 days.
- Cut the PDA medium with fungal mycelium into square cubes (side length 1.0-1.2 cm).
2. Preparation of poplar materials
- For 1-year-old poplar clones (cultivated > 3 months), select the newly matured, fully extended leaves (always 5-7th leaves, from the top of the sapling/branches) of the main branches as the inoculated materials, ensure they are free from pests, diseases, and mechanical damages.
- For 1-year-old branches of perennial poplar hybrid clones (cultivated > 2 months), select the top 5-7th leaves of the selected branches as the inoculated materials. Ensure the leaves are healthy and have the same light condition (shade or light) among all the tested poplar clones.
3. Pretreatment of inoculation leaves
- Spray the poplar leaves with clean water, and after drying, wipe the selected leaves with 75% alcohol 1 h or 1 day before the inoculation manipulations.
4. In vivo leaf inoculation
- For small leaves (leaf width < 8.0 cm), inoculate two square fungal mycelium cubes and PDA (or water agar, WA) cubes (1.0-1.2 cm in side length) onto the upper surface of the top 5-7 leaves of poplar clones; the mycelium faces the leaves. Ensure that the inoculation sites are located at the center of the half leaves, ~1-2 cm apart from the central veins, avoiding obscuring the secondary veins. Each clone inoculates 12 sites on 6 leaves (10 sites for mycelium and 2 sites for PDA inoculation).
- For large leaves (leaf width ≥ 8.0 cm), inoculate four square fungal mycelium cubes and PDA (or WA) cubes onto the top 5-7 poplar leaves. Each clone inoculates 12 sites at 3 leaves (10 sites for mycelium and 2 sites for PDA inoculation.
- Wrap the inoculated leaves with transparent adhesive tape (6.0 cm in width) and gently press them to make them adhesive to the leaves, preventing the moving and water loss of the mycelium (or PDA) cubes during the experiment.
- Pierce the leaves and mycelium (or PDA) cubes at five sites until the needles penetrate the cubes from the upper to the lower surface of the poplar leaves. One site lies at the center, and the other four lie 1-2 mm near the four vertices of the cubes.
NOTE: A 3 person team collaboration is recommended for fungal inoculation manipulation. The inoculation manipulation of a poplar hybrid population (>100 genotypes) can be conducted in 4 h through teamwork. The piercing manipulations were conducted after all the tested leaves were inoculated and wrapped to alleviate the impact of different piercing times on the disease severity. - Inspect the location shift and water loss of the mycelium inoculants, and observe the onset of necrotic lesions around the pierce wound sites from the lower surface of leaves until 3rd day of inoculation.
- Within 3 days of inoculation, observe the location shift and water loss of the mycelium cubes. Define and mark the moved and dried mycelium cubes as ineffective inoculations and discard them from the final identification of pathotypes while defining the other cubes as effective inoculations.
NOTE: This protocol defines the tested poplar clones with more than five effective inoculation cubes as invalid inoculated clones; therefore, each poplar clone provided at least 30 effective inoculation sites, which will develop into 30 independent necrotic spots.
- Within 3 days of inoculation, observe the location shift and water loss of the mycelium cubes. Define and mark the moved and dried mycelium cubes as ineffective inoculations and discard them from the final identification of pathotypes while defining the other cubes as effective inoculations.
- Pick off all inoculated leaves at the end of the experiment (~5-7 days after inoculation), bring them back into the laboratory in plastic sample bags, and store them at 4 °C.
5. Leaf pathotype acquisition and assessment of poplar resistance to stem canker diseases in the laboratory
- Carefully remove the transparent adhesive tapes and mycelium (or PDA) inoculants from the leaves.
- Observe and identify the leaf pathotypes of each poplar clone, including the shape and color of necrotic spots, and may include the fungal hyphae-like structure, pycnidia, and conidia that formed on the surface of the leaves around the pierce sites.
- Photograph the leaves (with an additional ruler) with a camera, or scan the leaves (with an additional ruler) using a scanner to obtain images with a resolution of over 300 dpi, and then save the images in JPEG, TIFF, or PNG format.
6. Identification and statistical analysis of disease occurrence
- Open the images of diseased leaves in the software ImageJ 1.54g (http://imagj.org).
- Set the scale according to the ruler in the leaf images.
- Identify and measure the lesions with the wand (tracing) tool.
- Record and export the area values as a spreadsheet after the areas of all necrotic spots are measured.
- Set the criteria for disease determination:
- If the area of lesion around the mycelium cubes covered pierce sites is significantly larger than that covered by PDA cubes, then define these sites as disease sites.
- If the morphological characteristics of the mycelium cubes covered pierce sites significantly changed when compared with the PDA-covered sites, including colors of the lesion, producing hyphae-like structure, pycnidia, and conidia, define the site also as a disease site.
- Calculate the average areas of each poplar hybrid clone's effective PDA lesion spots. According to the average areas, divide all the mycelium inoculated spots of one poplar clone into two categories: onset and non-onset. Then, calculate the tested poplar clone's disease incidence rate (Formula 1: Disease incidence rate (%) = Number of diseased prick sites/Total number of efficient prick sites × 100).
- Calculate the average area of the diseased spots in leaves (n = 50). According to the values of average areas of diseased spots of leaves in all tested poplar clones, set a 5-level disease grading standard.
- Calculate the disease index of each poplar clone (Formula 2) based on the above disease grading standard (Formula 2: Disease index = ∑(Number of prick sites by level × Values of severity at all levels)/(Value of the highest severity level × Total number of efficient prick sites) × 100).
- Verify the normal distribution of the numbers of poplar clones across different resistance levels using the Shapiro-Wilk test using any appropriate data analysis software.
- According to the disease index, identify all tested poplar clones as five (or seven) groups: very high resistance (VHR), high resistance (HR), resistance (R), no resistance and no susceptibility (NRNS), susceptibility (S), high susceptibility (HS), and very high susceptibility (VHS) group5.
Wyniki
In this protocol, the schematic workflow was conducted in 48 poplar hybrid clones infected by the stem canker pathogen C. chrysosperma (Figure 1). The poplar hybrid clones are part of the hybridization progeny of P. deltoides, cultivated in the nursery at the Chinese Academy of Forestry (CAF), Beijing.
The stem canker pathogen C. chrysosperma isolate CZC is a typical fungal strain (with middle pathogenicity) used for the physiological research of poplar canker disease6,7,8,9 and deposited in our laboratory. Results showed that inoculation of stem canker pathogen C. chrysosperma induced necrotic lesions (Figure 2B,C) and even pycnidia-like structures on poplar leaves (Figure 2D-F). Moreover, results also illustrated that the leaf positions (or leaf ages and leaf ontogeny) (Figure 2G-I) and light conditions of leaves (Figure 2J-L) impact the disease severity of the leaves inoculated by stem canker pathogen C. chrysosperma isolate CZC.
The pathogenicity of fungal inoculants is a crucial factor in the resistance screening of unknown hybrid populations. However, there have been two opposing views on the selection of virulent strains in breeding: using the most virulent strain10 and the moderately virulent strains11. In the preliminary experiment of this protocol, we selected C. chrysosperma to isolate CZC from the pathogenicity detection of 10 fungal isolates in hybrid poplar "Bofeng 3". Encouragingly, the results indicate that the distribution of the poplar clones across different resistance levels in the 48 poplar hybrid clones is normal, as confirmed by the Shapiro-Wilk test analysis in SPSS, suggesting that this isolate is favorite to resistance screening of this current poplar hybridization population. Partial results of resistance screening of 48 poplar clones are shown in Figure 3A-C. Moreover, results showed that six isolates of pathogen B. dothidea have differential virulence to hybrid poplar "Bofeng 3", and isolates SD47 and SD60 are the most virulent isolates in the tested fungal strains (Figure 3D-F), suggesting that the leaf inoculation method is also to used in the virulence screening of poplar stem canker disease pathogen.
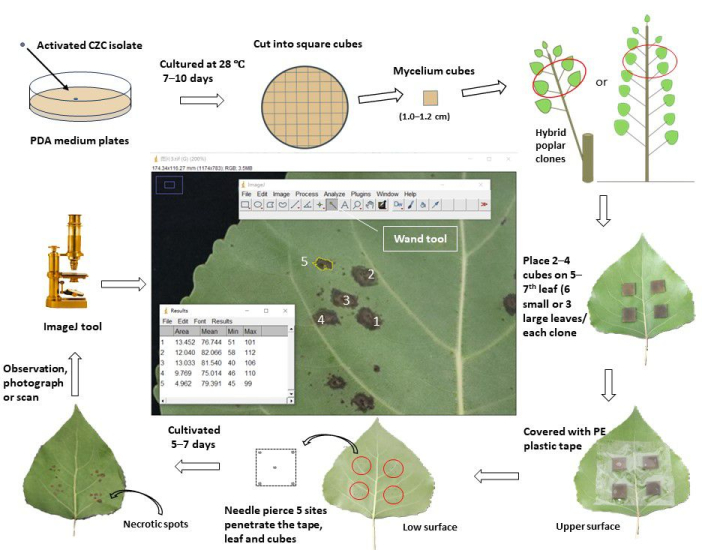
Figure 1: A schematic workflow of assessing stem canker resistance through in vivo leaf inoculation in poplar clones. The activated stem canker pathogen Cytospora chrysosperma isolate CZC was cultured in PDA plates at 28 °C, in darkness, for 10 days. Then, the C. chrysosperma mycelium was cut into square cubes (1.0-1.2 cm in side length) and inoculated on the upper surface of the top 5-7th leaves in 1-year-old poplar saplings or 1-year-old poplar branched on perennial poplars. The hyphae side of the mycelium cubes faced the leaves. For the small leaves (leaf width < 8.0 cm), two mycelium cubes were inoculated to one leaf, while four mycelium cubes were inoculated on the large leaves (leaf width ≥ 8.0 cm). However, the small and large leaves, ten mycelium cubes, and 2 PDA medium cubes were inoculated on the saplings/branches. Then, inoculated leaves were wrapped with 6.0-8.0 cm width plastic protective tape to fix the cubes and keep them from water loss. The leaves and cubes were pierced with needles to produce wounding sites at the center and the four vertices of the squares. The inoculated leaves were cultivated in the field/greenhouse and observed, photographed/scanned 5-7 days after inoculation. Then, the images were loaded into the ImageJ software to measure the average areas of the necrotic spots that developed from the pierced wounding sites of each poplar clone. Finally, the disease incidence rate and disease index were calculated based on the average areas of the necrotic spots of each poplar clone. According to the disease index, all tested poplar clones were divided into different resistance groups. Please click here to view a larger version of this figure.
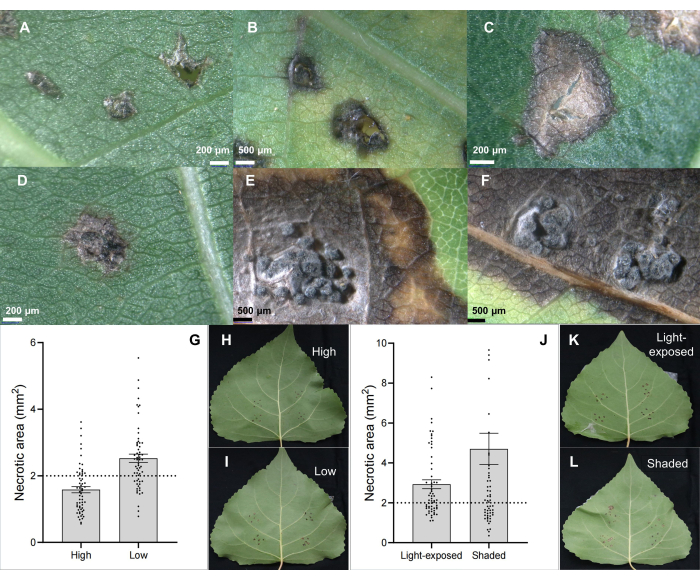
Figure 2: Classical leaf lesion characteristics on hybrid poplar clone B246 infected by canker pathogen C. chrysosperma through leaf inoculation. (A) inoculated by PDA inoculants; (B,C) necrotic spots; (D-F) necrotic spots with pycnidia structure; (G-I) positions of inoculated leaf in the 1-year-old sapling/branches influence the disease severity of stem canker pathogen C. chrysosperma on hybrid poplar cultivar "Bofeng 3"; (J-L) light conditions influence the disease severity on poplar cultivar "Bofeng 3". Please click here to view a larger version of this figure.

Figure 3: Resistance screening and susceptible detection. Resistance screening of (A-C) hybrid poplar clones and susceptible detection of pathogen Botryosphaeria dothidea isolates in (D-F) hybrid poplar clone "Bofeng 3" using the in vivo leaf inoculation method. Please click here to view a larger version of this figure.
| Severity grade | Grading criteria | Level |
| No disease | No symptoms, necrotic area 0–2.0 mm² | 0 |
| Extremely mild | Necrotic area 2.0–4.0 mm² | 1 |
| Mild disease | Necrotic area 4.0–6.0 mm² | 2 |
| Moderate disease | Necrotic area 6.0–8.0 mm² | 3 |
| Moderately severe disease | Necrotic area 8.0–10.0 mm² | 4 |
| Severe disease | Necrotic area greater than 10.0 mm² | 5 |
Table 1: Severity grading of necrotic symptoms induced by canker pathogens on leaves.
Dyskusje
This protocol provides a rapid and efficient inoculation method for poplar canker resistance pathogens, which is suitable for the research fields requiring large-scale disease resistance screening, such as hybrid breeding of poplar canker resistance and pathogenicity screening of stem canker pathogens.
The first key point of the method is to evaluate disease resistance by newly matured leaf inoculation instead of matured stems/branches. As a result, the selection of stem canker disease-resistance clones can be conducted in 1-2 years of the poplar hybrid breeding when using the leaf inoculation method, compared with the time-consuming resistance screening in the stem inoculation pathway (5-7 years after breeding), the leaf method sharply shortening the breeding cycle of poplar stem canker disease. The second crucial step of the leaf method is the selection of leaves. The disease resistance of leaves varies with their ontogeny (ages or position on the branches), named leaf ontogeny resistance of leaf stage-associated resistance12,13. So, the correlation of plant resistance between the tested leaves and stem/branches is crucial to the effectiveness of the leaf inoculation method. The leaf spot resistance on the "five middle leaves" is not related to the stem canker resistance in hybrid poplar-Septoria interaction13; however, Wei et al.'s study14 and our research illustrated that the leaf spot resistance on the top newly matured leaves or the top 5-7th leaves are consistent with the stem canker resistance in Malus-V. ceratosperma and poplars-C. chrysosperma interactions. Therefore, the in vivo leaf inoculation method can be used in the poplar breeding of stem, and the top 5-7 leaves are available inoculation materials. The third key point of this method is the production of sufficient wounding sites (30 sites are recommended and 50 sites in this protocol) through repeated needle piercing after pathogen inoculation, theoretically, which wounding sites will develop into 50 independent necrotic spots on three poplar leaves providing a more accurate assessment of the resistance to canker disease in poplar progeny. In addition, the moisture preservation and selection of the light conditions15 in leaves are beneficial in acquiring a more stable and accurate resistance assessment to poplar stem canker diseases.
In this protocol, both 1-year-old poplar saplings (cultivated > 3 months) and 1-year-old branches (cultivated > 2 months) of perennial poplar trees were used as the inoculation materials. The poplar saplings are ideal for disease-resistance screening because they are more consistent and easier to operate. On the contrary, selecting poplar branches or leaves (which should be free from pests, diseases, and mechanical damages and have the same environmental conditions, such as lighting or shading) is challenging.
Compared with the traditional in vitro stem inoculation method, the in vivo leaf inoculation method sharply improves the poplar canker-resistance breeding in many aspects: 1) providing a feasible disease-resistance screening method for the hybrid breeding of poplar stem canker diseases. According to our experience, a 3-person team can conduct the fungal inoculation of more than 200 poplar hybrid clones in 1 day and then obtain the results after 5-7 days. Thus, the breeders can screen the disease resistance of a large hybrid population (for example, 1,000 genotypes) in a short period (for example, 1 month) through the collaboration of 3-6 people. 2) The leaf inoculation method significantly advances the time of the first selection for the canker-resistance clones (from 5-7 years to 1-2 years after breeding), which benefits the early selection and sapling selection of resistant clones to stem canker diseases. 3) Using the leaf inoculation method, researchers can deeply reveal all hybrid progeny's resistance structure and diversity and obtain candidate resistance clones for production or breeding. 4) Moreover, combined with high-throughput sequencing technology, the leaf inoculation method benefits the mining of resistance-related genes and gene modules4and can be used in poplar disease-resistance breeding based on genomic selection (GS) technology4. Finally, for the continuous production of the top 5-7th leaves during the growth season of poplar, breeders can screen the poplar resistance to different pathogen strains or different disease pathogens; then, the leaf inoculation method also provides a feasible pathway for the multi-projective breeding of poplar, for example, obtain hybrid clone(s) that resist C. chrysosperma and B. dothidea canker diseases simultaneously. However, it should be noted that the leaf inoculation method developed from the screening for canker resistance in a relatively small hybrid poplar progeny (48 genotypes). Then, as a critical strategy for poplar canker resistance breeding, the effectiveness of this method still needs more robust validation from a larger population of poplar hybrids or cultivated poplar plantations.
This protocol will contribute to developing poplar canker diseases (for example, Cytospora, Botryosphaeria, and Septoria diseases) hybrid breeding, providing resistant poplar hybrid clones in the poplar afforestation in China and North America. Moreover, this protocol will contribute to our understanding of the pathogenicity of stem canker diseases, facilitate genetic assays and gene mining, and improve the development of poplar molecular breeding. In addition, the leaf inoculation method also implies an accurate and stable evaluation method for screening the susceptibility of canker pathogens and determining fungi pathogenicity-related genes.
Ujawnienia
The authors have nothing to disclose.
Podziękowania
This research was jointly funded by the Central Public-interest Scientific Institution Basal Research Fund of State Key Laboratory of Tree Genetics and Breeding (grant number CAFYBB2020ZY001-2) and the National Natural Science Foundation of China (grant number 32171776) to Jiaping Zhao.
Materiały
| Name | Company | Catalog Number | Comments |
| Aluminum foil | Biosharp | BS-QT-027B | |
| C. chrysosperma isolate | China Forestry Culture Collection Center | CFCC86775 | Separation and preservation by our laboratory |
| Cytospora chrysosperma | Plant Physiology Laboratory, Institute of Forestry New Technology, Chinese Academy of Forestry | NCBI accession number: MK994101 for rRNA-ITS and MN025273 for EF1α gene CGMCC number:40575 | Separation and preservation by our laboratory |
| Epson Perfection V370 Photo | Epson | V370 | Scanner; Scan the leaves into image |
| PDA (Potato Dextrose Agar) | Solarbio | P8931 | Provide nutrition for fungal growth |
| PE plastic film | To fix fungi on the leaves | ||
| Populus alba var. Pyramidalis | Plant Physiology Laboratory, Institute of Forestry New Technology, Chinese Academy of Forestry | Cultivated by our laboratory | |
| SPSS | IBM | Data analysis software | |
| Thermostatic incubator | Shanghai Kuntian Laboratory Instrument Co., Ltd | KTD-6000 | Provide an environment for fungal growth |
| Tough TG-6 camera | OLYMPUS | To take photos of diseased leaves |
Odniesienia
- Du, K., et al. Genetic analysis and seedling selection of the poplar progenies of Aigeiros section. Journal of Huazhong Agricultural University. 28 (5), 624-630 (2009).
- Shi, Y. . Identification of canker resistance to selection poplar clones [Master's thesis]. , (2014).
- Zhang, L., Zhang, H., Ou, D., Fan, J. Investigation on the resistance to canker of some new leuce hybrids inoculated with Botryosphaeria dothidea. Journal of Northwest Forestry University. 32 (6), 210-213 (2017).
- Du, C., et al. Genomic selection of seedling growth traits in a poplar hybrid population. For Res. 36 (6), 11-19 (2023).
- Li, Z., et al. A rapid and efficient in vivo inoculation method for introducing tree stem canker pathogens onto leaves: Suitable for large-scale assessment of resistance in poplar breeding progeny. BioRxiv. , (2024).
- Li, P., et al. Fungal canker pathogens trigger carbon starvation by inhibiting carbon metabolism in poplar stems. Sci Rep. 9 (1), 10111 (2019).
- Xing, J., et al. Fungal pathogens of canker disease trigger canopy dieback in poplar saplings by inducing functional failure of the phloem and cambium and carbon starvation in the xylem. Physiol Mol Plant Pathol. 112, 101523 (2020).
- Xing, J., et al. Comparisons of photosynthetic response and characteristics in leaves of Populus alba var. pyramidalis Infected by the stem canker pathogen Valsa sodida and Botryosphaeria dothidea at early stage. Scientia Silvae Sinicae. 57 (09), 121-129 (2021).
- Li, J., et al. Effects of Valsa sordida infection on photosynthetic characteristics and carbon-water metabolism in Populus alba var. pyramidalis. For Res. 34 (05), 58-68 (2021).
- Balendres, M. A., De Torres, R., Dela Cueva, F. Culture storage age and fungal re-isolation from host-tissue influence Colletotrichum spp. virulence to pepper fruits. J Phytopathol. 167 (9), 510-515 (2019).
- Ward, K. T., Ostry, M. E. Variation in Septoria musiva and implications for disease resistance screening of poplars. Plant Dis. 89 (10), 1077-1082 (2005).
- Asalf, B., et al. Ontogenic resistance of leaves and fruit, and how leaf folding influences the distribution of powdery mildew on strawberry plants colonized by Podosphaera aphanis. Phytopathology. 104 (9), 954-963 (2014).
- Dunnell, K. L., Le Boldus, J. M. The correlation between Septoria leaf spot and stem canker resistance in hybrid poplar. Plant Dis. 101 (3), 464-469 (2017).
- Wei, J., Huang, L., Gao, Z., Ke, X., Kang, Z. Laboratory evaluation methods of apple Valsa canker disease caused by Valsa ceratosperma sensu Kobayashi. Acta Phytopathol Sinica. 40 (1), 14-20 (2010).
- Shen, W., et al. Comparative study on the effectiveness of three inoculation methods for Valsa sordida in Populus alba var. pyramidalis. Biology. 13 (4), 251 (2024).
Przedruki i uprawnienia
Zapytaj o uprawnienia na użycie tekstu lub obrazów z tego artykułu JoVE
Zapytaj o uprawnieniaPrzeglądaj więcej artyków
This article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. Wszelkie prawa zastrzeżone