Method Article
细菌利用试金石比色法检测
摘要
We describe a protocol for colorimetric detection of E. coli using a modified litmus test that takes advantage of an RNA-cleaving DNAzyme, urease, and magnetic beads.
摘要
There are increasing demands for simple but still effective methods that can be used to detect specific pathogens for point-of-care or field applications. Such methods need to be user-friendly and produce reliable results that can be easily interpreted by both specialists and non-professionals. The litmus test for pH is simple, quick, and effective as it reports the pH of a test sample via a simple color change. We have developed an approach to take advantage of the litmus test for bacterial detection. The method exploits a bacterium-specific RNA-cleaving DNAzyme to achieve two functions: recognizing a bacterium of interest and providing a mechanism to control the activity of urease. Through the use of magnetic beads immobilized with a DNAzyme-urease conjugate, the presence of bacteria in a test sample is relayed to the release of urease from beads to solution. The released urease is transferred to a test solution to hydrolyze urea into ammonia, resulting in an increase of pH that can be visualized using the classic litmus test.
引言
Bacterial pathogens are one of the major causes of global morbidity and mortality. Outbreaks from hospital-acquired infections, food-borne pathogens, and bacterial contaminants in the environment pose serious and on-going threats to public health and safety. To prevent these outbreaks, effective tools are needed that permit pathogen detection in a timely fashion under a variety of settings. Simple but still effective tests that are portable and cost-effective are greatly coveted, especially in regions that are susceptible to outbreaks but cannot afford expensive testing facilities.1-3 Although there exists a multitude of methods to detect bacteria, many of them are not suitable as screening or on-site testing tools because they require long test times, expensive instruments and complicated testing procedures.
Colorimetric tests are particularly attractive for point-of-care or field applications as color changes can be easily detected by the naked eye. The litmus test for pH is simple, quick, and effective. Although it is a very old technology, it is still widely used today because of its simplicity and effectiveness. Surprisingly, this simple test had never been modified to achieve the detection of other analytes before we recently developed an approach of modifying this test for E. coli testing.4
The expanded litmus test for E. coli employs three additional components: an E. coli activated RNA-cleaving DNAzyme (EC1), 5 urease, and magnetic beads. DNAzymes refer to synthetic single-stranded DNA molecules with catalytic activity.6 They can be isolated from random-sequence DNA pools using in vitro selection.7,8 They are highly stable and can be produced cost-effectively using high-efficiency automated DNA synthesis.9 For these reasons, DNAzymes, particularly RNA-cleaving DNAzymes, have been widely examined for biosensing applications.6,10,11 RNA-cleaving DNAzyme sensors have been developed to detect metal ions,12-16 small molecules,17,18 bacterial pathogens5,19-21 and cancer cells.22 Given the great availability of target-induced RNA-cleaving DNAzymes, any assay that utilizes a DNAzyme can be potentially expanded to detect a diverse range of analytes.
Urease is chosen for its ability to hydrolyze urea into ammonia,23,24 resulting in a pH increase. Urease is also highly efficient, stable and amenable for conjugation to other biomolecules. Therefore, we postulated that a conjugate of an RNA-cleavage DNAzyme with urease would allow the use of litmus test for the detection of other targets.5
The action of the RNA-cleaving DNAzyme is relayed to urease-mediated increase of pH through the use of magnetic beads that are immobilized with the DNAzyme-urease conjugate. Because the activity of the DNAzyme under investigation is strictly dependent on E. coli, the presence of this bacterium in the test solution will result in the release of urease from the magnetic beads to the solution, which is then taken and used to hydrolyze urea in a reporter solution that contains a pH-sensitive dye. The final outcome of this procedure is a color change that can be conveniently reported by the dye or pH paper.
研究方案
1.试剂和缓冲液的制备
- 的0.5M乙二胺四乙酸(EDTA)的
- 在2L烧杯中,186.1克EDTA添加到800毫升去离子蒸馏水的(DDH 2 O的)。调节溶液至8.0用NaOH粒料的pH值。添加DDH 2 O的以1.0升的最终体积,并在4℃下将溶液转移到一个高压灭菌玻璃瓶高压灭菌和储存。
- 10×Tris-硼酸盐-EDTA(10×TBE)
- 在一个4升的塑料烧杯中,使用搅棒,直到所有的组分432克Tris-碱将200克硼酸中,加入80ml的0.5M EDTA(pH 8.0)中和DDH 2 O的的添加4 L.混合的最终体积溶解。在4℃下将溶液转移至1升的玻璃瓶高压灭菌和储存。
- 10%的变性聚丙烯酰胺股票
- 在4升的塑料烧杯中,加入1681.7克尿素,加入400ml 10X TBE,1升40%的丙烯酰胺/双丙烯酰胺(29:1)解决方案和DDH 2 O的,直到在FINAL量为4 L.混合使用搅拌棒直到尿素溶解。在4℃下将溶液转移至1L的琥珀色玻璃瓶和存储。
- 2X凝胶缓冲液(2X GLB)
- 在200毫升玻璃烧杯中,加入44克尿素8克蔗糖,10毫克溴酚蓝,10毫克二甲苯青FF,400微升10%十二烷基硫酸钠的,和4毫升10×TBE的。添加DDH 2 O的以40 ml的终体积,并在50℃下溶解有轻度加热的部件,同时用磁力搅拌棒混合。传输1毫升一份到1.5 ml离心管和储存在4℃。
注意:使用前需要在90℃加热简要重新溶解固体的任何。
- 在200毫升玻璃烧杯中,加入44克尿素8克蔗糖,10毫克溴酚蓝,10毫克二甲苯青FF,400微升10%十二烷基硫酸钠的,和4毫升10×TBE的。添加DDH 2 O的以40 ml的终体积,并在50℃下溶解有轻度加热的部件,同时用磁力搅拌棒混合。传输1毫升一份到1.5 ml离心管和储存在4℃。
- 的1M Tris盐酸(Tris-盐酸)(pH7.5)中
- 在玻璃瓶中,添加12.1克Tris-碱和70ml DDH 2 O的的并混合直到固体溶解。调整使用1M盐酸(HCl)将pH值至7.5。添加DDH 2 O的至100ml,高压釜和STO终体积再在4℃
- 的5M氯化钠(NaCl)中
- 在玻璃瓶中,溶解58.4克NaCl的150毫升的DDH 2 O的调节音量至200毫升的DDH 2 O保存在4℃。
- DNA洗脱缓冲液
- 在玻璃瓶中,混合2.0毫升的1M的Tris-HCl(pH为7.5),8.0毫升5摩尔NaCl和0.4ml的0.5M的EDTA(pH8.0)中的。调节音量到200毫升的DDH 2 O,高压灭菌器和储存在4℃。
- 1M的4-(2-羟乙基)-1-哌嗪乙磺酸(HEPES)(pH 7.4)中
- 在玻璃瓶中,溶解2.38克HEPES的80ml的DDH 2 O的调整用5N氢氧化钠将pH至7.4,并添加DDH 2 O的至100ml的最终体积。
- 1 1M氯化镁( 氯化镁 )
- 在玻璃瓶中,添加2.03克的MgCl 2 -6H 2 O和使体积为100毫升的DDH 2 O
- 反应缓冲液(RB)
- 在50ml锥形管中,加入50微升的1M HEPES(pH7.4)中,将1.5ml 5M的NaCl,0.75毫升的1摩尔的 MgCl 2,和5μl吐温-20组成。添加的DDH 2 O至50ml终体积。混合溶液中,并使用注射器驱动的滤器(0.22μm),并储存在4℃直至使用过滤到另一个锥形管中。
- 结合缓冲液(BB)
- 在一个50毫升锥形管,加入500微升的1M的Tris-HCl(pH7.5)中的,8.8克NaCl,50微升的1M 的 MgCl 2,和5μl吐温20的添加DDH 2 O的至最终体积为50毫升。混合溶液中,并使用注射器驱动的滤器(0.22μm),并储存在4℃直至使用过滤到另一个锥形管中。
- 底物溶液(SS)
- 在50ml锥形管中,加入5.8克NaCl的3毫升的1M MgCl 2的,1.5克尿素,和40毫升的DDH 2 O的调节溶液至5.0用10mM的HCl的pH值。由于该解决方案不进行缓冲,使用盐酸调节pH小心通过添加盐酸小等分。添加的DDH 2 O至50ml终体积。
- 混合溶液中,并使用注射器驱动的滤器(0.22μm),并储存在4℃直至使用过滤到另一个锥形管中。
- 卢里亚BERTANI(LB)肉汤
- 在烧杯中,溶解20.0克LB粉1升的DDH 2 O的转移到玻璃瓶中,高压釜中,并储存在4℃。
- 1.5%LB琼脂
- 在250毫升烧瓶中,添加1.5克琼脂和100ml LB肉汤中。高压灭菌器和储存在4℃。
- 琼脂平板
- 再溶解在微波的LB琼脂并冷却溶液至〜50℃。将溶液倒入皮氏培养皿,使得〜5块板,并允许它们固化。
2.合成和E的纯化coli-响应核酶EC1
- 通过模板介导的酶连接EC1的合成
- 净化COMMercially合成的寡核苷酸BS1,DE1,和T1 10%,根据标准方法变性聚丙烯酰胺凝胶电泳(dPAGE)( 表1中提供了序列)。
- 准备BS1,DE1,和T1的100微米的股票。储存在-20℃直到使用。
- 在1.5ml微量离心管中,添加38.5微升DDH 2 O的,5微升DE1和5微升10×T4多核苷酸激酶反应缓冲液中由酶的供应商(500毫摩尔Tris盐酸(pH值7.6),100mM的MgCl 2的设置,的50mM二硫苏糖醇(DTT),1.0mM的亚精胺)。
- 加入1μl三磷酸腺苷(ATP)(100毫米)的。加入5单位T4多核苷酸激酶(10U /微升)。通过仔细吹打反应混合物混合。
- 孵育在37℃下进行30分钟的反应。
- 淬火加热5分钟,将反应至90℃。
- 加入118微升的DDH 2 O,5微升BS1和5微升T1的。加热反应至90℃2英里n和然后在10分钟内冷却至室温。
- 加入20μl的酶的供应商提供的10×T4 DNA连接酶反应缓冲液(400毫摩尔Tris盐酸(pH值7.8),100mM的MgCl 2的,100毫摩尔DTT,5毫摩尔ATP)。加10单位T4 DNA连接酶(5U /微升)和通过吸液仔细混合。
- 在室温下孵育2小时。
- 加入20μl的3M乙酸钠(NaOAc)(pH为5.2),500微升冷的100%乙醇。混合通过涡旋该溶液,然后将管在-20℃下冷冻30分钟。
- 离心机离心在20000 XG在4℃下20分钟。小心通过移液除去上清液。
- 在4℃下以20,000 xg离心再次洗用冷70%乙醇,离心沉淀10分钟。再次去除上清液并干燥在真空下将沉淀10分钟。
- 重悬DNA与15微升的DDH 2 O,然后加入15微升2×GLB的。
- 涡大力并加热至90℃下2分钟。如下所述通过10%dPAGE纯化全长DNA。
- 设置10%dPAGE
- 清洁两块玻璃板(一个完整的板和一个切口),两个0.75毫米厚间隔物,和一个12孔的梳子。放置在平坦的表面上的一个玻璃板上每侧的两个间隔件和在顶部的切口板。剪辑在一起使用由供应商提供的四个片段的两大板块。
- 在150毫升的塑料烧杯中,倒入40含10%dPAGE的,四甲基乙二胺的40微升(TEMED)和400微升10%过硫酸铵(APS)的。混合组分和倾慢慢板之间的溶液。
- 插入梳子,然后使凝胶聚合在10分钟内。一旦凝胶聚合,慢慢取出梳子,用的DDH 2 O冲洗井
- 安装在板上的凝胶电泳装置。使用金属板来消散所产生,以防止过热的热量。
- 倾1×TBE上的顶部和底部腔室并确保井在缓冲器井淹没。冲洗使用吸液管或注射器用1×TBE的孔中。
- 设置装载样品前15分钟在35马润的设备和预运行。
- 从10%dPAGE结扎EC1的溶出
- 以下步骤2.2.6,运行在35毫安直到底部染料(溴酚蓝)凝胶到达玻璃板的底部。这大约需要1.5小时。关闭电源,并从该装置中删除所述板。
- 躺在纸巾几片板并小心地从玻璃板取下隔离物。小心取出顶部玻璃板并用保鲜膜包住凝胶。
- 翻转凝胶上以除去第二玻璃板,并再次用保鲜膜覆盖。请注意,以避免保鲜膜的皱纹。
- 通过使用UV遮蔽(260纳米),这将产生4个不同的DNA条带(完全连接的EC1,DE1,BS1和T1)可视化的连接产物。
- 消费用无菌刀片并转移到1.5ml的微离心管的顶部带(EC1)。粉碎用无菌枪头(200微升针尖大小),凝胶,直到它变成一个精膏。加入550微升DNA洗脱缓冲液,覆盖铝箔管,以保护嵌入荧光团,振摇15分钟。
- 离心以20,000 xg离心凝胶溶液5分钟,并小心地加入400μl的上清液转移至另外的150 ml离心管。避免在移液吸胶块。
- 为了与转移的上清液的离心管中添加40微升的3M醋酸钠(pH值5.2)和1.0冷100%乙醇的溶液中。混合通过涡旋该溶液,然后将管在-20℃下冷冻30分钟。
- 离心机离心在20000 XG在4℃下20分钟。小心通过移液除去上清液。
- 在4℃下以20,000 xg离心再次洗用冷70%乙醇,离心沉淀10分钟。再次,删除上清液并干燥在真空下将沉淀10分钟。
- 通过在260nm处测量UV吸光度确定EC1的浓度。使10μM的库存和样品储存在-20℃直到使用。
3.尿素酶的DNA的共轭
- 琥珀酰亚胺4-(N-马来酰亚胺甲基)环己烷-1-羧酸酯(SMCC)股票的制备
- 溶于676微升DMSO的1毫克SMCC。涡置于冰上直至使用。
- 股票脲酶的制备
- 在1毫升1×PBS(无镁离子和钙离子 )的溶解1毫克尿素酶。在冰上放置直至使用。
注:结晶尿素酶溶解缓慢且需要温和的混合,以避免变性。
- 在1毫升1×PBS(无镁离子和钙离子 )的溶解1毫克尿素酶。在冰上放置直至使用。
- 脲酶DNA的合成(UrDNA)
- 加10μl100μMLD1至2.5 ml离心管。加入140微升的DDH 2 O,40微升10X的PBS,旋涡。
- 添加80.5微升SMCC的S滴答,DMSO,旋涡,短暂离心的159.5微升使用台式离心机。
- 孵育在37℃下60分钟。避免离心帽下冷凝。
- 加入200μl1×PBS中的60微升的3M醋酸钠(pH值5.2)和1.5冷100%乙醇的溶液中。混合通过涡旋该溶液并在-20℃下孵育30分钟。
- 离心在4℃下以20,000 xg离心该溶液20分钟。除去上清液,并在真空下干燥沉淀。避免过度干燥。
- 向干燥的沉淀中添加400μl的脲酶的库存中,并在室温下孵育5小时。
- 转移200μl的粗共轭物的到预先洗涤100k的MWCO离心过滤柱。在14000 xg离心离心柱5分钟。
- 传输剩余200μl的粗共轭到柱和离心机在14,000rpm xg离心5分钟。删除列,把它倒挂在一个新的2.0 ml离心管("收集管")。
- CentrifUGE的收集管(与倒置列)在1000 xg离心2分钟。取下收集管,并添加30微升1×PBS中的离心柱来洗膜对偶联物的额外的恢复。
- 再次反转柱放回收集管。在1000 xg离心离心收集管(与倒置列)2分钟。移走和处置列。
- 存储UrDNA在4℃直至使用。
4. EC1和UrDNA装配到磁珠
- 混合磁珠(MB)的股票以及100微升MB悬挂转移到1.5 ml离心管中。放置离心管上用于隔离所述MB的磁性架子保持部。
- 通过移液除去上清液,并添加150微升的结合缓冲液(BB)的管中。从保持器中取出管,并仔细挖掘管悬浮MB到均匀的溶液。避免飞溅悬挂到T管或盖的运算。如果发生这种情况,可使用台式离心机,以旋转简要残余物中回悬浮液中。
- 重复步骤4.2两次。
- 在此悬浮液加入10微升10μMEC1的。小心地敲打管子混用。
- 孵育温和振摇30分钟的溶液中。挖掘管每隔2-3分钟,以避免对MB的沉淀。
- 放置离心管背面上的磁性机架隔离MB和通过移液除去上清液。用的BB 150微升(如在步骤4.2中所述)洗MB的三倍。
- 一旦在洗涤完成后,暂停所述MB在总共150微升的BB。向此溶液中,添加15微升UrDNA和加热该溶液至45℃持续2分钟。冷却该溶液至室温,并孵育2小时。
- 放置离心管背面上的磁性机架隔离MB和通过移液除去上清液。
- 加入100微升反应缓冲液(RB)的。除去叔他从磁架离心管,小心地悬浮MB。
- 通过重复步骤4.9洗MB三次。
注意:洗涤上清液可以快速测试,以确定未杂交UrDNA仍然存在,这可能会导致假阳性信号。测试可以通过加入50mM的尿素的10微升和10微升0.04%酚红的洗涤溶液来完成。继续洗MB直到洗涤液中不引起颜色变化。在100微升的悬浮液贮存于4℃直至使用。
5.制备细菌细胞20的
- E.培养大肠杆菌从股票
- E.板大肠杆菌 K12(MG1655)上从一个甘油下火焰或在生物安全柜的LB琼脂平板上。
- 使用无菌移液管尖端,轻轻触摸甘油和轻轻条纹的琼脂平板的表面上,以避免刺穿LB琼脂。
- 反转挑染板孵育在37℃下14小时。
- 密封在4℃下用封口膜板和存储最多3周。
- E.培养大肠杆菌的细胞计数
- 在无菌14毫升培养管,取2毫升LB肉汤。
- 使用无菌移液管尖端,从在步骤5.1中制备的划线琼脂平板挑取单菌落,并将其转移到培养管中。
- 孵育培养在37℃和以230rpm 14小时摇动。
- 连续稀释细菌培养的10倍区间。
- 对于每个稀释样品,均匀地镀上分开的LB琼脂平板个100微升等分试样。倒置平板,并且在37°C孵育14小时。
- 算每个样品的细胞,以获得每个稀释的平均细胞浓度。
注:10 7个细胞经常被用来设置为E.基准石蕊试大肠杆菌作为这个级别的E.大肠杆菌可引发快速的陈色GE。然而,正确执行试金石可检测低至500个细胞,如结果部分讨论。
- E.准备大肠杆菌细胞试验
- 对于所需的细胞悬液,转移1毫升培养股票到1.5 ml离心管。
- 离心细胞以6000 xg离心在4℃下10分钟。小心取出上清液,而不会干扰细胞沉淀。
- 添加10微升反应缓冲液中的细胞沉淀重悬细胞。超声处理的细胞悬浮液5分钟。细胞悬液转移到冰盒5分钟。
- 声波处理另一个5分钟的细胞悬浮液。
- 离心细胞悬浮液以13,000rpm xg离心在4℃下10分钟。用于测试的上清液(10微升)。
6.试金石
- 在1.5 ml离心管中,通过在离心管中添加和涡旋100微升反应缓冲液(RB)的的预洗管弃去缓冲液。
- 转移15微升组装EC1(协议4)向洗涤微量离心管。
- 通过将离心管上的磁性机架洗涤磁珠。通过移液除去上清液。从机架上卸下的离心管中,添加的RB的100微升,并小心地重悬磁珠。
- 通过重复步骤6.3洗MB两次以上。
- 放置离心管放回磁架,除去上清液,并添加10微升大肠杆菌从步骤5.3编写的大肠杆菌样本。
- 通过在离心管轻轻拍打仔细混合样品和磁性珠粒。
- 在室温下孵育1小时的反应。
- 向反应中,添加90微升DDH 2 O的和离心管放置到一个磁架。
- 后大约3分钟磁选,仔细转移85微升上清液至0.5 ml离心管。慢慢拔出上清避免收集任何磁珠。
- 向上述离心管加入15微升的0.04%酚红,和100微升的底物溶液。
- 采取以特定时间间隔的照片以记录颜色变化。
注:在pH值的变化也可以使用pH计用微电极监测。起始pH值应为大约5.2-5.5(溶液是黄色)。如果不是,则溶液可以通过添加1mM醋酸缓冲液pH 5.0)进行调整。
结果
细菌石蕊试验的原理在图1中说明的测试使用了三个关键材料:由一特定细菌酶,脲酶和磁珠活化的RNA切割核酶。所述核酶被用作分子识别元件,以实现所关注的细菌具有高度特异性的检测。脲酶和磁性珠粒用来实现DNA核酶的RNA的切割活性的信号转导。这涉及含有尿素酶结合物核酶磁珠的创建。在目标细菌的存在,所述核酶裂解其RNA的联动。这个动作引起脲酶从磁珠的解离。被释放的尿素酶可以从磁珠容易分离并用于产生在一个记者溶液,其中包含脲和pH敏感染料的颜色变化。尿素酶水解尿素成氨,伴随着触发℃pH值的增加染料的olor变化。
图2给出细菌试金石EC1的地方,一个E.大肠杆菌响应性的RNA切割核酶,用作核酶和酚红被用作pH值报告染料。 EC1先前由我们的组使用体外选择的技术中一个随机序列的DNA池隔离。5我们先前的研究已经表明,EC1为大肠杆菌高度特异性大肠杆菌和表现出最小的活动对其他细菌。5,19业已发现,EC1由来自大肠杆菌的蛋白质分子活化大肠杆菌。虽然这种蛋白质生物标记的身份还没有被破译,高识别特异性表明,这种蛋白是唯一的大肠杆菌大肠杆菌 。记者溶液被设置为具有5.5的初始pH值。在此pH,酚红呈现黄色。作为脲酶水解尿素成氨,记者溶胶的碱度ution增加。这是通过颜色从黄色到粉红色逐渐变化反映出来。颜色变化的深度依赖于以下两个参数,通过图2所示: 大肠杆菌的数量在核酶活化步骤和所允许的尿素水解步骤时所使用的大肠杆菌细胞。更多E.大肠杆菌细胞产生了更强的色彩变化,通过一个渐进的黄色到粉红色过渡时E.观察反映大肠杆菌细胞被连续从增加的5至5×10 7(10倍,每次增加)。同时,允许用于检测大肠杆菌的人数较少的尿素水解的时间较长大肠杆菌细胞(5,000个细胞在1小时的反应和500个细胞在2小时的反应)。
细菌石蕊测试的pH值的变化,也可以使用手持式pH计监测和代表性的结果在图3中示出。这WA作者发现10 7 E.存在的大肠杆菌细胞产生了由3个单位在10分钟内pH值的逐渐增加。与此相反,不存在E的大肠杆菌细胞没有造成相同的设置下可检测的pH变化。
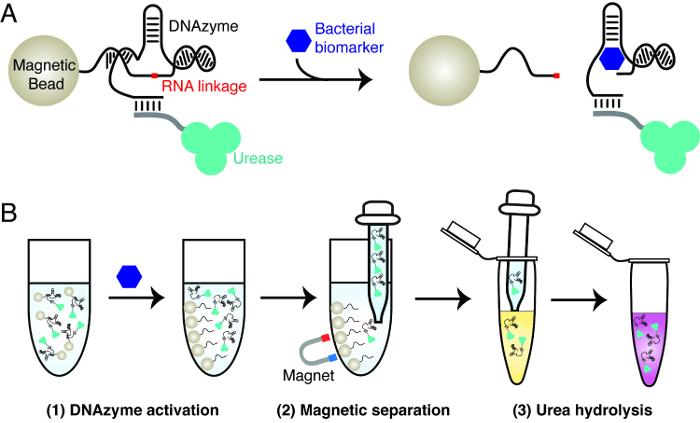
图1: 细菌试金石的设计原理 (A)由感兴趣的细菌的生物标志物特定的RNA裂解脱氧核酶的激活。在生物标志物的存在下,该RNA切割核酶固定于磁珠裂解RNA的联动,导致从磁性珠溶液中标记的脲酶的释放。 (B)的三步骤检测程序。第1步:激活核酶,如面板A.步骤2中所述:磁选 - 释放的脲酶是从磁珠分离。第3步:尿素水解 - [R12;被释放的尿素酶加入到一个含脲 - 报道溶液。尿素酶水解尿素成氨,导致pH的变化,可以通过pH敏感的染料进行报告。 请点击此处查看该图的放大版本。

图 2:E. 试金石 使用 大肠杆菌 E.大肠杆菌 响应性脱氧核酶EC1代表性的颜色变化结果与大肠杆菌的不同数量每个试管上方设置的大肠杆菌细胞。酚红被用作pH敏感染料。 E.没有一个测试大肠杆菌用作阴性对照。更多E.预期的大肠杆菌细胞以引起更多的脲酶分子的释放,伴随bŸ较强的颜色变化。 请点击此处查看该图的放大版本。
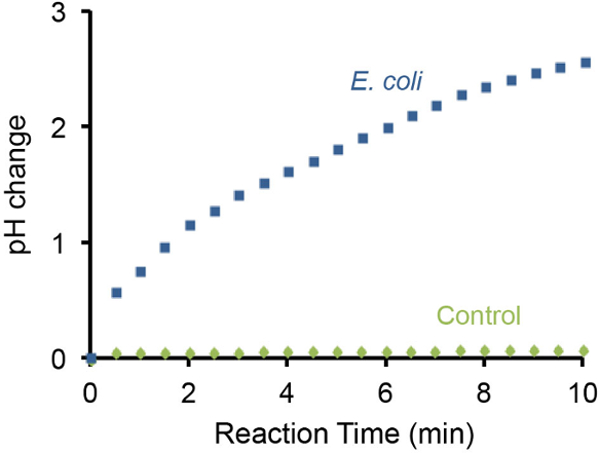
图3: 使用pH计监测pH上升引起的10 7 大肠杆菌的pH的变化。使用便携式pH计的大肠杆菌细胞进行监控。 E.没有一个测试大肠杆菌用作阴性对照。 10 E. 7的存在大肠杆菌细胞中的测试解决方案可以增加3〜pH单位的碱度在10分钟内。 请点击此处查看该图的放大版本。
| 名称 | 硒quence(5'-3') | 注意 |
| BS1 | BTTTT TTTTT TTTAC TCTTC CTAGC FRQGG TTCGA TCAAG一个 | A:5'-生物素; R:腺嘌呤核苷酸; F:荧光胸苷;问:DABCYL胸苷 |
| DE1 | GATGT GCGTT GTCGA GACCT GCGAC CGGAA CACTA CACTG TGTGG GGATG GATTT CTTTA CAGTT GTGTG TTGAA CGCTG TGTCA AAAAA AAAA | |
| T1 | GACAA CGCAC ATCTC TTGAT CGAACÇ | |
| LD1 | XTTTT TTTTT TTTTT TTGAC ACAGC GTTCA一个 | X:5'-NH 2的 |
表1: 合成的寡核苷酸的序列。
讨论
的细菌响应核酶到石蕊试验的RNA裂解活性的作用的翻译是通过使用脲酶和磁分离的成为可能,由图1所示。虽然改性石蕊试验的细菌检测示范是与E.完成大肠杆菌依赖性的RNA切割核酶,5,19,20设计通常可延伸为任何的RNA切割核酶。定为不同的分析物,并且各种方法的RNA切割DNA核酶的极大可用性来隔离从新的目标随机序列池新的RNA切割DNA核酶,我们预计,改性石蕊测试平台可以扩展到检测的感兴趣的多样目标。
为E.的试金石大肠杆菌的检测可以检测5000和500个细胞时,报告的反应时间被设定为1和2小时,分别。流行的聚合酶链反应(PCR)和夹心酶联免疫吸附测定(ELISA)方法可达到约10 4 -10 5 大肠杆菌的检测限大肠杆菌细胞在类似的测试时间。25,26因此,细菌试金石提供可比的检测灵敏度。
虽然细菌石蕊测试是容易进行,并且能够产生鲜明的色彩的变化,有几个因素可以显著影响测试结果。首先,脲酶的质量是非常重要的。我们已经从不同的源中使用的脲酶,并发现这些检测结果可以显著变化。我们建议使用尿素酶从材料部分指定的来源。
核酶的/脲酶/磁珠大会需要特别注意。磁珠彻底清洗以除去未杂交的UrDNA必须防止假阳性结果。护理也需要采取避免升的内表面上的残留磁性珠的堆积微量离心管中,这可能是难以看到的标识。一旦出现,使磁珠不再进行磁分离,因此,可能会携带一些未杂交UrDNA,可导致在记者反应假阳性信号。同样重要的是,以避免在磁分离步骤留下的磁性机架离心管长于10分钟。珠粒可聚集或粘到离心管,这可以减少洗涤效率并引入批与批不一致性。在洗涤溶液0.01%吐温-20的夹杂物可以改善批与批之间的一致性,并应来实现。
磁珠涂覆有链霉亲和,将其用作锚组装核酶脲酶偶联物上的磁性珠粒。两个链霉和脲酶是可以储存期间变性的蛋白质分子。我们通常在4℃下保存组装的DNA酶脲酶磁珠长达4周并定期作出新的批次,以获得更一致的结果。
护理也需要注意避免意外服用磁珠在以下核酶活化的磁性分离步骤(步骤6.9)。从我们的经验,细胞碎片和在溶液中的其它颗粒可降低磁分离效率,并且因此,一些磁性珠可以无意中移液中取出。这将导致假阳性结果。我们建议采取以下措施来缓解这个问题:一个较长的分离时间(如5-10分钟),压力对吸管较慢的释放,使上清温柔的撤离,以及对上清液进行另一轮磁选。
最后,为了避免其中多个样品进行测试的实验过程中,记者原液通过脲酶的意外污染是重要的。由于u的高反应性rease,这种性质的污染可导致假阳性结果。
披露声明
The authors have nothing to disclose.
致谢
The funding for this research project was provided by the Natural Sciences and Engineering Research Council of Canada (NSERC) via a Discovery Grant to YL.
材料
| Name | Company | Catalog Number | Comments |
| Ethylenediaminetetraacetic acid (EDTA) | VWR AMRESCO | 0105 | |
| Sodium Hydroxide (NaOH) pellets | BIO BASIC CANADA INC. | SB6789 | |
| Tris-base | VWR AMRESCO | 0497 | |
| Boric acid | AMRESCO | 0588 | |
| Urea | VWR AMRESCO | M123 | |
| 40% acrylamide/bisacrylamide (29:1) solution | BIO BASIC CANADA INC. | A0007 | |
| Sucrose | Bioshop Canada inc. | SUC507 | |
| Bromophenol blue | Bioshop Canada inc. | BRO777 | |
| Xylenecyanol FF | SIGMA-ALDRICH | X-4126 | |
| 10% sodium dodecyl sulfate | Bioshop Canada inc. | SDS001 | |
| Hydrochloric Acid (HCl) | CALEDON LABORATORIES LTD | 6026 | |
| Sodium Chloride (NaCl) | Bioshop Canada inc. | SOD001 | |
| 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES) | Bioshop Canada inc. | HEP001 | |
| Magnesium Chloride (II) hexahydrate | VWR AMRESCO | 0288 | |
| Tween 20 | Bioshop Canada inc. | TW508 | |
| Adenosine Triphospahte (ATP) | AMRESCO | 0220 | |
| Sodium Acetate trihydrate (NaOAc) | SIGMA-ALDRICH | S8625 | |
| Ethanol | Commercial Alcohols | P016EAAN | |
| Tetramethyleneethylenediamine (TEMED) | AMRESCO | 0761 | |
| 10% Ammonium persulfate (APS) | BIO BASIC CANADA INC. | AB0072 | |
| Succinimidyl 4-(N-maleimidomethyl) cyclohexane-1-carboxylate (SMCC) | ThermoFisher SCIENTIFIC | 22360 | |
| Dimethyl sulfoxide (DMSO) | CALEDON LABORATORIES | 803540 | |
| Urease | SIGMA-ALDRICH | U0251 | |
| 1x Phosphate Buffered Saline (PBS) | ThermoFisher SCIENTIFIC | 70011-069 | |
| 0.04% Phenol red | SIGMA-ALDRICH | P3532 | |
| 10x T4 polynucleotide kinase reaction buffer | Lucigen | 30061-1 | |
| 10x T4 DNA ligase reaction buffer | Bio Basics Canada | B1122-B | |
| T4 DNA ligase (5 U/μl) | Thermo Fischer Scientific | B1122 | |
| Luria Bertani (LB) Broth | AMRESCO | J106 | |
| Agar | AMRESCO | J637 | |
| T4 polynucleotide kinase (10 U/μl) | Lucigen | 30061-1 | |
| E. coli K12 (MG1655) | ATCC | ATCC700926 | |
| Centrifuge | Beckman Coulter, Inc. | 392187 | |
| Glass plates | CBS scientific | ngp-250nr | |
| 0.75 mm thick spacers | CBS scientific | VGS-0725r | |
| 12-well comb | CBS scientific | VGC-7512 | |
| UV Lamp | UVP | 95-0017-09 | |
| Spectrophotometer (NanoVue) | GE Healthcare | N/A | |
| Metal plate | CBS scientific | CPA165-250 | |
| Vortex | VWR International | 58816-123 | |
| Gel electrophoresis apparatus | CBS scientific | ASG-250 | |
| Petri dishes | VWR International | 25384-342 | |
| 100 kDa MWCO centrifugal filters | EMD Millipore | UFC510024 | |
| Magnetic Bead (BioMag) | Bangs Laboratories Inc | BM568 | |
| Magnetic Seperation Rack | New England BioLabs | S1506S | |
| Microfuge tubes | Sarstedt | 72.69 | |
| Syringe filter (0.22 μm) | VWR International | 28145-501 | |
| 14 ml culture tube | VWR International | 60818-725 | |
| Cell culture incubator | Eppendorf Scientific | M13520000 | |
| Branson Ultrasonic cleaner | Branson | N/A | |
| Camera (Canon Powershot G11) | Canon | N/A | |
| 50 ml conical tube | VWR International | 89004-364 |
参考文献
- Daar, A. S., et al. Top ten biotechnologies for improving health in developing countries. Nat. Genet. 32, 229-232 (2002).
- Newman, J. D., Turner, A. P. Home blood glucose biosensors: a commercial perspective. Biosens. Bioelectron. 20, 2435-2453 (2005).
- Turner, A. P. Biosensors: sense and sensibility. Chem. Soc. Rev. 42, 3184-3196 (2013).
- Tram, K., Kanda, P., Salena, B. J., Huan, S. Y., Li, Y. F. Translating Bacterial Detection by DNAzymes into a Litmus Test. Angew. Chem. Int. Ed. 53, 12799-12802 (2014).
- Ali, M. M., Aguirre, S. D., Lazim, H., Li, Y. Fluorogenic DNAzyme probes as bacterial indicators. Angew. Chem. Int. Ed. 50, 3751-3754 (2011).
- Schlosser, K., Li, Y. Biologically inspired synthetic enzymes made from DNA. Chem. Biol. 16, 311-322 (2009).
- Tuerk, C., Gold, L. Systematic evolution of ligand by exponential enrichment: RNA ligands to bacteriophage T4 DNA polymerase. Science. 249, 505-510 (1990).
- Ellington, A. D., Szostak, J. W. In vitro selection of RNA molecules that bind specific ligands. Nature. 346, 818-822 (1990).
- Breaker, R. R. Making catalytic DNAs. Science. 290, 2095-2096 (2000).
- Liu, J., Cao, Z., Lu, Y. Functional nucleic acid sensors. Chem. Rev. 109, 1948-1998 (2009).
- Navani, N. K., Li, Y. Nucleic acid aptamers and enzymes as sensors. Curr. Opin. Chem. Biol. 10, 272-281 (2006).
- Li, J., Lu, Y. A highly sensitive and selective catalytic DNA biosensor for lead ions. J. Am. Chem. Soc. 122, 10466-10467 (2000).
- Liu, J., Lu, Y. A colorimetric lead biosensor using DNAzyme-directed assembly of gold nanoparticles. J. Am. Chem. Soc. 125, 6642-6643 (2003).
- Liu, Z., Mei, S. H. J., Brennan, J. D., Li, Y. Assemblage of signaling DNA enzymes with intriguing metal specificity and pH dependence. J. Am. Chem. Soc. 125, 7539-7545 (2003).
- Liu, J., et al. A catalytic beacon sensor for uranium with parts-per-trillion sensitivity and millionfold selectivity. Proc. Natl. Acad. Sci. USA. 104, 2056-2061 (2007).
- Huang, P. J., Vazin, M., Liu, J. In vitro selection of a new lanthanide-dependent DNAzyme for ratiometric sensing lanthanides. Anal. Chem. 86, 9993-9999 (2014).
- Chiuman, W., Li, Y. Simple fluorescent sensors engineered with catalytic DNA 'MgZ' based on a non-classic allosteric design. PLoS One. 2, e1224(2007).
- Xiang, Y., Lu, Y. Using personal glucose meters and functional DNA sensors to quantify a variety of analytical targets. Nat. Chem. 3, 697-703 (2011).
- Aguirre, S. D., Ali, M. M., Salena, B. J., Li, Y. A sensitive DNA enzyme-based fluorescent assay for bacterial detection. Biomolecules. 3, 563-577 (2013).
- Aguirre, S. D., Ali, M. M., Kanda, P., Li, Y. F. Detection of Bacteria Using Fluorogenic DNAzymes. J. Vis. Exp. (63), e3961(2012).
- Shen, Z., et al. A catalytic DNA activated by a specific strain of bacterial pathogen. Angew. Chem. Int. Ed. 54, (2015).
- He, S., et al. Highly specific recognition of breast tumors by an RNA-cleaving fluorogenic DNAzyme probe. Anal. Chem. 87, 569-577 (2015).
- Sumner, J. B., Hand, D. B. Isoelectric point of crystalline urease. J. Am. Chem. Soc. 51, 1255-1260 (1929).
- Karplus, P. A., Pearson, M., Hausinger, R. P. 70 Years of crystalline urease: What have we learned. Acc. Chem. Res. 30, 330-337 (1997).
- Omiccioli, E., Amagliani, G., Brandi, G., Magnani, M. A new platform for Real-Time PCR detection of Salmonella spp., Listeria monocytogenes and Escherichia coli O157 in milk. Food Microbiol. 26, 615-622 (2009).
- Cui, S., Schroeder, C. M., Zhang, D. Y., Meng, J. Rapid sample preparation method for PCR-based detection of Escherichia coli O157:H7 in ground beef. J. Appl. Microbiol. 95, 129-134 (2003).
- Ibekwe, A. M., Watt, P. M., Grieve, C. M., Sharma, V. K., Lyons, S. R. Multiplex fluorogenic real-time PCR for detection and quantification of Escherichia coli O157:H7 in dairy wastewater wetlands. Appl. Environ. Microbiol. 68, 4853-4862 (2002).
- Strachan, N. J., Ogden, I. D. A sensitive microsphere coagulation ELISA for Escherichia coli O157:H7 using Russell's viper venom. FEMS Microbiol Lett. 186, 79-84 (2000).
- de Boer, E., Beumer, R. R. Methodology for detection and typing of foodborne microorganisms. Int. J. Food Microbiol. 50, 119-130 (1999).
- Gracias, K. S., McKillip, J. L. A review of conventional detection and enumeration methods for pathogenic bacteria in food. Can. J. Microbiol. 50, 883-890 (2004).
转载和许可
请求许可使用此 JoVE 文章的文本或图形
请求许可探索更多文章
This article has been published
Video Coming Soon
版权所属 © 2025 MyJoVE 公司版权所有,本公司不涉及任何医疗业务和医疗服务。