Method Article
Capturing Common Fragile Site Breaks by Native γH2A.X ChIP
In This Article
Summary
We present a rapid and efficient method to detect common fragile site breaks through native γH2A.X chromatin immunoprecipitation (ChIP). This approach significantly reduces both the time and labor associated with traditional γH2A.X ChIP assays while maintaining high reproducibility and reliability of results.
Abstract
Replication stress induced by exposure to extrinsic agents can lead to DNA breaks at common fragile sites, which are regions in the genome known to be prone to structural instability. The γH2A.X chromatin immunoprecipitation (ChIP) assay serves as a powerful tool in genotoxicity studies, as γH2A.X phosphorylation is a well-established marker for DNA double-strand breaks. Traditional γH2A.X ChIP assays, however, are often labor-intensive and involve multiple, time-consuming steps. In this study, we present a simplified yet effective method that combines subcellular fractionation with native ChIP to isolate γH2A.X-associated complexes. This approach is particularly suitable for analyzing γH2A.X-chromatin interactions with enhanced specificity and efficiency. Using subcellular fractionation, chromatin-unbound materials are effectively removed, resulting in a purified chromatin fraction. Subsequent micrococcal nuclease (MNase) digestion under mild conditions allows chromatin fragmentation while preserving physiological interactions between γH2A.X and its associated protein complexes. This preservation is essential for studying native interaction partners involved in DNA damage response pathways. This optimized native ChIP protocol substantially reduces the time and labor associated with conventional γH2A.X ChIP assays. The streamlined procedure not only simplifies the workflow but also yields highly reproducible results, making it particularly advantageous in settings where high-throughput processing of multiple samples is required. This method has broad applicability in studies focused on genome stability, DNA repair, and chromatin biology, where accurate and efficient detection of DNA damage sites is critical. By employing optimized protocols and streamlined steps, this method enables the detection of DNA damage at fragile sites with improved sensitivity and minimal sample handling, making it a valuable tool for studies on genome stability and DNA damage response.
Introduction
Common fragile sites (CFSs) are large chromosomal regions found on every human chromosome prone to breaking during metaphase. Under replication stress, replication at these regions is significantly delayed, preventing their complete duplication before mitotic entry1, which ultimately results in site-specific gaps and breaks. CFSs are hotspots for chromosomal instability and are a major cause of chromosomal rearrangements during early cancer development. Replication stress, which is often present under tumorigenic conditions, can lead to the loss of tumor suppressor genes and amplification of oncogenes-collectively referred to as copy number variation (CNV)2,3,4,5,6. Additionally, CFSs are highly prone to viral integration, further promoting cancer development7,8,9,10. Multiple homozygous deletions of tumor suppressor genes have been detected in CFS regions during pan-cancer analyses of primary tumors. The most commonly affected CFSs in cancer include FRA2F, FRA3B, FRA4F, FRA5H, and FRA16D11. CFSs are particularly vulnerable to breakage in the presence of extrinsic carcinogenic agents12. To assess the detrimental carcinogenic effects of environmental contaminants, a fast and reliable method for quantifying CFS break occurrence is needed.
Phosphorylation of H2A.X at the serine residue 139 (γH2A.X) by Ataxia Telangiectasia and Rad3-Related Protein (ATR) or Ataxia Telangiectasia Mutated (ATM) is a key event in signaling replication fork stalling13. γH2A.X serves as an indicator of stalled replication forks prior to double-strand break (DSB) formation13, creating a favorable chromatin environment to facilitate the efficient recruitment of repair proteins to stalled sites. Additionally, γH2A.X can be recruited to break sites following fork collapse14,15, consistent with its primary role in DSB repair. Since CFS breaks are closely associated with chromosomal aberrations that drive cancer progression, detecting these breaks can be instrumental in understanding the early stages of tumorigenesis. The presence of γH2A.X at CFSs can be used as a biomarker to detect early events of genomic instability. This information can help identify potential carcinogens and evaluate the risk associated with exposure to various extrinsic agents. By measuring DNA breaks at CFSs induced by extrinsic agents, γH2A.X chromatin IP (ChIP) can provide insight into how such agents contribute to the mechanisms underlying tumorigenesis.
In the conventional ChIP (i.e., Cross-linked ChIP, X-ChIP), the association of γH2A.X with its target DNA sequences is stabilized by reversible formaldehyde crosslinking. Chromatin is subsequently sheared to fragments of approximately 500 base pairs (bp) through sonication, and the resulting solution is cleared of debris by sedimentation16,17,18. A ChIP-grade γH2A.X antibody is then added to the cleared chromatin fraction, followed by the addition of Protein A/G agarose beads to enrich for γH2A.X-bound chromatin regions16,17,18. The immune complexes (i.e., beads-antibody-γH2A.X-targeted DNA complex) are washed multiple times with stringent washing buffers to remove nonspecifically bound DNA fragments16,17,18. After washing, the specifically bound DNA is eluted from the immune complexes. The formaldehyde cross-links are then reversed, followed by protein digestion using proteinase K, after which the enriched DNA is purified and concentrated16,17,18. To assess the γH2A.X-associated regions, PCR, quantitative PCR (qPCR), or direct sequencing is used16,17,18. The occupancy of γH2A.X at specific regions, such as CFS, is determined by the intensity of the PCR or qPCR signal, which is proportional to the amount of γH2A.X bound at that location, providing insights into site-specific DNA damage and repair events16,17,18.
Despite being a powerful experimental approach, the X-ChIP has several significant limitations: (i) it requires a large number of cells, typically in the range of 1 x 107 to 5 x 107, due to the inefficiency of antibody precipitation associated with fixation, which increases the overall cost of the experiment19; (ii) the process of reversing formaldehyde cross-links and subsequent DNA purification is time-consuming and labor-intensive, making it challenging to maintain consistency and reliability in results; and (iii) γH2A.X-DNA interactions with minor functional significance may not be distinguished from those with greater significance because the cross-linking step can stabilize transient interactions, leading to the detection of interactions that may not be biologically relevant19.
Native chromatin immunoprecipitation (Native ChIP or N-ChIP) is an essential biochemical technique used to study protein-DNA interactions within their native chromatin context under physiological salt conditions. It has been instrumental in elucidating the spatial and temporal organization of chromatin, transcription factor binding, and histone modifications. Native ChIP has a long-standing role in the broader field of chromatin biology and epigenetics, providing unique advantages and limitations compared to X-ChIP. This method, introduced in the late 1980s20, involves the isolation of chromatin from cells by methods that preserve its native structure, such as digestion with micrococcal nuclease (MNase)21. This preserves the inherent protein-DNA and histone-DNA contacts, which makes Native ChIP particularly well-suited for studying histone modifications and nucleosome positioning in their natural chromatin setting22. High-resolution Native ChIP studies have demonstrated the use of MNase digestion to reduce chromatin to individual nucleosomes, which facilitates the mapping of histone modifications with greater accuracy23. Furthermore, because no chemical cross-linking is involved, the risk of introducing biases or artifacts that might misrepresent the protein-DNA interactions is minimized24.
In contrast to X-ChIP, where formaldehyde or other cross-linking agents are used to fix protein-DNA interactions, Native ChIP provides a more realistic view of chromatin by avoiding potential cross-linking artifacts. However, while X-ChIP is generally better suited for detecting transient or dynamic interactions between DNA and regulatory proteins25, Native ChIP is ideal for stable protein-DNA interactions, such as histones or other chromatin-bound proteins26,27. One of the limitations noted for Native ChIP is the inability to capture low-affinity or transient binding events, which are often stabilized through cross-linking in X-ChIP25.
A significant body of work in epigenetics has leveraged Native ChIP to uncover histone modifications in diverse biological settings28. These efforts have been crucial in defining the histone code - the pattern of histone modifications that regulate gene expression and chromatin dynamics29. Although H2A.X is a less strongly associated linker histone, the native H2A.X ChIP method has been successfully applied in embryonic stem cells30. In this study, we optimized a chromatin extraction procedure to perform Native ChIP of γH2A.X in human 293T cells (Figure 1). Hydroxyurea and aphidicolin are widely used in research to investigate DNA replication stress, damage, and genomic instability31. In this study, these agents were applied to cells to induce replication stress and generate DNA breaks at CFS.
Using starting material of approximately 1 x 106 to 5 x 106 cells, this method can be divided into four main stages: (i) subcellular fractionation to isolate chromatin, (ii) micrococcal nuclease (MNase) digestion to fragment chromatin, (iii) immunoprecipitation and elution, and (iv) DNA analysis by quantitative PCR (qPCR). Conducting ChIP following subcellular fractionation provides several benefits and has been well-documented in numerous studies32,33,34,35. This approach allows for the removal of chromatin-unbound proteins and other cellular debris, resulting in a highly purified chromatin fraction. By isolating chromatin before immunoprecipitation, subcellular fractionation helps maintain native chromatin interactions and reduces background noise from non-chromatin-associated proteins, which leads to more specific and reliable results, as only chromatin-bound complexes are retained for analysis. Moreover, subcellular fractionation enables milder conditions for chromatin digestion, thereby preserving physiological protein-DNA interactions and offering a more accurate representation of chromatin dynamics within the native cellular environment.
Using native ChIP of γH2AX to measure the impact of extrinsic agents on common fragile site breakage holds significant potential for cancer research. This technique enables the detection of DNA damage induced by exposure to environmental carcinogens, providing insights into the molecular mechanisms by which pollutants contribute to genomic instability and cancer development. By preserving the native chromatin context, this method facilitates the accurate assessment of DNA damage patterns associated with carcinogenic exposure, aiding in the evaluation of environmental risks and the study of pollution-driven tumorigenesis.
Protocol
1. Cell harvesting
- Seed about 5 x 105 HEK 293T cells into each of the four 6 cm dishes, each containing 4 mL of complete DMEM medium.
- After 24 h, treat one dish with 2 µL of 1 mM Aphidicolin (refer to Table of Material) stock solution (final concentration of 0.5 µM) and another dish with 20 µL of 1 M hydroxyurea (refer to Table of Material) stock solution (final concentration of 5 mM) to induce replication stress. Add DMSO to the remaining two dishes to serve as controls.
- After 24 h of treatment, discard the culture media. One 6 cm plate typically yields approximately 2 x 106 cells at 60%-70% confluence.
- Rinse each dish 2x with 5 mL of ice-cold 1x PBS. Use cell scrapers to detach the cells and transfer the cell suspension to four individual 1.5 mL tubes. Gently pipette up and down with a P1000 pipette to dissociate any cell clumps.
- Centrifuge the cells at 500 x g for 5 min at 4 °C, then discard the supernatant. Place the cells on ice.
2. Subcellular fractionation
- Resuspend the cell pellet in 500 µL of freshly prepared cold Buffer A (refer to Table 1), ensuring the complete dissociation of cell clumps by gentle pipetting.
- Incubate the lysates on ice for 5-10 min. Check lysis progression under a microscope to make sure complete cell lysis.
- Take a small aliquot of the lysate (around 5 - 10 µL) and place it on a clean microscope slide. Cover it with a coverslip to avoid contamination.
- Use a light microscope with an appropriate magnification (e.g., 20x - 40x) to visualize cells or debris. Compare with an un-lysed control sample to differentiate between intact cells and lysed material.
NOTE: A properly lysed sample will have no distinct cell outlines, only diffuse chromatin or cellular material. Adjust the focus to clearly observe the lysate. If needed, apply mechanical force during the lysis step, such as using a Dounce homogenizer, when working with certain cell types.
- Centrifuge at 500 x g for 5 min at 4 °C, once cells are completely lysed. Carefully discard the supernatant. Resuspend the nuclei pellet in 500 µL of cold Buffer A using wide-orifice pipette tips.
NOTE: Wide-orifice tips help minimize shear forces and protect delicate samples like chromatin. Make wide-bore tips by cutting the end of standard tips with a sharp blade. - Centrifuge at 500 x g for 5 min at 4 °C. Carefully discard the supernatant.
- Prewarm an incubator to 37 °C and prepare a stopping buffer (100 mM EDTA, pH 8.0; Table 1).
- Optimize Micrococcal Nuclease (MNase, refer to Table of Material) concentrations and incubation times in advance.
- Divide 40 µL of testing chromatin sample into several equal aliquots to test different MNase concentrations and incubation times.
- Use a range of MNase concentrations (e.g., 0.0625 U, 0.125 U, 0.25 U, 0.5 U, 1 U, 2 U, 4 U, 8 U per reaction) and test multiple incubation times (e.g., 2, 5, 10, and 15 min).
- Add MNase Buffer (refer to Table 1) containing various concentrations of MNase to the chromatin aliquots and incubate samples at 37 °C for the specified times.
- Terminate the reaction by adding 1/4 volume of stopping buffer (final concentration: 20 mM EDTA) immediately after the desired incubation time.
- Isolate DNA from the digested chromatin samples using a phenol/chloroform/isoamyl alcohol extraction method.
- Run the extracted DNA on a 1.5% agarose gel to visualize digestion patterns: Under-digestion will show high molecular weight bands (Figure 2, lane 1-4); over-digestion will result in a smear or very short fragments (Figure 2, lane 6-8), and optimal digestion will yield a clear nucleosomal ladder pattern (Figure 2, lane 5, e.g., mono-, di-, tri-nucleosomes).
- Identify the conditions that produce the desired nucleosomal resolution without excessive over-digestion.
NOTE: CaCl2 acts as a cofactor for MNase activity. Optimize the digestion by adjusting the CaCl2 concentration between 1 mM and 5 mM.
- Gently resuspend the intact nuclei with 100 μL of MNase Buffer by pipetting 5 - 10 times with wide-orifice tips. Immediately add the pre-determined amount of MNase to the samples (1.25 U MNase/100 µL MNase Buffer).
NOTE: When working with multiple samples, digest each one individually to avoid over-digestion. - Place the tubes on a rotator and incubate for 5 min at 37 °C. Immediately return the tubes to ice and terminate MNase digestion by adding EDTA to a final concentration of 20 mM and mix by vortexing.
- Add 500 µL of Buffer B (refer to Table 1) to each sample and mix thoroughly by pipetting up and down 5x - 10x. Solubilize proteins by incubating on ice for 5 min.
NOTE: The salt and detergent in Buffer B help dissociate weak chromatin-bound proteins and expose the epitopes for immunoprecipitation. - Pellet the insoluble material by centrifuging at maximum speed for 5 min at 4 °C. Transfer the clear supernatant to new 1.5 mL tubes labeled as the native chromatin fraction. The samples can either be stored at -80 °C or used to validate the efficiency of chromatin fragmentation.
NOTE: Avoid frequent freeze-thaw cycles, as they may disrupt protein-DNA interactions of interest. Minimize freeze-thaw cycles whenever possible.
3. Verification of chromatin fragmentation
- Aliquot 10 µL of the supernatant from each sample into a new 1.5 mL tube. Mix with 20 µL of distilled water and 30 µL of phenol/chloroform/isoamyl alcohol (25:24:1).
- Close the tubes tightly and vortex vigorously for 15-30 s. Centrifuge at 20,000 x g (or the maximum speed of the centrifuge) for 10 min at 4 °C. After centrifugation, three distinct layers will be observed: a clear top layer, a white middle layer, and a yellow bottom layer.
- Carefully transfer 20 µL of the upper aqueous phase (containing DNA) to a fresh tube. Separate the purified DNA in 1.5% agarose gel for 30 min at 100 V and visualize the digestion patterns. Ensure that the size of chromatin fragments is primarily between 200 and 1000 base pairs.
NOTE: Proper chromatin fragment sizes are crucial for the success of native ChIP and depend on MNase treatment conditions, including enzyme units, incubation time, and CaCl2 concentration. MNase digestion efficiency can also vary based on cell type and number. The chromatin fragmentation pattern shown in Figure 2 (lane 5) is recommended for this ChIP assay.
4. Immunoprecipitation
- Aliquot 20 µL of digested chromatin from each sample into a fresh 1.5 mL tube and mix with 180 µL of Elution Buffer (refer to Table 1). Label these tubes as Input samples and store at -20 °C.
- Transfer 400 µL of chromatin sample into another 1.5 mL tube for ChIP.
- Add γH2A.X antibody (refer to Table of Material) to one DMSO-treated, one Aphidicolin-treated sample, and one hydroxyurea-treated sample. Add the same amount of normal IgG (refer to Table of Material) to another DMSO-treated sample as a negative control for the ChIP assay.
NOTE: Here, 1 μg of primary antibody is typically used for 400 μL of chromatin (i.e., antibody final concentration is 2.5 µg/mL). However, the optimal amount should be empirically determined for different γH2A.X antibodies. - Place the ChIP tubes on a rotator at 4 °C and incubate for at least 5 h, or preferably overnight.
- Meanwhile, aliquot 100 µL of ChIP-grade magnetic Protein A/G beads (refer to Table of Material) into a new 1.5 mL tube. Use wide-orifice tips and pipette slowly to ensure accurate measurement of the beads. Place the tube on a magnetic stand for at least 1 min, then carefully discard the liquid.
- Resuspend the beads in 1 mL of 1x PBS containing 0.5% BSA. Rotate at 4 °C for approximately 4 h. Place the tube on a magnetic stand for at least 1 min and discard the supernatant.
- Wash the beads again with 1 mL of 1x PBS containing 0.5% BSA. Place the tube on the magnetic stand for 1 min to pellet the magnetic beads, then discard the supernatant.
NOTE: Steps 4.5 to 4.7 are the pre-coating of beads to reduce non-specific binding of antibodies to magnetic beads. - Resuspend the pre-coated beads in 100 µL of Buffer B using wide-orifice tips. Add 25 µL of the pre-coated magnetic bead suspension to each ChIP sample tube. Rotate at 4 °C for 2 h.
- Place the ChIP tubes on the magnetic stand and wait until the beads are completely attached to the side of the tube and the solution becomes clear.
- Discard the clear supernatant without disturbing the magnetic beads. Resuspend the beads with 1 mL of Wash Buffer (refer to Table 1) and rotate at 4 °C for 10 min.
- Place the tubes back on the magnetic stand and wait until the solution becomes clear. Discard the wash buffer. Repeat washing for a total of four washes.
- Discard the wash buffer after final wash and briefly centrifuge the tubes at 400 x g for 30 s at 4 °C to spin down any residual liquid. Place the tubes back on the magnetic stand and carefully remove any remaining liquid from the bottom of the tube.
5. Elution and DNA precipitation
NOTE: Antibody efficiency may vary among different batches. It is important to confirm the binding affinity of a new antibody by checking the immunoprecipitated samples through Western blot analysis.
- Verify ChIP antibody pull-down efficiency using Western blot (WB) as described below.
- Take a small aliquot of the ChIP sample for analysis (i.e., usually 10% of the ChIP sample). Include Input chromatin (pre-immunoprecipitation) and negative control (e.g., IgG pull-down) for comparison.
- Elute proteins from the antibody-bound beads by heating in 20 µL of 1x SDS-PAGE loading buffer (refer to Table of Material) at 95 °C for 5 min.
- Load the IP samples, input, and controls onto a 15% SDS-PAGE gel. Run the gel.
- Transfer the proteins to a 0.2 µm nitrocellulose (refer to Table of Material) or PVDF membrane using a wet or semi-dry transfer system.
- Block the membrane with 5% non-fat milk or BSA in TBST (refer to Table 1) for 1 h at room temperature to prevent non-specific binding.
- Incubate the membrane with the primary antibody against the γH2A.X (refer to the Table of Material) diluted in blocking buffer for 1-2 h at room temperature or overnight at 4 °C.
- Wash the membrane 3x with TBST to remove unbound antibodies. Incubate the membrane with an HRP-conjugated secondary antibody (refer to the Table of Material) for 1 h at room temperature. Wash the membrane again to remove excess secondary antibodies.
- Develop the membrane using a chemiluminescent substrate and visualize the signal with an imager. Compare the signal intensity between the IP, input, and control lanes to assess the efficiency and specificity of the pull-down.
NOTE: A band corresponding to the target protein in the IP lane confirms successful antibody pull-down. This approach ensures you can evaluate the efficacy of the antibody in capturing the target protein during the ChIP experiment.
- Add 50 µL of Elution Buffer (refer to the Table 1) to each of the remaining ChIP samples. Place the tubes on a thermomixer and shake for 15 min at room temperature.
- Place the tubes on the magnetic holder for at least 1 min. Collect the elute into new tubes. Repeat 1x and collect the elute in the same tubes.
- Add additional 100 µL of Elution Buffer to each ChIP elution sample and 180 µL of Elution Buffer to each Input sample.
- Add 200 µL of phenol/chloroform/isoamyl alcohol (25:24:1) to each sample and vortex vigorously. Centrifuge the samples at 20,000 x g (or maximum speed) for 10 min at 4 °C.
- Add 19 µL of 3M sodium acetate (NaOAc, pH 5.2; refer to the Table 1) and 2 µL of glycogen solution (20 mg/mL, refer to the Table of Material) to each new 1.5 mL centrifuge tube.
- After centrifugation, carefully transfer the upper aqueous layer (approximately 190 µL) to the tubes containing NaOAc and glycogen and mix by vortexing.
- Add 500 µL of 100% ethanol and vortex. Precipitate the DNA by incubating the samples at -20 °C for at least 2 h or overnight.
- Centrifuge the tubes at 20,000 x g (or maximum speed) for 10 min at 4 °C. Discard the supernatant, taking care not to disturb the white pellet. Resuspend the pellet in 1 mL of 70% ethanol and vortex thoroughly.
- Centrifuge the tubes at 20,000 x g (or maximum speed) for 5 min at 4 °C. Carefully remove the supernatant. Briefly centrifuge the tubes again to spin down any residual ethanol. Carefully remove the ethanol using a P20 pipette. Air dry the DNA pellets for 2-3 min.
NOTE: Avoid over-drying the pellet, as this can make the DNA difficult to re-dissolve. - For ChIP samples, resuspend the DNA in 400 µL of TE buffer (refer to Table 1). For Input DNA, resuspend in 1000 µL of TE buffer. The eluted samples can now be stored at -20°C.
6. qPCR quantification
- Perform qPCR using a commercial kit (refer to Table of Material) with technical triplicates for each sample. Confirm the presence of a single specific PCR product by conducting melting curve analysis to ensure the specificity of amplification36.
- Data analysis
NOTE: In relative quantification analysis, the test sample is expressed as a fold change relative to a control sample (immunoprecipitated using normal purified IgG or mock IP). DNA loci known to be unoccupied by the immunoprecipitated protein (negative locus) can be used in this manner as a reference gene compared to known, occupied, positive control DNA loci36.- Calculate the percent of input for each ChIP using the formula below
%Input = 2(-ΔCt [normalized ChIP]) - Normalize the positive locus ΔCt values to negative locus (ΔΔCt) by subtracting the ΔCt value obtained for the positive locus from the ΔCt value for the negative locus using the formula below
(ΔΔCt = ΔCtpositive - ΔCtnegative) - Calculate the fold enrichment of the positive locus sequence in ChIP DNA over the negative locus using the formula below
Fold enrichment =2ΔΔCt
The sequences of the qPCR primers used for analysis are provided in Table 2. The genomic organization of FRA3B and FRA16D37is depicted in Figure 3A,B.
- Calculate the percent of input for each ChIP using the formula below
- Statistical analysis
- Analyze the results statistically using Student's paired t-test. A p-value of ≤0.05 is considered statistically significant, indicating that the observed differences are unlikely to be due to random variation38.
Results
The size of chromatin fragments is crucial for the success of Native ChIP, as it directly impacts the accessibility of DNA regions for antibody binding. To determine the optimal MNase concentration for chromatin fragmentation, we prepared a series of microcentrifuge tubes containing varying concentrations of MNase (i.e., 0.0625 U, 0.125 U, 0.25 U, 0.5 U, 1 U, 2 U, 4 U, 8 U per reaction) and 40 µL of isolated nuclei. Each reaction was incubated at 37 °C for 5 min to achieve a range of chromatin fragment sizes. The results of the MNase digestion are presented in Figure 2.
As shown in Figure 2, higher concentrations of MNase led to more extensive digestion of chromatin, resulting in a predominance of mono-nucleosome fragments (Figure 2, lanes 1, 2, and 3). In contrast, at lower MNase concentrations, the majority of chromatin fragments were larger, often exceeding 1 kb (Figure 2, lanes 6, 7, and 8), indicating insufficient digestion for downstream applications. With an MNase concentration of 1.6 units and a digestion time of 5 min, we obtained chromatin fragments primarily in the range of 200 to 1000 base pairs (Figure 2, lane 5). This fragment size is ideal for Native ChIP, as it ensures efficient enrichment of chromatin-associated DNA while maintaining accessibility to specific epitopes. The appropriate fragment size facilitates efficient immunoprecipitation and downstream analysis, such as quantitative PCR or sequencing, making it suitable for detecting protein-DNA interactions with high resolution.
The binding affinity of the antibody is a critical factor for the success of Native ChIP. Ensuring that the antibody efficiently and specifically binds to its target epitope is essential to achieve reliable results. Therefore, it is crucial to verify the antibody's binding efficiency in advance. To confirm the efficiency of γH2A.X immunoprecipitation, we conducted a western blot (WB) assay.
We compared γH2A.X levels between DMSO-treated cells (control), aphidicolin-treated and hydroxyurea-treated cells (replication-stressed) using western blotting. As shown in Figure 4A (Input), the γH2A.X levels were significantly higher in the aphidicolin-treated cells compared to the DMSO-treated cells. This increase in γH2A.X is consistent with the induction of replication stress by aphidicolin, which leads to increased double-strand break formation and subsequent γH2A.X phosphorylation. After performing ChIP, we verified the specificity of the immunoprecipitation by comparing the γH2A.X enrichment in the IgG control and γH2A.X IP samples. The IgG control did not pull down any detectable γH2A.X, indicating minimal non-specific binding (Figure 4B, N-ChIP, and X-ChIP). In contrast, the γH2A.X IP sample successfully enriched γH2A.X from the aphidicolin-treated cells, demonstrating efficient and specific binding of the antibody to γH2A.X (Figure 4, N-ChIP and X-ChIP). These results confirm that the γH2A.X antibody has sufficient binding affinity and specificity for use in Native ChIP experiments, enabling the reliable detection and enrichment of γH2A.X-bound chromatin regions. This validation step is essential to ensure the quality and accuracy of subsequent ChIP analyses.
We used the common fragile sites (CFSs) FRA16D and FRA3B to assess the specificity of γH2A.X binding to DNA in response to replication stress. FRA16D and FRA3B are well-known CFS regions prone to instability under conditions of replication stress, making them ideal candidates for validating γH2A.X/DNA interactions. Figure 3A,B illustrate the genomic organization of these two CFSs, along with the specific qPCR primers used for analysis.
To determine whether γH2A.X associates with these CFS regions in response to replication stress, we performed ChIP-qPCR analysis in control (DMSO-treated), aphidicolin-treated cells, and hydroxyureas-treated cells. The qPCR results showed that in the DMSO-treated control cells, γH2A.X was not enriched at either FRA16D or FRA3B loci, suggesting a lack of significant DNA damage or replication stress at these regions (Figure 3C). However, in cells treated with aphidicolin or hydroxyurea, which induces replication stress by inhibiting DNA polymerase, we observed significant enrichment of γH2A.X at both FRA16D and FRA3B (Figure 3C). This indicates that γH2A.X was recruited to these CFS regions specifically in response to DNA replication stress. These findings demonstrate that the occupancy of γH2A.X at CFSs is triggered by replication stress, supporting its role as a marker of DNA damage response and replication fork stalling. The specific recruitment of γH2A.X to FRA16D and FRA3B during aphidicolin treatment further validates the specificity of our ChIP assay and confirms that γH2A.X is a reliable indicator of DNA damage and replication stress at fragile sites.
To compare the efficiency of native ChIP with crosslinked ChIP (X-ChIP), we conducted γH2A.X X-ChIP using the same starting number of cells, following the protocol described by Lyu et al.39. γH2A.X recruitment to FRA16D was increased by treatment with aphidicolin and hydroxyurea, as shown in Figure 3D. However, the relative enrichment of γH2A.X at FRA16D in X-ChIP was noticeably lower than that observed with native ChIP, indicating a reduced efficiency of X-ChIP under these conditions. Furthermore, at FRA3B, the increase in γH2A.X enrichment was statistically insignificant, providing additional evidence that X-ChIP is less efficient when working with a limited number of cells. These results suggest that native ChIP may be more suitable for detecting γH2A.X enrichment at fragile sites when sample size is constrained. Analyze the results statistically using Student's paired t-test. A p-value of ≤ 0.05 is considered statistically significant.

Figure 1: Workflow for performing a native γH2A.X ChIP assay. (i) Cells are cultured and subjected to specific treatments (e.g., DNA-damaging agents like hydroxyurea) to induce γH2A.X signaling. (ii) Treated cells are collected for processing. (iii) The cells are fractionated to isolate the chromatin-bound γH2A.X, separating it from other cellular components. (iv) Micrococcal nuclease (MNase) digestion is performed to fragment the chromatin into nucleosome-sized pieces. (v) The size of the chromatin fragments is validated by running a sample on an agarose gel, ensuring fragments are of the appropriate length for ChIP. (vi) An antibody specific for γH2A.X is used to immunoprecipitate chromatin fragments that are marked by γH2A.X. (vii) Protein A/G beads are used to pull down the antibody-chromatin complexes, enriching for γH2A.X-bound DNA. (viii) The γH2A.X-marked DNA fragments are eluted and purified, preparing them for downstream analyses. (ix) Downstream Analysis: qPCR and sequencing and Data Analysis. Please click here to view a larger version of this figure.

Figure 2: Assay to optimize MNase digestion condition. Chromatin fragments were prepared from HEK 293T cells using varying MNase digestion conditions. The resulting purified DNA from the fragmented chromatin was separated on a 2% agarose gel and run at 100 V for 30 min. Lanes 1 to 8 represent samples treated with increasing concentrations of MNase (i.e., 0.0625 U, 0.125 U, 0.25 U, 0.5 U, 1 U, 2 U, 4 U, 8 U per reaction), demonstrating a gradient of chromatin digestion. The DNA ladder (indicated by M) was included for size reference. DNA fragments were visualized using Midori Green Advance DNA staining solution. The band labeled corresponds to mono-nucleosome-sized DNA fragments, highlighting the digestion efficiency at each MNase concentration. Please click here to view a larger version of this figure.
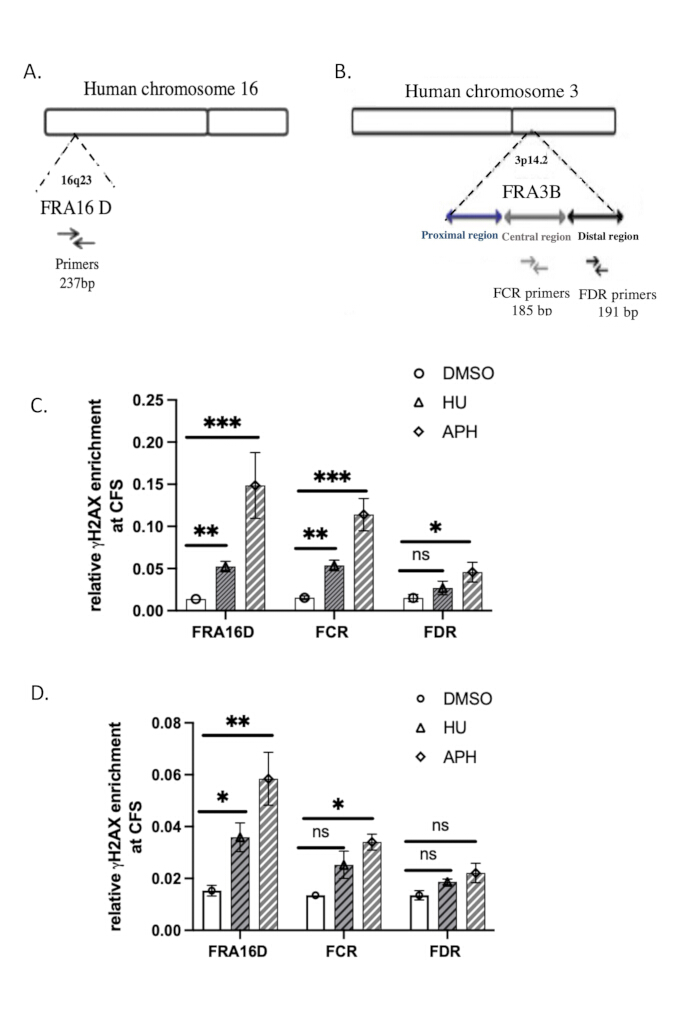
Figure 3:qPCR verification of γH2A.X ChIP. (A) Diagram of genomic organizations of the FRA16D. (B) Diagram of genomic organizations of the FRA3B region. Primer sets used for qPCR analyses of FRA16D, distal (FDR), and central (FCR) regions within the FRA3B locus are indicated. (C) The relative enrichment of γH2A.X at common fragile sites (CFS), specifically FRA3B and FRA16D, was assessed using native ChIP followed by qPCR analysis after treatment with aphidicolin and hydroxyurea. (D) The relative enrichment of γH2A.X at common fragile sites (CFS), specifically FRA3B and FRA16D, was assessed using X-ChIP followed by qPCR analysis after treatment with aphidicolin and hydroxyurea. *** indicates a statistically significant result with p < 0.001; ** indicates p < 0.01 and * indicates p < 0.05, determined by a t-test; n = 3. Please click here to view a larger version of this figure.
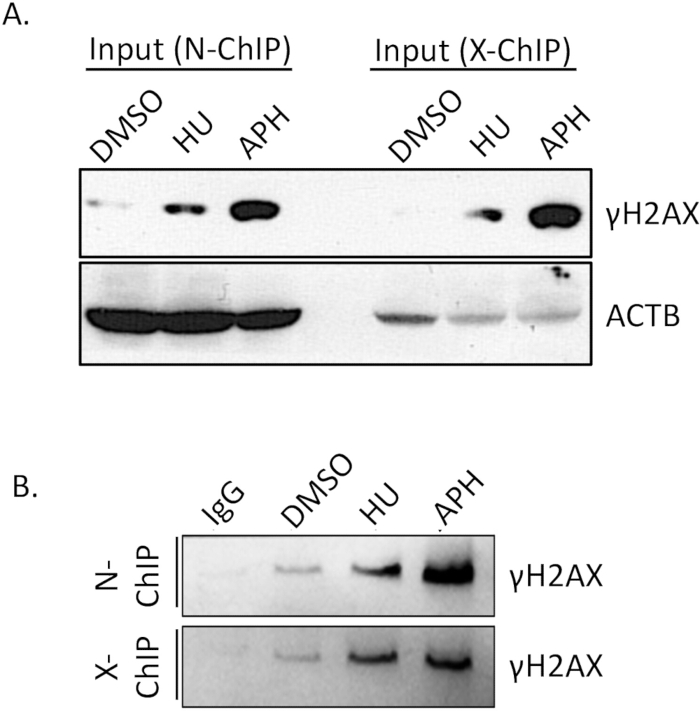
Figure 4: Verification of ChIP antibody pull-down efficiency using Western blot. From the total sample, 10% of the ChIP sample, along with input controls, was separated on a 15% SDS-PAGE gel to resolve the polypeptides. Following electrophoresis, the separated proteins were transferred onto a 0.22 µm PVDF membrane using a standard transfer protocol. The membrane was then sequentially probed with a specific primary antibody targeting γH2A.X and ACTB, followed by an appropriate HRP-conjugated secondary antibody. The signal was subsequently visualized using chemiluminescence detection, allowing for the assessment of antibody specificity and pull-down efficiency in the ChIP assay. (A) γH2A.X levels between DMSO-treated cells (control), aphidicolin-treated and hydroxyurea-treated cells (replication-stressed) with ACTB as the loading control. (B) γH2A.X enrichment in the IgG control and IP samples. Abbreviations: APH = aphidicolin, HU = hydroxyurea. Please click here to view a larger version of this figure.
| Buffer A (1X) | |
| Reagent | Working Concentration |
| PIPES pH 6.8 | 10 mM |
| NaCl | 100 mM |
| MgCl2 | 3 mM |
| EGTA pH 7.6 | 1 mM |
| Store at -20 ˚C for up to 1 year | |
| MNase buffer (1X) | |
| Reagent | Working Concentration |
| Tris-HCl, pH 7.5 | 50 mM |
| CaCl2 | 1 mM |
| MgCl2 | 4 mM |
| Store at -20 ˚C for up to 1 year | |
| Buffer B (1X) | |
| Reagent | Working Concentration |
| Sodium phosphate pH 7. 0 | 20 mM |
| Sodium pyrophosphate10.H2O | 30 mM |
| KCl | 250 mM |
| EDTA, pH 8.0 | 5 mM |
| Glycerol | 10% |
| Triton X-100 | 0.10% |
| Protease Inhibitor cocktail | 1x |
| Phosphatase Inhibitor cocktail | 1x |
| DTT | 0.5 mM |
| Store at -20 ˚C for up to 1 year | |
| Elution Buffer | |
| Reagent | Working Concentration |
| NaHCO3 | 50 mM |
| SDS | 1% |
| Freshly prepared | |
| Washing Buffer | |
| Reagent | Working Concentration |
| MNase buffer | x 0.5 |
| Buffer B | x 0.5 |
| Fresh prepared | |
| Stop Buffer | |
| EDTA | 100 mM , pH8.0 |
| 3 M Sodium acetate , pH 5.2 | |
| 408.24 g Sodium acetate | |
| ajust pH with acetic acid to pH 5.2 | |
| djusting the volume to 1 L | |
| Sterilize the buffer by filtration or autoclaving | |
| TBST buffer | |
| Tris Base, pH 8.0 | 10 mM |
| NaCl | 150 mM |
| Tween 20 | 0.1% (v/v) |
| TE buffer | |
| Tris-HCl, pH 8.0 | 10 mM |
| EDTA, pH 8.0 | 1mM |
Table 1: Buffer composition.
| Gene loci | Forward primer | Reverse primer |
| FRA16D | TCCTGTGGAAGGGATATTTA | CCCCTCATATTCTGCTTCTA |
| FRA3B | TGTTGGAATGTTAACTCTATCCCAT | ATATCTCATCAAGACCGCTGCA |
| FCR | ||
| FRA3B | CAATGGCTTAAGCAGACATGGT | AGTGAATGGCATGGCTGGAATG |
| FDR | ||
| ACTB (negative control) | GACGCAGGATGGCATGGG | ACGCCTCTGGCCGTACCAC |
Table 2: Primer sequence.
| Low recovery of DNA | Potential issue | Possible solution |
| Poor quality of antibody | Use ChIP grade antibody. | |
| Insufficient antibody | Use 1-10 µg of ChIP antibody per 25 µg chromatin. | |
| Insufficient incubation time | Perform the immunoprecipitation step overnight at 4 °C. | |
| Overly stringent washes | Do not use a concentration of NaCl higher than 500 mM in wash buffer. | |
| Low quality beads | Ensure that Protein A or G is compatible with the ChIP antibody. | |
| Follow bead product datasheet for optimal volume of beads to antibody ratio. | ||
| Insufficient starting | Prepare a separate plate of cells to accurately determine cell number. | |
| Sample | Increase number of cells used if target is low abundance. | |
| Inadequate cell Lysis | Optimize buffer composition and lysis time to improve efficiency. | |
| Apply mechanical force during the lysis step, such as using a Dounce homogenizer, when working with certain cell types. | ||
| Degradation of nuclei occurred | Ensure nuclei isolation is gentle to preserve chromatin integrity and accessibility. | |
| Use wide-bore pipette tips to minimize shearing of nuclei during handling. | ||
| Degradation of sample occurred | Perform all steps on ice or at 4 °C. | |
| Include protease inhibitors in all buffers and ensure that all buffers are freshly prepared. | ||
| Insufficient MNase digestion | Titrate the MNase concentration (e.g., 0.1–5 units) to determine the optimal amount for specific sample type; and start with a range of concentrations to identify the ideal digestion conditions. | |
| MNase over-digestion | Experiment with different incubation times (e.g., 1–10 min) at 37 °C and monitor the fragmentation pattern to ensure sufficient digestion without over-digestion. | |
| Disrupted Phosphorylation | Add phosphatase inhibitor cocktail to the lysis buffer, and ensure that all buffers are freshly prepared. | |
| High background in negative control | Nonspecific binding to beads | Include a pre-clearing step before immunoprecipitation step, and use magnetic ChIP-grade beads, which generally exhibit reduced non-specific binding. |
| Insufficient washing | Increase the number or stringency of washes by adjusting salt and detergent concentration. | |
| Insufficient fragmentation of chromatin | Optimize fragmentation to achieve fragments of 200-750 bp. | |
| Optimization is necessary for each cell or tissue type | ||
| PCR troubleshooting | No amplification of Input sample | (1) Over-fragmented: signal is diminished for amplicons of over 150 bp if chromatin is fragmented to mono-nucleosome length. |
| (2) Optimize PCR conditions. | ||
| (3) Design primers to amplify a smaller (<150 bp) region. | ||
| Low resolution | DNA fragment size too large | Optimize fragmentation to achieve fragments of 200-750 bp. |
Table 3: Chromatin Immunoprecipitation Troubleshooting.
Discussion
Environmental pollution is a significant contributor to human cancers. Many pollutants are carcinogenic, meaning they can cause genetic damage that leads to the development of cancer40,41. However, determining whether a particular substance is tumorigenic is a challenging task. A fast, reliable, and cost-efficient method for identifying carcinogenic potential would empower scientists to efficiently screen environmental pollutants and assess their impact on genomic stability. In this study, we focus on a modified histone variant, γH2A.X, which is widely recognized as a highly specific marker for DSBs. γH2A.X is formed when the histone variant H2A.X is phosphorylated at serine 139 in response to DNA damage.
By detecting the presence of γH2A.X by Native ChIP at CFSs, we can effectively assess the extent of DNA damage caused by environmental contaminants42. The ability to quantitively measure γH2A.X at CFSs provides a rapid, sensitive, and reliable way to evaluate the potential of environmental pollutants to induce tumorigenic effects. In contrast to traditional genotoxicity assays, which often measure indirect markers of DNA damage (such as mutations, micronucleus formation, or cellular transformation), native γH2AX ChIP allows for precise mapping of breakage events at specific genomic loci42. This provides a deeper understanding of the region most vulnerable to environmental pollutants and helps in the identification of potential carcinogenic mechanisms.
Successful Native ChIP requires careful attention to several critical steps to ensure the effective enrichment of target regions. Below are the critical steps involved in the native ChIP of γH2AX. Cell preparation: Start with an adequate number of cells. Treat the cells with a DNA damage-inducing agent, such as aphidicolin, to induce replication stress as a positive control. Include appropriate untreated negative control samples for comparison. Chromatin isolation: Perform subcellular fractionation to isolate chromatin-bound proteins while minimizing contamination from cytoplasmic components. MNase optimization: Conduct pilot experiments to optimize micrococcal nuclease (MNase) concentration and incubation time. The aim is to obtain chromatin fragments between 200-1000 base pairs, ensuring good resolution for ChIP while maintaining nucleosome integrity. Immunoprecipitation: Utilize a high-quality ChIP-grade γH2AX antibody along with Protein A/G agarose to ensure efficient pull-down of the target chromatin while minimizing non-specific binding. Washing: Wash the beads with a buffer containing the appropriate concentration of salt and detergent to remove non-specific interactions while retaining the γH2AX-bound chromatin. Elution and DNA recovery: Efficiently recover the bound chromatin by thorough elution and precipitation of DNA, ensuring minimal loss of the enriched target chromatin. Compared to conventional X-ChIP, there are four main advantages of native γH2AX ChIP. Firstly, integrating subcellular fractionation reduces false-positive interactions and enhances ChIP specificity. Secondly, the protein-DNA complexes are preserved in their native state, minimizing the stabilization of transient γH2AX-chromatin interactions that may occur with cross-linking agents. Thirdly, omitting the fixation and time-consuming reverse cross-linking steps streamline the overall workflow. Lastly, less starting material is required, and no expensive equipment is needed for chromatin shearing, such as a water bath sonicator (e.g., Bioruptor) or focused ultrasonic sonicator (e.g., Covaris).
Although Native ChIP has its advantages, its limitations should also be acknowledged. One limitation is the potential for protein rearrangement during chromatin preparation and immunoprecipitation. This rearrangement could theoretically alter the interactions of chromatin-bound proteins and introduce variability in the results. Although we do not have direct evidence to support the occurrence of such rearrangements, it is important to include appropriate experimental controls to account for this potential artifact. Another limitation of the native ChIP assay is the cleavage bias of MNase, which can lead to inaccurate results. MNase preferentially cleaves A-T-rich regions of the genome, making these regions more likely to be fragmented and enriched during the ChIP process. This bias can cause overrepresentation of A-T-rich sequences and underrepresentation of G-C-rich regions, potentially resulting in false-negative signals for genomic regions that are less accessible to MNase digestion. If the loci of interest are G-C-rich, careful optimization of MNase digestion conditions is necessary. Alternatively, incorporating additional controls, such as chromatin fragmentation by sonication, can help mitigate the impact of MNase bias and improve the reliability of the results.
Despite its certain limitations, native γH2AX ChIP is particularly useful for identifying the gen loci where specific pollutants induce DNA damage. By enriching DNA fragments bound to γH2AX, researchers can identify fragile regions of the genome, particularly CFSs by qPCR, that are more prone to breakage under replication stress induced by pollutants. This technique also enables researchers to establish a direct relationship between exposure to a specific carcinogen and the formation of DNA damage in particular genomic regions, providing insight into the genotoxic profile of pollutants and their potential role in initiating carcinogenesis43,44.
The troubleshooting table (Table 3) provides detailed solutions for common issues encountered during the ChIP assay, such as low DNA yield, insufficient chromatin fragmentation, high background noise, or poor antibody performance. Each issue is paired with specific recommendations to ensure the protocol's reliability and reproducibility.
By using automated liquid handling systems and quantitative PCR or sequencing technologies, native γH2AX ChIP can efficiently identify agents that induce DNA damage, thus serving as a valuable tool in toxicological studies and environmental monitoring. By employing native γH2AX ChIP on samples derived from populations exposed to different levels of environmental pollutants, researchers can identify specific DNA damage patterns and determine the level of risk associated with exposure to particular agents. In conclusion, native γH2AX ChIP offers significant advantages for evaluating the carcinogenic potential of environmental pollutants, providing a fast, cost-effective, and high-specificity approach to detect DNA damage at specific genomic loci. Its future applications in high-throughput screening, personalized risk assessment, and biomarker development make it a valuable tool for advancing our understanding of environmental carcinogenesis and developing strategies for cancer prevention and intervention.
Disclosures
The authors have no conflicts of interest to disclose.
Acknowledgements
This work was supported by University of South China's startup funding.
Materials
| Name | Company | Catalog Number | Comments |
| 0.2 µm nitrocellulose membrane | Amersham | 10600011 | |
| Actin B | proteintech | 20536-1-AP | |
| Aphidicolin | MedChemExpress | HY-N6733 | |
| ChIP-grade magnetic Protein A/G beads | ThermoFisher | 26162 | |
| Clarity Western ECL Substrate | Bio-Rad | #1705061 | |
| Glycogen, molecular biology grade | ThermoFisher | Cat. No. R0561 | |
| HRP-conjugated secondary antibody | proteintech | SA00001-2 | |
| hydroxyurea | MedChemExpress | HY-B0313 | |
| Micrococcal Nuclease | NEB | M0247S | |
| normal IgG | Santa Cruz | sc-2025 | |
| Taq Universal SYBR Green Supermix | BioRad | 1725120 | |
| γH2A.X antibody (for ChIP) | Sigma-Aldrich | 05-636 | |
| γH2A.X antibody (for WB) | Cell Signaling | #25955 |
References
- Glover, T. W., Berger, C., Coyle, J., Echo, B. DNA polymerase alpha inhibition by aphidicolin induces gaps and breaks at common fragile sites in human chromosomes. Hum Genet. 67 (2), 136-142 (1984).
- Bignell, G. R., et al. Signatures of mutation and selection in the cancer genome. Nature. 463 (7283), 893-898 (2010).
- Hellman, A., et al. A role for common fragile site induction in amplification of human oncogenes. Cancer Cell. 1 (1), 89-97 (2002).
- Kotzot, D., et al. Parental origin and mechanisms of formation of cytogenetically recognisable de novo direct and inverted duplications. J Med Genet. 37 (4), 281-286 (2000).
- Miller, C. T., et al. Genomic amplification of MET with boundaries within fragile site FRA7G and upregulation of MET pathways in esophageal adenocarcinoma. Oncogene. 25 (3), 409-418 (2006).
- Zack, T. I., et al. Pan-cancer patterns of somatic copy number alteration. Nat Genet. 45 (10), 1134-1140 (2013).
- Gao, G., et al. Common fragile sites (CFS) and extremely large CFS genes are targets for human papillomavirus integrations and chromosome rearrangements in oropharyngeal squamous cell carcinoma. Genes Chromosomes Cancer. 56 (1), 59-74 (2017).
- Thorland, E. C., Myers, S. L., Gostout, B. S., Smith, D. I. Common fragile sites are preferential targets for HPV16 integrations in cervical tumors. Oncogene. 22 (8), 1225-1377 (2003).
- Matovina, M., Sabol, I., Grubisić, G., Gasperov, N. M., Grce, M. Identification of human papillomavirus type 16 integration sites in high-grade precancerous cervical lesions. Gynecol Oncol. 113 (1), 120-127 (2009).
- Yu, T., et al. The role of viral integration in the development of cervical cancer. Cancer Genet Cytogenet. 158 (1), 27-34 (2005).
- Bignell, G. R., et al. Signatures of mutation and selection in the cancer genome. Nature. 463 (7283), 893-898 (2010).
- Thavathiru, E., Ludes-Meyers, J. H., MacLeod, M. C., Aldaz, C. M. Expression of common chromosomal fragile site genes, WWOX/FRA16D and FHIT/FRA3B is downregulated by exposure to environmental carcinogens, UV, and BPDE but not by IR. Mol Carcinog. 44 (3), 174-182 (2005).
- Sirbu, B. M., et al. Analysis of protein dynamics at active, stalled, and collapsed replication forks. Genes Dev. 25 (12), 1320-1327 (2011).
- Barlow, J. H., et al. Identification of early replicating fragile sites that contribute to genome instability. Cell. 152 (3), 620-632 (2013).
- Petermann, E., Orta, M. L., Issaeva, N., Schultz, N., Helleday, T. Hydroxyurea-stalled replication forks become progressively inactivated and require two different RAD51-mediated pathways for restart and repair. Mol Cell. 37 (4), 492-502 (2010).
- Rogakou, E. P., Pilch, D. R., Orr, A. H., Ivanova, V. S., Bonner, W. M. DNA double-stranded breaks induce histone H2AX phosphorylation on serine 139. J Biol Chem. 273 (10), 5858-5868 (1998).
- Shanbhag, N. M., Rafalska-Metcalf, I. U., Balane-Bolivar, C., Janicki, S. M., Greenberg, R. A. ATM-dependent chromatin changes silence transcription in cis to DNA double-strand breaks. Cell. 141 (6), 970-981 (2010).
- Stiff, T., et al. ATR-dependent phosphorylation and activation of ATM in response to UV treatment or replication fork stalling. EMBO J. 25 (24), 5775-5782 (2006).
- Nelson, J. D., Denisenko, O., Bomsztyk, K. Fast chromatin immunoprecipitation assay. Nuc Acids Res. 34 (5), e2 (2006).
- Dorbic, T., Wittig, B. Isolation of oligonucleosomes from active chromatin using HMG17-specific monoclonal antibodies. Nuc Acids Res. 14 (8), 3363-3376 (1986).
- Dorbic, T., Wittig, B. Chromatin from transcribed genes contains HMG17 only downstream from the starting point of transcription. EMBO J. 6 (8), 2393-2399 (1987).
- Hebbes, T. R., Thorne, A. W., Crane-Robinson, C. A direct link between core histone acetylation and transcriptionally active chromatin. EMBO J. 7 (5), 1395-1402 (1988).
- Hebbes, T. R., Thorne, A. W., Clayton, A. L., Crane-Robinson, C. Histone acetylation and globin gene switching. Nuc Acids Res. 20 (5), 1017-1022 (1992).
- Hebbes, T. R., Clayton, A. L., Thorne, A. W., Crane-Robinson, C. Core histone hyperacetylation co-maps with generalized DNase I sensitivity in the chicken beta-globin chromosomal domain. EMBO J. 13 (8), 1823-1830 (1994).
- Orlando, V. Mapping chromosomal proteins in vivo by formaldehyde-crosslinked-chromatin immunoprecipitation. Trend Biochem, Sci. 25 (3), 99-104 (2000).
- Myers, F. A., Evans, D. R., Clayton, A. L., Thorne, A. W., Crane-Robinson, C. Targeted and extended acetylation of histones H4 and H3 at active and inactive genes in chicken embryo erythrocytes. J Biol Chem. 276 (23), 20197-20205 (2001).
- Litt, M. D., Simpson, M., Recillas-Targa, F., Prioleau, M. N., Felsenfeld, G. Transitions in histone acetylation reveal boundaries of three separately regulated neighboring loci. EMBO J. 20 (9), 2224-2235 (2001).
- Madisen, L., Krumm, A., Hebbes, T. R., Groudine, M. The immunoglobulin heavy chain locus control region increases histone acetylation along linked c-myc genes. Mol Cell Biol. 18 (11), 6281-6292 (1998).
- Clayton, A. L., Hebbes, T. R., Thorne, A. W., Crane-Robinson, C. Histone acetylation and gene induction in human cells. FEBS Lett. 336 (1), 23-26 (1993).
- Tseng, Z., Wu, T., Liu, Y., Zhong, M., Xiao, A. Using native chromatin immunoprecipitation to interrogate histone variant protein deposition in embryonic stem cells. Methods Mol Biol. 1176, 11-22 (2014).
- Durkin, S. G., Glover, T. W. Chromosome fragile sites. Ann Rev Genetics. 41, 169-192 (2007).
- Lee, J. B., Keung, A. J. Chromatin immunoprecipitation in human and yeast cells. Methods Mol Biol. 1767, 257-269 (2018).
- Miyamoto, R., Yokoyama, A. Protocol for fractionation-assisted native ChIP (fanChIP) to capture protein-protein/DNA interactions on chromatin. STAR Protoc. 2 (2), 100404 (2021).
- Mendez, J., Stillman, B. Chromatin association of human origin recognition complex, cdc6, and minichromosome maintenance proteins during the cell cycle: Assembly of prereplication complexes in late mitosis. Mol Cell Biol. 20 (22), 8602-8612 (2000).
- Nowak, D. E., Tian, B., Brasier, A. R. Two-step cross-linking method for identification of NF-κB gene network by chromatin immunoprecipitation. BioTechniques. 39 (5), 715-725 (2005).
- Schmittgen, T. D., Livak, K. J. Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc. 3 (6), 1101-1108 (2008).
- Lu, X., Parvathaneni, S., Hara, T., Lal, A., Sharma, S. Replication stress induces specific enrichment of RECQ1 at common fragile sites FRA3B and FRA16D. Mol Cancer. 12, 29 (2013).
- Ruijter, J. M., et al. Amplification efficiency: linking baseline and bias in the analysis of quantitative PCR data. Nucl Acids Res. 37 (6), e45 (2009).
- Lyu, X., Chastain, M., Chai, W. Genome-wide mapping and profiling of γH2AX binding hotspots in response to different replication stress inducers. BMC Genomics. 20, 579 (2019).
- Farmer, P. B., et al. Molecular epidemiology studies of carcinogenic environmental pollutants. Effects of polycyclic aromatic hydrocarbons (PAHs) in environmental pollution on exogenous and oxidative DNA damage. Mutat Res. 544 (2-3), 397-402 (2003).
- Phillips, D. H., Arlt, V. M. Genotoxicity: damage to DNA and its consequences. EXS. 99, 87-110 (2009).
- Nitsch, S., Schneider, R. Native ChIP: Studying the genome-wide distribution of histone modifications in cells and tissue. Meth Mol Biol. 2846, (2024).
- Nikitina, T., Wang, D., Gomberg, M., Grigoryev, S. A., Zhurkin, V. B. Combined micrococcal nuclease and exonuclease III digestion reveals precise positions of the nucleosome core/linker junctions: implications for high-resolution nucleosome mapping. J Mol Biol. 425 (11), 1946-1960 (2013).
- Teves, S. S., Henikoff, S. Salt fractionation of nucleosomes for genome-wide profiling. Methods Mol Biol. 833, 421-432 (2012).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved