Method Article
Identifying PD-1/PD-L1 Inhibitors with Surface Plasmon Resonance Technology
In This Article
Summary
This protocol describes a blockade assay for PD-1/PD-L1 inhibitors using surface plasmon resonance technology. It employs a dual-step immobilization strategy and a tailored buffer system to accurately measure response units, facilitating the assessment of blockade rates for compounds or biologics. Additionally, it supports the high-throughput identification of PD-1/PD-L1 inhibitors.
Abstract
The disruption of the PD-1/PD-L1 interaction is a promising strategy for cancer immunotherapy. Reliable screening platforms are essential for evaluating the efficacy of PD-1/PD-L1 inhibitors. A previously established human PD-1/PD-L1 blockade assay utilizing Surface Plasmon Resonance (SPR) technology (first-generation PD-1/PD-L1 inhibitor SPR screening platform) demonstrated results comparable to those obtained through Homogeneous Time-Resolved Fluorescence (HTRF) and cell-based assays, with potential for large-scale screening. Herein, an optimized version of this assay (second-generation PD-1/PD-L1 inhibitor SPR screening platform) is presented, featuring a dual-step coupling process that combines amine and bio-streptavidin coupling to enhance PD-1 orientation control on the chip and reduce PD-1 protein consumption. The updated platform was successfully validated using the PD-1/PD-L1 inhibitor BMS-1166, showing blockade effects comparable to the previous SPR-based method and other established techniques such as ELISA. These results confirm the reliability of the approach. This optimized SPR screening platform offers a high-throughput and reliable tool for identifying novel PD-1/PD-L1 inhibitors, advancing cancer immunotherapy research, and highlighting the potential of SPR in immune checkpoint inhibitor screening.
Introduction
Immune checkpoint blockade therapies, particularly those targeting Programmed Cell Death-1 (PD-1) and Programmed Cell Death-Ligand 1 (PD-L1), stand at the forefront of cancer immunotherapy strategies. Anti-PD-1/PD-L1 therapies have received approval for use in various cancer types, such as hematological, cutaneous, pulmonary, hepatic, urinary bladder, and renal cancers1. PD-1 is a transmembrane glycoprotein belonging to the immunoglobulin superfamily, characterized by a single immunoglobulin variable (IgV)-like domain at the N-terminal, a roughly 20-amino acid stalk separating the IgV domain from the plasma membrane, a transmembrane domain, and a cytoplasmic tail containing tyrosine-based signaling motifs2. PD-L1, identified as one of the ligands for PD-1, is a type I transmembrane protein featuring a transmembrane region, two extracellular domains-immunoglobulin constant (IgC) and IgV-and a relatively short cytoplasmic domain that triggers intracellular signaling pathways3. The PD-1/PD-L1 inhibitory pathway serves as a critical immune checkpoint that regulates T cell activation and autoimmunity4. PD-1 is expressed on T cells, where it interacts with PD-L1, inhibits T cell receptor signaling, and blocks the stimulation of CD28 and CD80 molecules on antigen-presenting cells and T cells5. Cancer tissues exploit this physiological mechanism by overexpressing PD-L1 during the escape phase, thus creating an immunosuppressive environment that promotes tumor growth and progression6. Inhibitors of PD-1 and PD-L1 disrupt this interaction, enabling the immune system to evade tumor-induced suppression and reinitiate the T-cell-mediated tumor-cell death process7.
Building on the foundation laid by the prominent role of immune checkpoint blockade therapies, the development of PD-1/PD-L1 inhibitors has marked a significant advancement in cancer immunotherapy. The U.S. Food and Drug Administration (FDA) has endorsed nine immune checkpoint inhibitors that specifically target the PD-1/PD-L1 pathway. These include six PD-1 inhibitors-pembrolizumab, dostarlimab, nivolumab, cemiplimab, oripalimab, and tislelizumab-and three PD-L1 inhibitors-atezolizumab, avelumab, and durvalumab8,9. These therapies have been effectively utilized to treat a variety of cancers, such as melanoma, lung cancer, urothelial cancer, cervical cancer, gastric or gastroesophageal cancer, and other solid tumors10. Despite their efficacy, monoclonal antibody-based therapies face significant limitations, including low response rates, high costs, prolonged half-lives, severe immune-related adverse events, and restrictions to intravenous or subcutaneous delivery11,12,13. Consequently, research is increasingly focused on developing small-molecule inhibitors targeting the PD-1/PD-L1 axis. These small molecules offer distinct advantages, such as improved cellular penetration, modulation of diverse biological targets, enhanced oral bioavailability, and reduced costs, with the goal of achieving comparable therapeutic outcomes with fewer adverse effects14. However, the development of small molecule inhibitors targeting the PD-1/PD-L1 interaction is in its early stages, primarily due to the lack of a reliable high-throughput screening platform. Such platforms are essential for rapidly evaluating vast libraries of small molecules and identifying lead compounds for further validation and optimization. Overcoming this challenge is critical to advancing cancer immunotherapy.
Surface Plasmon Resonance (SPR) technology is extensively employed in detecting various biomolecules, including antibody antigens, enzymes, nucleic acids, and drugs, and is particularly effective in small molecule drug screening15,16. Unlike other biophysical techniques, SPR offers label-free detection, real-time kinetic data, and a broad detection range. In contrast, Isothermal Titration Calorimetry lacks real-time kinetic insights and requires larger sample volumes, limiting throughput. Microscale Thermophoresis is prone to buffer interference and cannot provide kinetic data, while Biolayer Interferometry has application-specific limitations based on molecular size and properties. Homogeneous Time-Resolved Fluorescence requires labeling and is susceptible to fluorescent interference. We acknowledge that HTRF is another suitable technology to explore PD-1/PD-L1 inhibitors. One inherent limitation of HTRF, compared to SPR, is fluorescence quenching caused by external interactions with the intramolecular excitation process (e.g., electron transfer, FRET, and bleaching), the sensitivity is too low in the drug screening process because of the small window range, and interference from fluorescent library compounds or biological proteins17. These features position SPR as a superior tool for drug discovery. Our previous studies have demonstrated that SPR is able to determine the blockade effect of small molecules against PD-1/PD-L1, which is advantageous over other techniques that require high labeling technology requirements, multiple steps, poor specificity, and high cost in the drug discovery process18.
This study introduces an optimized SPR-based platform, integrating a dual-step coupling process that utilizes both amine and bio-streptavidin coupling to enhance PD-1 orientation on the chip and minimize protein usage. This updated approach was successfully validated using the PD-1/PD-L1 inhibitor BMS-1166 as a positive control binder, demonstrating blockade effects comparable to both our previous SPR method and other established techniques such as ELISA19,20. This not only confirms the reliability and reproducibility of our protocol but also illustrates the effectiveness of our modified platform in facilitating high-throughput screening of PD-1/PD-L1 inhibitors. The incorporation of the bio-streptavidin capturing step provides site-directed rather than random protein orientation, allowing for reduced PD-1 concentration (40 µg/mL vs. 10 µg/mL) and cost savings by enabling the end user to immobilize streptavidin (SA) to a CM5 chip, a less expensive alternative to commercialized pre-immobilized SA chips. This makes it advantageous for large-scale, cost-effective screenings of compound/peptide libraries. Although additional characterization methods, including in silico, in vitro, and in vivo assays, are essential to evaluate the clinical potential of PD-1/PD-L1 inhibitors against cancer, our enhanced SPR-based screening platform stands out as an efficient tool for large-scale screening of PD-1/PD-L1 inhibitors.
Protocol
The reagents and equipment are listed in the Table of Materials.
1. Immobilization of the streptavidin (SA) protein on the CM5 chip
- Setup the immobilization method on the SPR instrument: Open/new wizard template, choose immobilization, set the chip type to CM5, and flow cells per cycle to 1. Check immobilize flow cell 1 and flow cell 2.
- Set Amine as the immobilization method. Set aim for immobilized level, ligand concentration: 40 µg/mL streptavidin, target level: 2000 RU, wash solution: 50 mM NaOH. Next, check prime before running.
- Prepare the following tubes: R2 B1 and R2 C1 - 40 µg/mL streptavidin; R2 B2 and R2 C2: 50 mM of NaOH; R2 B3 and R2 C3: EDC; R2 B4 and R2 C4: NHS; R2 B5 and R2 C5: Empty; R2 B6 and R2 C6: Ethanolamine.
NOTE: EDC and NHS activate the carboxyl groups on the CM5 chip, allowing covalent coupling with amines on the ligand. Ethanolamine is used as a blocking buffer to prevent nonspecific binding during immobilization. - Dilute 20 mL of HBS-EP+ buffer 10× in 180 mL of deionized (DI) water to prepare 200 mL of 1× HBS-EP+ running buffer solution.
- Add 1 mL of DNase-free water to 1 mg of streptavidin and incubate at room temperature for 30 min. Next, dilute the streptavidin solution to 40 µg/mL in acetate buffer (pH 4.5). Prepare the Amine Coupling reagent kit reagents according to the manufacturer's instructions and place all of the relevant reagent's layouts as indicated in step 1.2.
- Replace the maintenance sensor chip with the CM5 chip, then place Tube A into the prepared 1× HBS-EP+ buffer, reopen the immobilization method, and insert tubes according to step 1.2 layout. Run the method and save the results file.
- Post-run maintenance: replace the CM5 chip with the maintenance sensor chip, place Tube A into a bottle filled with deionized water, and run the prime. After taking out the CM5 chip, wash the chip with a few drops of DI water and air dry. Place the chip into a 50 mL tube at 4 °C.
NOTE: The immobilized SA enables the CM5 chip to function as an SA chip.
2. Immobilization of PD-1 protein on the SA chip
- Set the immobilization method on the SPR instrument: Open/new wizard template, choose immobilization, set chip type to SA, and flow cells per cycle to 1. Check immobilize flow cell 1 and cell 2.
- Set SA-biotin capture as the method. For cell 1 set blank immobilization, for cell 2 set aim for immobilized level, 10 µg/mL PD-1 as the ligand, target level: 4000 RU, then check prime before running.
NOTE: Prepare the following tubes - R2 B1 and R2 C1: 1 M of NaCl, 50 mM of NaOH; R2 B2 and R2 C2: 50% Isopropanol/50 mM of NaOH/1 M of NaCl; R2 C3: 10 µg/mL PD-1.
- Set SA-biotin capture as the method. For cell 1 set blank immobilization, for cell 2 set aim for immobilized level, 10 µg/mL PD-1 as the ligand, target level: 4000 RU, then check prime before running.
- Dissolve 58.44 mg of NaCl in 1 mL of 50 mM NaOH to prepare the 1 M of NaCl and 50 mM of NaOH solution. Dissolve 58.44 mg of NaCl and 4.0 mg of NaOH in 500 µL water, then add 500 µL of isopropanol to prepare the 50% Isopropanol/50 mM NaOH/1 M NaCl solution.
- Prepare PD-1 solution: Add 200 µL of DNase-free water to 100 µg of Biotinylated Human PD-1 (Fc and Avitagged) and stabilize at room temperature for 30 min, then dilute to 10 µg/mL in acetate buffer (pH 5.0).
- Place all the reagent layouts as described in step 2.1. Place Tube A into the 1× HBS-EP+ running buffer solution, then eject the maintenance sensor chip and insert the SA chip (CM5 chip coated by streptavidin protein from step 1). Reopen the immobilization method, insert reagent rack 2, and check the positions, then run the method for the estimated run time.
- Repeat the step 1.6.
3. Regeneration scouting for PD-1 and PD-L1
- Setup the regeneration scouting method: Open/new wizard template, choose Regeneration Scouting, set flow path: 2-1, 4-3, chip type to SA, check run conditioning cycle, record the solution as HBS-EP+, contact time as 30 s, number of injections as 3, solution as PD-L1, contact time: 30 s, flow rate: 30 µL/min.
- For regeneration parameters, the flow rate is 30 µL/min, and the stabilization period is 300 s. In the experimental design, set the number of conditions as 4, and the number of cycles for each condition as 2. Set the conditions as 4, regeneration solution: Glycine 1.5, 2.0, 2.5, 3.0, contact times: 30 s, then check prime before running.
- Set each PD-L1 concentration as a separate sample well position in a 96-well microplate layout: R1 A1 to R1 A9: 1 µM PD-L1; R1 A10 to R1 A12: HBS-EP+ buffer; R1 B1: Glycine 1.5; R1 B2: Glycine 2; R1 B3: Glycine 2.5; R1 B4: Glycine 3.
NOTE: Glycine buffer with different pH is used as the regeneration buffer. - Prepare PD-L1 solution: Add 200 µL of DNase-free water to 100 µg of Human PD-L1 Fc Tag protein (9.42 µM), then dilute to 1 µM in HBS-EP+ buffer.
- Place all the relevant reagents in the layout as described in step 3.2. Place Tube A into the 1× HBS-EP+ buffer, then replace the maintenance sensor chip with the SA chip. Reopen the Regeneration Scouting method, follow the tube position from step 3.2, insert the reagent rack, and then run the method for the estimated run time.
- Repeat the step 1.6.
4. Validation of PD-1/PD-L1 interaction
NOTE: For validation, a previously published report18 was followed with minor adjustments.
- Use the same parameters as the published report under General Settings, Assay Steps, and Cycle Types, set contact time to 60 s, dissociation time to 60 s, and flow rate to 30 µL/min. Under method variables, evaluation variables, and commands, use the same setting, except for using Glycine 2.0 as Regeneration, setup flow rate to 30 µL/min, goes through the flow path of 1, 2, 3, 4.
- Then set up run, and choose the flow path: 2-1, 4-3. Input PD-L1 with concentrations from 0 µM, 0.037 µM, 0.111 µM, 0.333 µM, and a molecular weight of 51,300 Da. Check all the assay steps for verification and select prime before the run.
- Then set each PD-L1 concentration as a separate sample well position in a Reagent Rack 2 layout: R2 B1: PD-L1 0 µM; R2 B2: PD-L1 0.037 µM; R2 B3: PD-L1 0.111 µM; R2 B4: PD-L1 0.333 µM; R2 A1: Glycine 2.0 as regeneration buffer; R2 A2: HBS-EP+ as startup buffer.
- Prepare 200 mL of 1× HBS-EP+ running buffer solution. Prepare PD-L1 concentrations: dilute PD-L1 protein at 9.42 µM to 0.333 µM, 0.111 µM, and 0.037 µM in HBS-EP+. Then place all the relevant reagents in the layout as described in step 4.1.2.
- Insert Tube A into the 1× HBS-EP+ running buffer solution, eject the maintenance sensor chip, and insert the SA chip. Reopen the regeneration scouting method, follow the tube positioning from step 4.1, insert reagent rack 2, and run the method for the estimated duration.
- Repeat the step 1.6.
5. PD-1/PD-L1 blockade assay with small molecule inhibitor: BMS-1166
NOTE: For the blockade assay, a previously published report18 was followed with minor adjustments.
- Use the same parameters as the published report under General Settings, Assay Steps, and Cycle Types, set contact time to 60 s, dissociation time to 60 s, and flow rate to 30 µL/min. Under method variables, evaluation variables, and commands also use the same setting, except for using Glycine 2.0 as Regeneration, setup flow rate to 30 µL/min, goes through the flow path of 1, 2, 3, 4.
- Then set up run, and choose flow path: 2-1, 4-3. Input the sample solution: PD-L1 (0.111 µM, molecular weight of 51,300 Da) with BMS-1166 at 0 µM, 0.125 µM, 0.625 µM, 3.125 µM. Check all the assay steps for verification and select prime before the run.
- Then set each PD-L1 concentration as a separate sample well position in a Reagent Rack 2 layout: R2 B1: PD-L1 (0.111 µM) + BMS-1166 0 µM R2 B2: PD-L1 (0.111 µM) + BMS-1166 0.125 µM; R2 B3: PD-L1 (0.111 µM) + BMS-1166 0.625 µM; R2 B4: PD-L1 (0.111 µM) + BMS-1166 3.125 µM; R2 A1: Glycine 2.0 for regeneration.
- Prepare 200 mL of 1× HBS-EP+ running buffer solution. Prepare BMS-1166/PD-L1 mixture: Dissolve 5 mg of BMS-1166 in 77.99 µL Dimethyl sulfoxide (DMSO) to prepare a 100 mM stock solution. Dilute the stock solution with PD-L1 protein (0.11 µM) to the target concentrations of BMS-1166 at 0 µM, 0.125 µM, 0.625 µM, and 3.125 µM in HBS-EP+. Place all the relevant reagents layout as described in step 5.1.2.
- Place Tube A into the 1× HBS-EP+ running buffer solution, then eject the maintenance sensor chip and insert the SA chip. Reopen the regeneration scouting method, follow the tube position from 5.1 and, insert reagent rack 2, then run the method for the estimated run time.
- Repeat the step 1.6.
Results
Immobilization of SA on CM5 chip
Data were analyzed via the output from the SPR instrument and associated analysis software indicating successful achievement of the target RU (2000 RU) of SA protein on flow cell 1 and flow cell 2. Flow cells 1 and 2 were immobilized with SA (40 μg/mL) on the CM5 chip surface with a final response of 1902.3 RU on flow cell 1 (Figure 1A) and 1900.7 RU on flow cell 2 (Figure 1B).
Immobilization of PD-1 on SA chip
Data analysis based on the output from the SPR instrument and associated software indicated a low response unit (RU) for the blank cell on flow cell 1 and successful attainment of the target RU (4000 RU) for the PD-1 ligand on flow cell 2. Flow cell 1 was immobilized as a blank, yielding a final response of -161.0 RU (Figure 2A), while flow cell 2 was immobilized with biotinylated PD-1 protein (10 µg/mL) coated on the SA chip, resulting in a final response of 3698.5 RU (Figure 2B).
Regeneration scouting for PD-1 and PD-L1.
Regeneration scouting was conducted with PD-1 immobilized on flow cell 2, and PD-L1 in solution (0.1 μM) at various Glycine pH levels (1.5, 2, 2.5, and 3) to determine the regeneration solution resulting in a stable baseline and sample response. Data were analyzed via the output from the SPR instrument and analysis software, resulting in Glycine (pH 2.0) as the optimal regeneration condition due to minimal changes in response for both the baseline and sample response, indicating minimal PD-1 protein loss and successful removal of the PD-L1 protein from the chip surface. At Glycine pH 2.0, the baseline response remained relatively constant, and the analyte response was stable and close to the response at the start of the experiment, indicating the most optimal regeneration buffer among the four tested glycine pH conditions. A higher pH is insufficient, and a lower pH is too harsh; pH 2.0 is identified as the most suitable regeneration condition (Figure 3).
Validation of PD-1/PD-L1 interaction
Data were analyzed using the corresponding evaluation software. Under the Kinetics/Affinity section, select "surface-bound," choose curve 2-1, set the kinetics model to 1:1 binding, and adjust the RI parameter to a constant fit to determine the association rate (ka), dissociation rate (kd), and equilibrium dissociation constant (KD). The binding interaction of PD-L1 at varying concentrations with PD-1 was quantified, yielding a measurable response (Figure 4). The analyzed binding parameters included an association rate (ka) of 3.611 × 104 (1/Ms), a dissociation rate (kd) of 0.0236 (1/s), and an equilibrium dissociation constant (KD) of 6.536 × 10-7 M.
PD-1/PD-L1 blockade assay with established small molecule inhibitor: BMS-1166
Data were analyzed by using the corresponding. Under Kinetics/Affinity, select surface-bound, choose the curve 2-1, then obtain the visualized curve of the result and response unit. The blockade effect of the PD-1/PD-L1 binding interaction was observed with BMS-1166 (0-3.125 µM) with 0.11 µM PD-L1 protein in HBS EP+ buffer (Figure 5). The highest response unit is identified by PD-L1 alone, whereas with increasing BMS-1166 concentration, the binding response unit decreases proportionally.

Figure 1: Immobilization of SA on CM5 chip. Immobilization curves of streptavidin protein on CM5 chip flow cell 1 (A) and flow cell 2 (B) are shown. First, five pre-concentrations of the SA protein flowed over flow cells 1 and 2, followed by a NaOH wash and establishment of a stable baseline. Next, EDC and NHS were added, and then an ethanolamine hydrochloride wash was conducted. Next, five pulses of PD-1 were performed to reach the target level (2000 RU), followed by an ethanolamine hydrochloride wash to remove electrostatically bound ligands and deactivate NHS-esters that remain unreactive. Please click here to view a larger version of this figure.
Figure 2: Immobilization of PD-1 on SA chip. Immobilization curves of (A) the blank flow cell 1 coated with SA only, and (B) the PD-1 protein on the SA chip on flow cell 2 are shown. For the blank (flow cell 1), 1 M of NaCl and 50 mM of NaOH were injected three times, followed by a wash with 50% isopropanol/1 M of NaCl/50 mM of NaOH. For the PD-1 protein (flow cell 2), 1 M of NaCl and 50 mM of NaOH were injected three times, followed by five pulses of PD-1 protein to reach the target level of 4000 RU and a final wash of 50% isopropanol/1 M of NaCl/50 mM of NaOH. Please click here to view a larger version of this figure.

Figure 3: Regeneration scouting for PD-1 and PD-L1. The baseline and sample response were obtained for four regeneration conditions with Glycine at pH 1.5, 2, 2.5, and 3 to determine the optimal regeneration solution prior to sample testing. Please click here to view a larger version of this figure.

Figure 4: Validation of PD-1/PD-L1 interaction. Binding kinetics of different concentrations of PD-L1 (0.037 µM, 0.111 µM, and 0.333 µM) to PD-1 on the streptavidin-coated chip surface. PD-L1 demonstrates distinct association (0-60 s) and dissociation phases (61-120 s) with PD-1. Please click here to view a larger version of this figure.
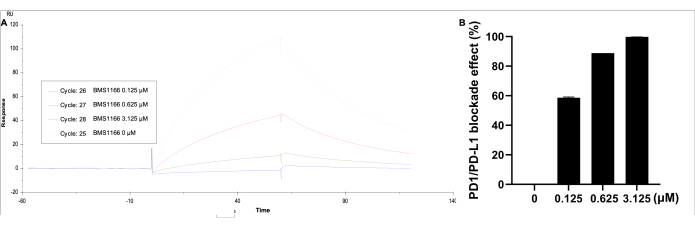
Figure 5: SPR analysis of PD-1 coated on an SA immobilized CM5 chip, with PD-L1 (0.11 µM) and BMS-1166 (0-3.125 µM) in solution. (A) A representative real-time SPR response of the binding reactions between PD-1/PD-L1 with BMS-1166. (B) The percentage blockade effect BMS-1166 on the PD-1/PD-L1 interaction binding kinetics. Please click here to view a larger version of this figure.

Figure 6: Comparison of PD-1 protein immobilization strategies. Amine coupling (A) vs. dual-step Streptavidin-biotin coupling (B). Amine coupling presents challenges, including limited availability of accessible binding sites, steric hindrance to binding, and potential modification of PD-1 binding sites during immobilization. In contrast, the dual-step Streptavidin-biotin approach enhances the availability of free binding sites on immobilized PD-1, facilitating improved interaction with PD-L1 in solution. Please click here to view a larger version of this figure.
Discussion
Over the past few decades, various immunotherapy approaches-including cancer vaccines, immune checkpoint inhibitors, and CAR T-cell therapies-have significantly advanced cancer treatment21. Immune checkpoints play a crucial role in preventing immune cell-mediated collateral damage during pathogenic responses and in suppressing autoimmunity. A key example is the interaction between PD-L1 and PD-1, which forms a major immune checkpoint, allowing cancer cells to evade immune surveillance. Targeting the PD-1/PD-L1 pathway with monoclonal antibodies has achieved remarkable success in clinical oncology. However, due to limitations associated with monoclonal antibody therapies and the increasing incidence of immune-related adverse events, there is growing interest in developing small-molecule inhibitors targeting PD-1/PD-L19,22.
Current screening strategies for small-molecule PD-1/PD-L1 inhibitors primarily focus on bioassay techniques such as ELISA, cell-based reporter assays, and T-cell assays. Biophysical techniques, including SPR and biolayer Interferometry (BLI) are widely used for the characterization of binding affinities, but their potential to be used as screening tools is underestimated23. This study developed an optimized SPR-based PD-1/PD-L1 blockade assay, which offers a high-throughput platform suitable for small-molecule PD-1/PD-L1 inhibitor discovery. SA was immobilized to ~2000 RU, followed by biotinylated PD-1 (~4000 RU) via the biotin-streptavidin interaction. This robust binding ensured secure PD-1 coating with optimal orientation, minimizing nonspecific binding and reducing protein usage (10 µg/mL). Site-directed immobilization improved efficiency compared to conventional methods. Glycine buffer (pH 2.0) was used to regenerate the sensor surface between cycles, maintaining experimental integrity and preventing nonspecific binding.
Compared to standard immunological methods and ELISA, this SPR approach offered real-time, label-free detection with high sensitivity and specificity. Moreover, it enables high-throughput screening with a runtime of 120 s/sample and complements bioassays in assessing the blockade efficiency of PD-1/PD-L1-targeting compounds and biologics. The immobilization level achieved was comparable to the previous platform (3698.5 RU vs. 3688.5 RU), with a similar PD-1/PD-L1 interaction affinity (KD = 6.536 × 10-7 M vs. 1.295 × 10-7 M). The BMS-1166 inhibitor demonstrated a higher dissociation rate at a lower concentration, with blockade effects comparable to the earlier platform (99.8% vs. 94.2% at 3.125 µM). BMS-1166 demonstrated PD-1/PD-L1 blockade rates supported by IC50 values of 1.4 nM and 96 nM in HTRF and cell-based assays, respectively24. Additionally, other studies reported IC50 values of 3.9 nM and 1574 nM using HTRF and immune checkpoint blockade co-culture assay methods25. Our previous results showed that the IC50 values of BMS-1166 were 85.4 nM, which is consistent with these earlier findings18. Another advantage of this screening platform is its robustness and high throughput. This method was extensively applied in high-throughput screening using a 384-well plate format, with BMS-1166 and BMS-202 included as positive controls for every 10 samples. Blockade rate ranges were 29.8%-38.1% for BMS-1166 and 6.0%-10.4% for BMS-202 at 10 nM (n = 11 per plate).
DMSO is likely to have varying effects on heterogeneous biological membranes, depending on their local composition and structure, potentially influencing membrane-associated biological functions. At relatively low concentrations, DMSO can alter protein properties in solution, leading to denaturation, aggregation, or degradation. Additionally, DMSO may modify the apparent binding properties of proteins26,27. Furthermore, to address previous concerns regarding DMSO interference, we reduced the DMSO concentration to 0.003% (0.01% DMSO was used in the earlier platform) in this protocol.
In a previous study, PD-1 proteins were immobilized on sensor chips using amine coupling, which employs EDC/NHS chemistry to activate carboxymethyl groups on the chip, forming covalent bonds with amine groups on PD-1. Due to the presence of multiple amine groups across PD-1's 288 amino acids, this method inherently results in random orientations. To achieve a more specific orientation, we employed a capture coupling strategy with biotinylated human PD-1 (Fc, Avitag) in this study. The single lysine residue in the Avitag is enzymatically biotinylated on the Fc region of PD-1, enabling precise immobilization through the high-affinity interaction between biotin and streptavidin (Figure 6). Theoretically, this approach ensures that the variable domain of PD-1 remains exposed, facilitating optimal interaction with PD-L1 in the buffer. However, this assumption requires further validation using techniques such as cryo-EM or crystallography to confirm protein orientation.
Glycine 2.0 was used as a mild regeneration buffer, effectively removing PD-L1 without reducing the immobilized PD-1. However, for tightly bound inhibitors or protein aggregates, stronger regeneration buffers, such as 0.5% SDS or 50-100 mM of NaOH, may be required to prevent interference with subsequent samples.
This study has several limitations. First, the high cost of SPR instrumentation can limit accessibility, although the protocol can often be adapted for other SPR systems with similar capabilities. Parameters such as protein concentration and association/dissociation times can be adjusted to fit specific SPR platforms. Another limitation is its reliance on recombinant proteins, which may not completely replicate native protein interactions. These factors should be considered when interpreting SPR data and comparing results with complementary techniques, such as cell-based assays or in vivo models.
Despite these limitations, the optimized SPR method offers a rapid, real-time, high-throughput, and label-free approach for screening small-molecule inhibitors of the PD-1/PD-L1 interaction. It significantly enhances biophysical techniques for characterizing small molecules and biologics targeting PD-1/PD-L1 interaction. With its high sensitivity, the platform is particularly valuable for studying immune checkpoint interactions and broader protein-protein interactions (PPI), making it a powerful tool for advancing PPI drug discovery.
Disclosures
The authors have nothing to disclose.
Acknowledgements
The authors acknowledge the RI-INBRE core facility at the University of Rhode Island, supported by Grant P20GM103430 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH). This research was supported by a Pilot Grant Award from the College of Pharmacy at the University of Rhode Island, a Small Grant Award from the Rhode Island Life Science Hub (RILSH), and a Rhode Island Foundation grant, all awarded to Chang Liu, Ph.D.
Materials
| Name | Company | Catalog Number | Comments |
| 50 mM NaOH | Cytiva Life Sciences | 100358 | |
| 50 mM NaOH | Fisher Scientific | 905376 | |
| 96-Well Polystyrene Microplates | Cytiva Life Sciences | BR100503 | |
| Amine Coupling Kit | Cytiva Life Sciences | 35120 | |
| Biacore T200 SPR System and Evaluation Software 3.2 | Cytiva Life Sciences | 28975001 | |
| Biotinylated Human PD-1 Fc, Avitag Protein | Acro Biosystems | PD1-H82F1 | |
| BMS1166 | MedChemExpress | HY-102011 | |
| Dimethyl Sulfoxide (DMSO) | Sigma-Aldrich | 276855 | |
| DNase Free Water | Fisher Scientific | 188506 | |
| Glycine 1.5 | Cytiva Life Sciences | BR100354 | |
| Glycine 2.0 | Cytiva Life Sciences | BR100355 | |
| Glycine 2.5 | Cytiva Life Sciences | BR100356 | |
| Glycine 3.0 | Cytiva Life Sciences | BR100357 | |
| HBS-EP+ Buffer | Cytiva Life Sciences | BR100669 | |
| Human PD-L1 Fc Tag Protein | Acro Biosystems | PD-1-H5258 | |
| Isopropanol | Fisher Scientific | BP2618-1 | |
| Microplate Foil, 96-Well | Cytiva Life Sciences | 28975816 | |
| NaCl | Sigma-Aldrich | 746398 | |
| Plastic Vials 7 mm | Cytiva Life Sciences | BR100212 | |
| Rubber Caps, Type 3 | Cytiva Life Sciences | BR100502 | |
| Series S Sensor Chip CM5 | Cytiva Life Sciences | 29149603 | |
| Sodium Acetate 4.5 | Cytiva Life Sciences | 100350 | |
| Sodium Acetate 5.0 | Cytiva Life Sciences | 100351 | |
| Streptavidin | Sigma-Aldrich | S4762 |
References
- Ribas, A., Wolchok, J. D. Cancer immunotherapy using checkpoint blockade. Science. 359 (6382), 1350-1355 (2018).
- Zhang, X., et al. Structural and functional analysis of the costimulatory receptor programmed death-1. Immunity. 20 (3), 337-347 (2004).
- Freeman, G. J., et al. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J Exp Med. 192 (7), 1027-1034 (2000).
- Han, Y., Liu, D., Li, L. PD-1/PD-L1 pathway: Current researches in cancer. Am J Cancer Res. 10 (3), 727-742 (2020).
- Lyu, N., et al. Recognition of PDL1/L2 by different induced-fit mechanisms of PD1: A comparative study of molecular dynamics simulations. Phys Chem Chem Phys. 22 (3), 1276-1287 (2020).
- Taube, J. M., et al. Colocalization of inflammatory response with B7-H1 expression in human melanocytic lesions supports an adaptive resistance mechanism of immune escape. Sci Trans Med. 4 (127), 127ra37 (2012).
- Sun, X., et al. Immune-related adverse events associated with programmed cell death protein-1 and programmed cell death ligand 1 inhibitors for non-small cell lung cancer: A PRISMA systematic review and meta-analysis. BMC Cancer. 19 (1), 558 (2019).
- Alkholifi, F. K., Alsaffar, R. M. Dostarlimab an inhibitor of PD-1/PD-L1: A new paradigm for the treatment of cancer. Medicina. 58 (11), 1572 (2022).
- Uzar, W., et al. An updated patent review on PD-1/PD-L1 antagonists (2022-present). Expert Opin Ther Pat. 34 (8), 627-650 (2024).
- Ai, L., et al. Research status and outlook of PD-1/PD-L1 inhibitors for cancer therapy. Drug Des Devel Ther. 14, 3625-3649 (2020).
- Conroy, M., Naidoo, J. Immune-related adverse events and the balancing act of immunotherapy. Nat Commun. 13 (1), 392 (2022).
- Guzik, K., et al. Small-molecule inhibitors of the Programmed Cell Death-1/Programmed Death-Ligand 1 (PD-1/PD-L1) interaction via transiently induced protein states and dimerization of PD-L1. J Med Chem. 60 (13), 5857-5867 (2017).
- Sifniotis, V., Cruz, E., Eroglu, B., Kayser, V. Current advancements in addressing key challenges of therapeutic antibody design, manufacture, and formulation. Antibodies. 8 (2), 36 (2019).
- Beck, H., Härter, M., Haß, B., Schmeck, C., Baerfacker, L. Small molecules and their impact in drug discovery: A perspective on the occasion of the 125th anniversary of the Bayer Chemical Research Laboratory. Drug Discov Today. 27 (6), 1560-1574 (2022).
- Nguyen, H. H., Park, J., Kang, S., Kim, M. Surface plasmon resonance: A Versatile technique for biosensor applications. Sensors (Basel, Switzerland). 15 (5), 10481-10510 (2015).
- Liu, C., Seeram, N. P., Ma, H. Small molecule inhibitors against PD-1/PD-L1 immune checkpoints and current methodologies for their development: A review. Cancer Cell Int. 21 (1), 239 (2021).
- Hu, K., Li, X. -. J., Asmamaw, M. D., Shi, X. -. J., Liu, H. -. M. Establishment of high-throughput screening HTRF assay for identification small molecule inhibitors of Skp2-Cks1. Sci Rep. 11 (1), 21105 (2021).
- Puopolo, T., et al. Establishment of human PD-1/PD-L1 blockade assay based on surface plasmon resonance (SPR) biosensor. Bio-protoc. 13 (15), e4765 (2023).
- Ding, M., Chen, Y., Lang, Y., Cui, L. The role of cellular prion protein in cancer biology: A potential therapeutic target. Front Oncol. 11, 742949 (2021).
- Li, H., Seeram, N. P., Liu, C., Ma, H. Further investigation of blockade effects and binding affinities of selected natural compounds to immune checkpoint PD-1/PD-L1. Front Oncol. 12, 995461 (2022).
- Kamrani, A., et al. New immunotherapeutic approaches for cancer treatment. Pathol Res Pract. 248, 154632 (2023).
- Yan, Y., Zhang, L., Zuo, Y., Qian, H., Liu, C. Immune checkpoint blockade in cancer immunotherapy: mechanisms, clinical outcomes, and safety profiles of PD-1/PD-L1 inhibitors. Arch Immunol Ther Exp. 68 (6), 36 (2020).
- Chandrasekharan, G., Unnikrishnan, M. High throughput methods to study protein-protein interactions during host-pathogen interactions. Eur J Cell Biol. 103 (2), 151393 (2024).
- Zhang, Y., et al. BMS-202, a PD-1/PD-L1 inhibitor, decelerates the profibrotic effects of fibroblasts derived from scar tissues via ERK and TGFβ1/Smad signaling pathways. Immun Inflamm Dis. 10 (1), e591 (2022).
- Surmiak, E., et al. PD-L1 inhibitors: Different classes, activities, and mechanisms of action. Int J Mol Sci. 22 (21), 11797 (2021).
- Feoli, A., Sarno, G., Castellano, S., Sbardella, G. DMSO-Related effects on ligand-binding properties of lysine methyltransferases G9a and SETD8. ChemBioChem. 25 (4), e202300809 (2024).
- Tjernberg, A., Markova, N., Griffiths, W. J., HalléN, D. DMSO-Related effects in protein characterization. SLAS Discov. 11 (2), 131-137 (2006).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionExplore More Articles
This article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved