Method Article
Application of Laparoscopic Partial Splenectomy with Total Blood Flow Occlusion in Benign Splenic Lesions
In This Article
Summary
In this study, we describe an intraoperative hemorrhage control technique for laparoscopic partial splenectomy, improving spleen resection's safety and precision.
Abstract
Laparoscopic partial splenectomy (LPS) is gradually becoming the preferred method for treating benign splenic lesions. However, due to the abundant blood supply and its soft, fragile tissue texture, especially when the lesion is located near the splenic hilum or is particularly large, performing partial splenectomy (PS) in clinical practice is extremely challenging. Therefore, we have been continuously exploring and optimizing hemorrhage control methods during PS, and we here propose a method to perform LPS with complete spleen blood flow occlusion.
This study describes an optimized approach to control intraoperative hemorrhage during LPS. First, it involves the thorough dissection of the splenic ligaments and careful separation of the pancreatic tail from the spleen. With complete exposure to the splenic hilum, we temporarily occlude the entire blood supply of the spleen using a laparoscopic bulldog clip. Subsequently, we employ intraoperative ultrasound to identify the boundary of the lesion and resect the corresponding portion of the spleen under complete blood flow control. This approach embodies the essence of 'spleen preservation' through effective hemorrhage control and precise resection. It is particularly suitable for laparoscopic surgery and deserves further clinical promotion.
Introduction
With a profound understanding of the physiological functions of the spleen, the research underscores its pivotal role in the body's immune response, hematopoiesis, and clearance of red blood cells1. Complications following splenectomy, such as overwhelming post-splenectomy infections (OPSIs), pulmonary hypertension, and thromboembolism, significantly influence the choice of surgical methods in clinical practice2,3. According to the literature, patients after total splenectomy exhibit a decreased capacity to clear malaria-parasitized RBCs and a higher risk of developing meningitis and sepsis following infections with Streptococcus pneumoniae, Neisseria meningitidis, and Haemophilus influenzae type B4. PS preserves splenic function while ensuring treatment effectiveness, making it widely applied in clinical practice.
In 1959, the first successful PS was reported by Cristo5. The spleen is a fragile organ comprising well-defined splenic segments, each with its distinctive arterial and venous supply, demarcated by relatively avascular regions6. These factors collectively establish the anatomical foundation for PS. However, conventional open surgery carries inherent drawbacks, including significant trauma, cosmetic disadvantages, and postoperative pain. In recent years, alongside the maturation of laparoscopic instruments and techniques, LPS has emerged as the preferred therapeutic modality for benign splenic lesions. Nonetheless, due to the spleen's rich blood supply, substantial intraoperative hemorrhage during laparoscopy may cause a conversion to open surgery. Romboli et al. reviewed 457 cases of LPS, revealing an average operative time of approximately 128 ± 43.7 min, and demonstrated that about 3.9% of patients required conversion due to hemorrhage, and the average postoperative stay is 4.9 ± 3.8 days7. Comprehensive knowledge of splenic anatomy and meticulous surgical skills have hindered the broad clinical application of LPS.
To mitigate the risk of intraoperative hemorrhage and expedite the learning curve, we try to perform LPS with complete blood flow occlusion. In this study, we present a 72-year-old female patient with a massive splenic vascular tumor located in the upper middle pole of the spleen and adjacent to the splenic hilum. This novel technique excels in effective hemorrhage control and ensures safety, efficacy, and a high level of reproducibility.
Protocol
This study follows the guidelines of the Ethics Committee of Shunde Hospital of Southern Medical University. Informed consent was obtained from the patient before the surgery for the data and video.
1. Patient selection
- Apply this surgical method in the following cases:
- Include patients experiencing abdominal pain or discomfort, coupled with radiological examinations confirming the presence of benign lesions.
- Do not impose specific restrictions on tumor size, but maintain a residual spleen volume exceeding 25%.
- Ensure patients exhibit normal levels of AFP, CEA, and CA-199 or confirm the absence of malignancy.
- Consider obesity, which results in increased visceral fat potentially affecting intraoperative anatomy, but does not view it as an absolute limitation.
- Exclude patients from this surgery in the following conditions:
- Exclude if the patient is highly suspected of splenic metastasis from malignant tumors.
- Exclude patients with splenomegaly resulting from hematologic diseases or lymphoma.
- Exclude patients with splenomegaly secondary to liver cirrhosis.
- Exclude patients having life-threatening traumatic spleen rupture.
- Exclude patients who have poor overall health and inability to withstand surgery.
2. Surgical technique
- Set up for the operation.
- Position the patient under general anesthesia in a reverse Trendelenburg posture with the left side of the body inclined approximately 10-30°.
NOTE: Make adjustments in position as needed throughout the surgical procedure. - Position the first surgeon and the assistant with the laparoscope on the patient's right side and the first assistant on the patient's left side.
- Establish and maintain pneumoperitoneum at 12-14 mmHg.
- Install four ports on the abdominal wall with the assistance of laparoscopic visualization, as depicted in Figure 1.
- Position the patient under general anesthesia in a reverse Trendelenburg posture with the left side of the body inclined approximately 10-30°.
- Examine the peritoneal cavity under laparoscopy to confirm the absence of malignancies.
- Perform temporary occlusion of the splenic artery.
- Use a vessel sealing system to dissect the gastrosplenic ligament along the greater curvature of the stomach,entering the lesser sac.
- Grasp the stomach and move it to the upper right for better exposure to the surgical field.
- Meticulously dissect the main trunk of the splenic artery at the superior edge of the pancreas and temporarily occlude it with a bulldog clip.
NOTE: Following the occlusion of blood flow, the spleen exhibits a decrease in volume and a softer texture, which will provide adequate surgical space.
- Dissect the perisplenic ligaments, including the splenocolic, splenorenal, and splenophrenic ligaments.
NOTE: Caution must be taken when separating the pancreatic tail from the spleen, particularly in cases of splenomegaly or massive splenic lesions, to prevent uncontrolled hemorrhage and the occurrence of postoperative pancreatic fistula (POPF). - Perform temporary occlusion of the splenic hilum.
- Expose the splenic hilum by resecting the attachments around it with an ultrasonic scalpel, then implement a temporary occlusion of the splenic hilum with a bulldog clip.
NOTE: In cases with variations in splenic blood vessels, merely occluding the splenic artery may not achieve the desired splenic ischemic changes. - Following this step, conduct a thorough reassessment of the spleen's color, size, and texture.
- Expose the splenic hilum by resecting the attachments around it with an ultrasonic scalpel, then implement a temporary occlusion of the splenic hilum with a bulldog clip.
- Perform intraoperative ultrasound for lesion boundary identification.
- Use intraoperative ultrasound to identify the boundary of the lesion during surgery.
- Apply electrocautery to mark the demarcation line at least 1 cm away from the lesion boundary.
- Perform spleen parenchyma dissection.
- Insert a bipolar radiofrequency device into the splenic parenchyma along the demarcation line for coagulation and ablation, setting the radiofrequency energy at 80 W.
- Use an ultrasonic scalpel to dissect the splenic parenchyma in the necrotic coagulation zone.
- Securely clamp thick ducts using Hem-o-lok vascular clips and then carefully cut, ensuring a safe resection of the upper spleen containing the lesion.
NOTE:Vessels supplying the removed portion of the spleen need to be carefully ligated and transected
- Remove the specimen.
- Release the bulldog clip, ensuring no bleeding from the splenic cut edge, and confirm adequate blood supply to the remnant spleen.
- Use bipolar electrocautery to cauterize the splenic cut edge and apply absorbable hemostatic agents over it.
- Position a drain tube in the splenic fossa.
- Place the specimen into a specimen bag, fragment it with oval forceps, and remove it through the enlarged port.
- Release the pneumoperitoneum and suture the puncture wounds.
3. Postoperative details
- Perform continuous electrocardiography for 24 h postoperatively. Monitor essential signs, including heart rate, blood pressure, respiration, oxygen saturation, central venous pressure, pupillary response, and level of consciousness, todetect early postoperative complications in a timely manner.
- Administer antibiotics (cefuroxime sodium, 1.5 g every 12 h) and proton pump inhibitors routinely following the surgery.
- Remove the gastric tube on the second postoperative day and allow the patient a full liquid diet.
- Remove the abdominal drain tube when the drainage output is less than 100 mL/24 h.
- Schedule a follow-up abdominal CT scan 2 months after the surgery to assess the abdominal condition.
Results
In this case, a 72-year-old female patient was admitted for a massive splenic lesion found on a routine examination at a local hospital. She had a history of previous abdominal surgery. Her medical history was unremarkable, and her BMI was normal (20.1 kg/m2). Abdominal contrast-enhanced CT showed a massive lesion located in the upper middle pole of the spleen, with a diameter of approximately 15 cm (Figure 2). Preoperative assessments revealed no evidence of malignancy. Due to the large size of the lesion, after full discussion, LPS with complete spleen blood flow occlusion was attempted. Representative intraoperative laparoscopic images are shown in Figure 3.
The operative time was 102 min, with an intraoperative blood loss of 30 mL, and approximately 65% of the spleen was resected. No intraoperative complications occurred, and there was no conversion to laparotomy. Intraoperative blood transfusion was not required. The abdominal drain was removed 4 days postoperatively, and the patient was discharged on the 7th day after the surgery. There were no postoperative complications, including postoperative pancreatic fistula, intraperitoneal infection, hemorrhage, portal thrombosis, or splenic infarction. Specific details can be seen in Table 1. The pathological diagnosis was hemangioma. The follow-up abdominal CT after 2 months showed that the remnant spleen had good blood supply, and no recurrence was observed.
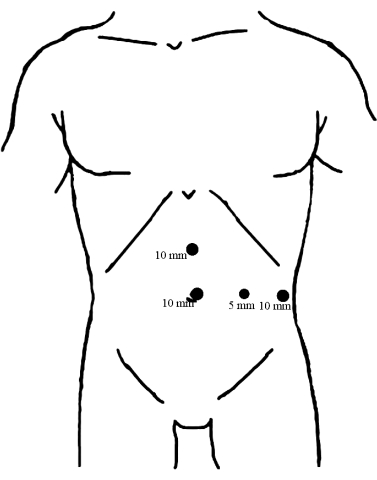
Figure 1: Distribution of trocars. Place a 10 mm trocar beneath the umbilicus as an observation port. The other two 10 mm trocars are inserted, one at the midpoint between the umbilicus and xiphoid process and the other on the left anterior axillary line, parallel to the umbilicus, both serving as the main operating ports. Then, the 5 mm trocar was inserted along the midline of the left clavicle at the level of the umbilicus as the assisted operating port. Please click here to view a larger version of this figure.
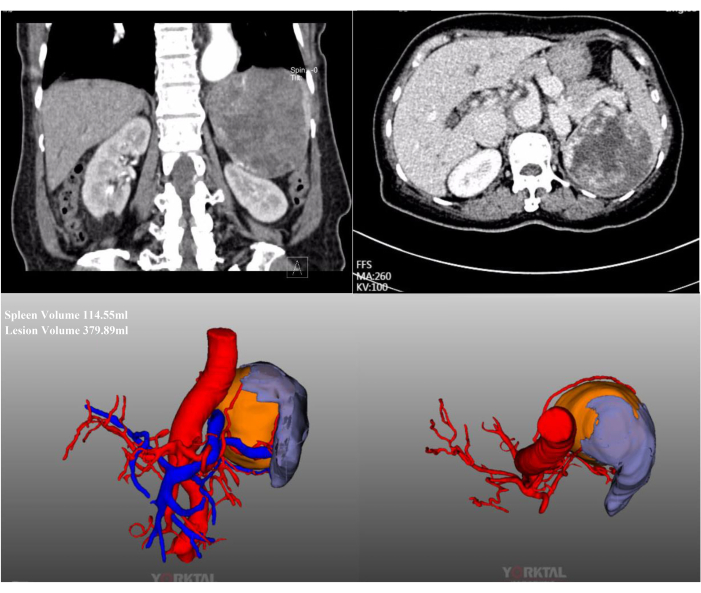
Figure 2: Contrast-enhanced CT and CT-3D reconstruction. Abdominal contrast-enhanced CT and CT-3D reconstruction show a massive lesion located in the upper middle pole of the spleen. Please click here to view a larger version of this figure.
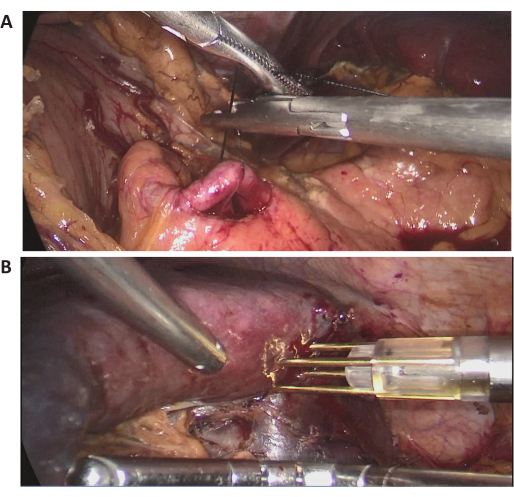
Figure 3: Intraoperative laparoscopic images. (A,B) Representative intraoperative laparoscopic images of the surgical procedure. Please click here to view a larger version of this figure.
| Items | Outcome |
| Operative time (min) | 102 |
| Intraoperative blood loss (mL) | 30 |
| Conversion to laparotomy | No |
| Intraoperative blood transfusion | No |
| Postoperative hospital stay (days) | 7 |
| Postoperative complications | No |
Table 1: Operative and postoperative details of LPS.
Discussion
For years, total splenectomy was the primary treatment for splenic tumors, splenomegaly, and hematological disorders. However, with extensive cases followed up, complications after total splenectomy, including infectious complications and thromboembolic complications, have gradually aroused attention8. Overwhelming post-splenectomy infections (OPSIs) are the most severe complication after splenectomy, characterized by rapid disease progression with a mortality rate of approximately 50%9. This heightened risk of bacterial infections in splenectomized patients is attributed to the role of IgM memory B cells in the spleen's marginal zone, which is crucial for clearing encapsulated bacteria10,11. Furthermore, postoperative complications such as portal thrombosis, caval system thrombosis, and pulmonary embolism need to be noted. Patients with cirrhosis who undergo splenectomy have a higher incidence of portal thrombosis12, possibly related to a hypercoagulable state13. PS can achieve therapeutic effects while preserving spleen function. Therefore, for eligible patients, PS has become a preferred alternative. Laparoscopic surgery, offering benefits such as minimal trauma, aesthetic outcomes, and shorter postoperative stays, has been widely used in PS in recent years.
LPS was first reported by Poulin in 199514. LPS involves selective vascular occlusion of specific spleen segments, followed by resection along the demarcation line. Typically, the terminal branches of the splenic artery divide into 2-3 branches before entering distinct spleen segments. Each segment is separated by relatively avascular regions15. However, the application of LPS is challenging due to uncontrolled intraoperative hemorrhage. Renato reviewed 344 cases of LPS surgery performed between 1960 and July 2017, indicating that the average intraoperative blood loss ranged from 0 to 1200 cc. Among the cases, 6.4% (22/344) required conversion to laparotomy, and 14 cases underwent total splenectomy due to intraoperative hemorrhage16. Based on our experience, the main reasons are as follows: (i) The spleen possesses a rich blood supply, and its delicate texture makes it prone to injury during surgery, rendering hemostasis challenging. (ii) Insufficient perisplenic ligament dissection complicates the mobilization of the spleen, leading to splenic parenchymal tears. (iii) Dissecting the branches of the splenic artery poses significant difficulty and carries the potential risk of damaging adjacent abdominal structures.
Numerous studies have reported methods to reduce intraoperative hemorrhage during LPS. There are mainly three main methods: occlusion of splenic blood supply, the use of radiofrequency devices, and robotic assistance. Borie reported the temporary occlusion of the splenic artery using a loop clamp to control intraoperative hemorrhage during LPS17. In Peng's study18, 46 patients underwent LPS with temporary occlusion of the splenic artery. They temporarily blocked the main splenic artery with a bulldog clip before dissecting the corresponding branches of the splenic artery. Unlike us, this study only included tumors located at the upper and lower poles of the spleen, with spleen sizes less than 20 cm. Preoperative vascular embolization has also been attempted for controlling hemorrhage in LPS19,20, demonstrating satisfactory clinical outcomes. Catalin et al.21 reviewed 10 cases of robotic-assisted PS, which had less intraoperative hemorrhage and shorter postoperative stays compared to LPS. However, its high cost limits its widespread use. Our center was the first to apply the radiofrequency device Habibi4X for splenectomy22. It creates a coagulative necrotic area through radiofrequency, allowing for bloodless splenectomy. It has become a standardized method at the center with consistent clinical effectiveness.
Considering the limitations of the above methods and the unique experience of our center, we attempted to use a bulldog clip for temporary occlusion of the splenic artery and hilum, performing LPS under complete splenic ischemia. The study reported that 95% of the main splenic artery trunk runs at the superior edge of the pancreas23. Temporary occlusion using a bulldog clip after intraoperative localization and isolation is easily feasible. However, it is essential to highlight that the dissection of the splenic hilum requires considerable laparoscopic surgical experience. The complexity of this procedure is influenced by factors such as a high BMI, a lesion located near the splenic hilum, a large lesion volume, and a history of abdominal surgery. Precise surgical technique is required to prevent injury to the pancreatic tail and short gastric vessels. A complete blockade of splenic blood flow can ensure the desired splenic ischemic effect, particularly when dealing with cases of variant blood vessels.
Radiofrequency devices demonstrate excellent performance in hemostasis24,25. We routinely use radiofrequency devices to insert into the splenic parenchyma along the pre-cut edge for coagulation, forming a coagulative necrotic area. Subsequently, we use an ultrasonic scalpel to dissect the splenic parenchyma, achieving bloodless partial splenectomy. In this study, we performed a large splenic lesion resection assisted with radiofrequency. The intraoperative blood loss was 30 mL, and the operative time was 102 min, with no conversion to open surgery. The patient was discharged 7 days after surgery without postoperative complications, which was significantly better than most other studies16,26. Upper abdominal CT follow-up at 2 months after the operation showed no thrombosis, and the remnant spleen had good blood supply, demonstrating a satisfactory therapeutic effect.
Teperman et al. indicate that the safe, warm ischemic time for the human spleen is 1 h27. Based on extensive surgical experience, we can complete the dissection of the splenic parenchyma within a safe time. Furthermore, postoperative abdominal CT scans have consistently shown no thrombosis or splenic infarction. This confirms the safety of LPS under complete splenic blood flow occlusion. However, we recommend that during the initial learning curve, challenging cases should be avoided, including those with BMI > 25 kg/m², a history of abdominal surgery, and oversized or near-splenic hilum lesions, to prevent prolonged ischemic time that may damage splenic function. The method described in this study is not applicable to lesions located at the splenic hilum. Postoperative monitoring is also equally important for detecting potential adverse events.
Compared to total splenectomy, PS offers the advantage of preserving the spleen's physiological function while achieving therapeutic efficacy and reducing long-term complication rates2. It is generally recommended that the remnant spleen volume should exceed 25% to effectively preserve splenic function. To assess the remnant spleen volume, we employ preoperative CT-3D reconstruction technology. In cases involving massive splenic lesions, the conventional approach of identifying and occluding corresponding splenic artery branches poses challenges in preserving sufficient splenic parenchyma. In this study, by combining preoperative CT-3D reconstruction and intraoperative ultrasound assessment, we optimized the approach to LPS to maximize the preservation of normal spleen tissue. Approximately 65% of the spleen was resected, which can achieve both therapeutic goals and maintaining splenic function.
In order to ensure the safety of this method, we recommend performing preoperative abdominal CT scans to clarify the relationship between the lesion, splenic vessels, and important abdominal structures, as well as the presence of variant blood vessels. If possible, CT-3D reconstruction can further enhance preoperative assessment. It is important to note that this method is suitable for patients with trauma confined to one side of the spleen, and we do not advise employing this method in cases of life-threatening traumatic splenic rupture or for patients with hematologic disorders. The former may pose a risk of unnecessary splenic preservation leading to death. For patients undergoing PS due to hematologic disorders, long-term follow-up has shown a high risk of recurrence and subsequent conversion to total splenectomy2. The ideal indication for this method is benign lesions of the spleen. This method does not require the dissection of splenic artery branches, thereby somewhat simplifying the learning curve for LPS. However, caution should be taken when selecting patients during the initial learning phase.
In conclusion, LPS under total splenic blood flow occlusion is a safe, feasible, and reproducible method that yields satisfactory results. However, further extensive research is still needed to assess its safety and effectiveness comprehensively.
Disclosures
None
Acknowledgements
None
Materials
| Name | Company | Catalog Number | Comments |
| Absorbable hemostat | Ethicon, LLC | W1913T | |
| Disposable trocar | Kangji Medical | 101Y.307,101Y.311 | |
| Endo bag | Medtronic | https://www.medtronic.com/covidien/en-us/search.html#q=endo%20bag | specimen bag |
| Jaw sealer/divider | Covidien Medical | LF1737 | |
| Laparoscopic radiofrequency device | AngioDynamics, Inc | Rita 700-103659 | |
| Laparoscopic system | Olympus | WM-NP2 L-RECORDOR-01 | |
| LigaSure | Medtronic | https://www.medtronic.com/covidien/en-us/products/vessel-sealing/ligasure-technology.html | vessel sealing system |
| Ligation clips (Hem-o-lok) | Teleflex Medical | 544240,544230,544220 | |
| Ultrasonic scalpel | ETHICON Medical | HAR36 |
References
- Lewis, S. M., Williams, A., Eisenbarth, S. C. Structure and function of the immune system in the spleen. Sci Immunol. 4 (33), eaau6085 (2019).
- Kristinsson, S. Y., Gridley, G., Hoover, R. N., Check, D., Landgren, O. Long-term risks after splenectomy among 8,149 cancer-free American veterans: a cohort study with up to 27 years follow-up. Haematologica. 99 (2), 392-398 (2014).
- Guizzetti, L. Total versus partial splenectomy in pediatric hereditary spherocytosis: A systematic review and meta-analysis. Pediatr Blood Cancer. 63 (10), 1713-1722 (2016).
- Bronte, V., Pittet, M. J. The spleen in local and systemic regulation of immunity. Immunity. 39 (5), 806-818 (2013).
- Christo, M. C. Partial regulated splenectomies. Preliminary note on the first 3 cases operated on. Hospital (Rio J). 56, 645-650 (1959).
- Redmond, H. P., Redmond, J. M., Rooney, B. P., Duignan, J. P., Bouchier-Hayes, D. J. Surgical anatomy of the human spleen. Br J Surg. 76 (2), 198-201 (2005).
- Romboli, A., et al. Laparoscopic partial splenectomy: A critical appraisal of an emerging technique. A review of the first 457 published cases. J Laparoendosc Adv Surg Tech A. 31 (10), 1130-1142 (2021).
- Buzelé, R., Barbier, L., Sauvanet, A., Fantin, B. Medical complications following splenectomy. J Visc Surg. 153 (4), 277-286 (2016).
- Bisharat, N., Omari, H., Lavi, I., Raz, R. Risk of infection and death among post-splenectomy patients. J Infect. 43 (3), 182-186 (2001).
- Kruetzmann, S., et al. Human immunoglobulin M memory B cells controlling Streptococcus pneumoniae infections are generated in the spleen. J Exp Med. 197 (7), 939-945 (2003).
- Leone, G., Pizzigallo, E. Bacterial infections following splenectomy for malignant and nonmalignant hematologic diseases. Mediterr J Hematol Infect Dis. 7 (1), e2015057 (2015).
- Romano, F., et al. Thrombosis of the splenoportal axis after splenectomy. Langenbecks Arch Surg. 391 (5), 483-488 (2006).
- Watters, J. M., et al. Splenectomy leads to a persistent hypercoagulable state after trauma. Am J Surg. 199 (5), 646-651 (2010).
- Poulin, E. C., Thibault, C., DesCôteaux, J. G., Côté, G. Partial laparoscopic splenectomy for trauma: technique and case report. Surg Laparosc Endosc. 5 (4), 306-310 (1995).
- Ignjatovic, D., Stimec, B., Zivanovic, V. The basis for splenic segmental dearterialization: a post-mortem study. Surg Radiol Anat. 27 (1), 15-18 (2005).
- Costi, R., et al. Partial splenectomy: Who, when and how. A systematic review of the 2130 published cases. J Pediatr Surg. 54 (8), 1527-1538 (2019).
- Borie, F. Laparoscopic partial splenectomy: Surgical technique. J Visc Surg. 153 (5), 371-376 (2016).
- Ouyang, G., et al. Laparoscopic partial splenectomy with temporary occlusion of the trunk of the splenic artery in fifty-one cases: experience at a single center. Surg Endosc. 35 (1), 367-373 (2021).
- Mignon, F., et al. Preoperative selective embolization allowing a partial splenectomy for splenic hamartoma. Ann Chir. 128 (2), 112-116 (2003).
- Zheng, L., et al. Treatment of hemangioma of the spleen by preoperative partial splenic embolization plus laparoscopic partial splenectomy: A case report. Medicine (Baltimore). 97 (17), e0498 (2018).
- Vasilescu, C., Stanciulea, O., Tudor, S. Laparoscopic versus robotic subtotal splenectomy in hereditary spherocytosis. Potential advantages and limits of an expensive approach. Surg Endosc. 26 (10), 2802-2809 (2012).
- Wang, W. -. D., et al. Partial splenectomy using a laparoscopic bipolar radiofrequency device: a case report. World J Gastroenterol. 21 (11), 3420-3424 (2015).
- Liu, D. L., et al. Anatomy of vasculature of 850 spleen specimens and its application in partial splenectomy. Surgery. 119 (1), 27-33 (1996).
- Habib, N. A. How we do a bloodless partial splenectomy. Am J Surg. 186 (2), 164-166 (2003).
- Gumbs, A. A., Bouhanna, P., Bar-Zakai, B., Briennon, X., Gayet, B. Laparoscopic partial splenectomy using radiofrequency ablation. J Laparoendosc Adv Surg Tech A. 18 (4), 611-613 (2008).
- Liu, G., Fan, Y. Feasibility and safety of laparoscopic partial splenectomy: A systematic review. World J Surg. 43 (6), 1505-1518 (2019).
- Teperman, S. H., et al. Bloodless splenic surgery: The safe warm-ischemic time. J Pediatr Surg. 29 (1), 88-92 (1994).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionExplore More Articles
This article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved