Method Article
Identifying Protein-protein Interaction in Drosophila Adult Heads by Tandem Affinity Purification (TAP)
In This Article
Summary
Drosophila is famous for its powerful genetic manipulation, but not for its suitability of in-depth biochemical analysis. Here we present a TAP-based procedure to identify interacting partners of any protein of interest from the fly brain. This procedure can potentially lead to new avenues of research.
Abstract
Genetic screens conducted using Drosophila melanogaster (fruit fly) have made numerous milestone discoveries in the advance of biological sciences. However, the use of biochemical screens aimed at extending the knowledge gained from genetic analysis was explored only recently. Here we describe a method to purify the protein complex that associates with any protein of interest from adult fly heads. This method takes advantage of the Drosophila GAL4/UAS system to express a bait protein fused with a Tandem Affinity Purification (TAP) tag in fly neurons in vivo, and then implements two rounds of purification using a TAP procedure similar to the one originally established in yeast1 to purify the interacting protein complex. At the end of this procedure, a mixture of multiple protein complexes is obtained whose molecular identities can be determined by mass spectrometry. Validation of the candidate proteins will benefit from the resource and ease of performing loss-of-function studies in flies. Similar approaches can be applied to other fly tissues. We believe that the combination of genetic manipulations and this proteomic approach in the fly model system holds tremendous potential for tackling fundamental problems in the field of neurobiology and beyond.
Introduction
Defining the molecular pathways or networks that mediate a particular biological process is one of the ultimate goals of biomedical research. Fly geneticists have depended heavily on forward genetics, especially modifier genetic screens (both enhancer and suppressor screens), to identify factors that work together, in parallel with, or upstream or downstream of a gene of interest. However, forward genetics screens often times fail to identify essential genes which, when mutated, cause lethality at early developmental stages, or genes with functional redundancy and compensation whose loss of function only cause subtle defects that are hard to score. One way to overcome this difficulty is to screen for direct protein-protein interactions. For more than a decade, a growing list of biochemical methods, including yeast two-hybrid, phage display, chemical cross-linking, Co-IP, Tandem Affinity Purification (TAP), etc. have been used to investigate protein-protein interactions. Each of these approaches has its own set of strengths and weaknesses in regards to sensitivity and specificity. Among them, the TAP method allows for detection of physical interaction under near-physiological conditions, preserves specificity and consistency2 and includes the ability to extend to high-throughput analyses3,4.
The TAP method was originally developed in yeast by Rigautand colleagues1. In this method, a protein of interest is expressed with a TAP tag. The TAP tag harbors two independent affinity-binding domains: a Protein A domain that binds to IgG and a calmodulin-binding domain. The two domains are separated by a TEV (Tobacco Etch Virus) cleavage site. Such a combination allows for two independent rounds of affinity purifications to sufficiently reduce nonspecific bindings and enrich specific bindings1. For this instance, the TAP method is a very powerful method to identify in vivo interactions of a given protein, although overexpressing the exogenous protein may make it more prone to associate with proteins that normally don't complex with its endogenous counterpart. Since its development, the TAP method has been applied in many other systems, including cell-culture-based systems5,6 and other in vivo model systems6-9. Here we describe the adaptation of the TAP method in Drosophila. We first generate pUAST-NTAP and pUAST-CTAP vectors to facilitate cloning and fusion of the TAP tag to either the N- or C-terminal of the gene of interest. The UAS-TAP-tagged transgene is then expressed in the nervous system under the control of a neuronal GAL4 driver10. Next, a large number of adult fly heads will be collected, which have high content of neural cells and are easy to separate from other body parts after freezing based on size differences. The adult heads are homogenized and cleared by sequential centrifugations, and the supernatant is subject to a TAP procedure described below.
Protocol
1. Generate UAS-TAP-tagged Transgenic Flies
- Generate pUAST-TAP-tagged DNA constructs.
- Decide which side (N- or C-terminus) of the bait protein the TAP tag should be fused to, based on the protein's structure/function. See discussion for more details.
- Subclone the cDNA coding region of the gene of interest into the multiple cloning sites (MCS) of the pUAST-NTAP or pUAST-CTAP vectors to generate N- or C-terminal-tagged UAS-TAP transgenes, respectively. See Figure 1 for detailed maps and usable restriction sites and reading frames.
- Generate UAS-TAP-tagged transgenic flies.
- Generate transgenic flies following standard protocols using P-element-mediated insertion11. A number of injection services are commercially available.
- Cross neuronal GAL4 driver (i.e. BG380-GAL4) to each individual transgenic line and determine the protein expression levels of each line by western blot (with Peroxidase Anti-Peroxidase antibody) and/or immunostaining (with anti-TAP antibody). In general, a transgenic line with a protein expression level that is close to the endogenous protein is recommended for TAP procedures. See discussion for more details.
- PerformGAL4/UAS-based rescue experiments to confirm the functionality of the TAP-tagged transgenes if loss-of-function mutants of the genes of interest are available. Choose a transgene that can substantially rescue the mutant phenotypes for the following TAP experiments.
2. Prepare Samples for TAP Procedure
- Generate a fly stock that carries both a neuronal GAL4 driver (e.g. BG380-Gal4) and the chosen TAP-tagged transgene in order to ease expansion of fly samples. Collect the F1 progenies of the GAL4 driver and the UAS-transgene cross in rare cases when the above combination causes survival and growth disadvantage.
- Collect small scale samples and optimize lysis condition for solubilizing the TAP-tagged protein.
- Make a series of lysis buffers using a combination of the nonionic detergents NP-40 (0.1-1%), NaDOC (0.1-1%) and Triton X-100 (0.05-0.5%). See Table 1 and discussion for more information.
- On top of a CO2 pad, use #5 forceps to dissect 20 adult heads from the TAP transgene expressing flies and collect them in a 1.5 ml tube to test each lysis buffer condition.
- Add 100 ul testing lysis buffer to the tube and homogenize the heads by stroking up and down with a plastic pestle, then add another 100 ul testing buffer.
- Spin the head lysate at 21,500 x g for 10 min (4 °C), and separate the supernatant and pellet after centrifugation. Add 25 ul 2x SDS loading buffer to the pellet and 10 ul 4x SDS loading buffer to 30 ul of supernatant respectively.
- Boil the two samples for 5 min and analyze them side-by-side using SDS-PAGE and subsequent Western Blot with the PAP antibody. Determine the solubility by the ratio of TAP-protein levels in supernatant vs the pellet.
- Prepare sample in large scale
- Expand and collect adult flies.
- Expand the neuronal-GAL4/UAS-TAP-transgene stock in bottles and flip the bottles every 3 days till accumulative 250 bottles are used for collection.
- Collect 1-3 days old adult flies into 50 ml conical tubes, put the tube in liquid nitrogen immediately to deep freeze the flies. Store the flies in a -80 °C freezer. Note that the volume of the flies must not exceed 2/3 of the 50 ml tube.
- Collect fly heads (perform this step on top of powdered dry ice).
- Take out the prechilled sieves and the mortar and pestle from the -80 °C freezer and put them on dry ice, ideally inside a large ice bucket. Stack two U.S.A. standard test sieves with a No. 25 on the top and No. 40 at the bottom.
- Take the frozen flies out and drop them in liquid nitrogen and keep the flies in there for about 10 min. Vortex or shake the tubes vigorously to break the heads, legs, and wings from the bodies.
- Pour the mixture to the top sieve, and then shake the sieves vigorously while holding both sieves together. After sieving, the bodies will stay on the top sieve, fly heads will be retained on the bottom sieve, and legs, wings, and other debris will fall to the dry ice. Separate the two sieves and carefully transfer the fly heads to the cold mortar.
- Homogenize the fly heads
- On top of dry ice, grind the heads with the mortar and the pestle to powdery particles, then transfer the powder to a 15 ml glass Dounce Tissue Grinder that was prechilled on ice.
- Measure the weights of the grinder before and after the head sample was poured into it, and then calculate how much the head sample weighs. A total of 6-15 grams of fly heads will be sufficient for each TAP experiment adjusting accordingly to the expression levels of the protein. Add 15 ml of ice-cold homogenization buffer (lysis buffer optimized in step 2.2) to the powder and then stroke with the large clearance pestle until it is easy for the pestle to go up and down. Keep the glass grinder on ice at all times.
- Prepare the supernatant for TAP
- Transfer the homogenate to a high-speed centrifuge tube and spin for 20 min at ~50,000 x g (4 °C). Transfer the supernatant to a new high-speed centrifuge tube and repeat the centrifugation one more time.
- Transfer the supernatant to an ultracentrifuge tube and perform a 40 min ~250,000 x g spin to further clear the supernatant. The supernatant is ready for the tandem affinity purification procedures after ultracentrifugation.
- Expand and collect adult flies.
3. TAP Purification
The following sections were derived from the Séraphin lab TAP protocol12 (http://web.as.uky.edu/Biology/faculty/rymond/BIO%20510/Bertran%20Seraphin%27s%20TAP%20page.pdf )
- Perform IgG bead affinity purification
- Prepare IgG sepharose bead while the samples are being centrifuged. Wash 400 µl IgG bead 3x in a 15 ml Falcon tube with 10 ml cold IgG washing buffer. For each wash, rock the tube gently for 2 min, and then spin down the beads at 1,000 x g for another 2 min. At the end of the third wash, remove the buffer and leave only the beads in the tube.
- Carefully transfer the cleared supernatant (~15 ml) into the 15 ml tube containing the IgG beads. Incubate the beads and brain lysate mix at 4 ºC on a nutator for 2 hr.
- Set up a clean and empty micro column with about 15 ml total volume in the cold room. Load the IgG bead mixture by steadily pouring the mix into the column; try not to trap any air bubbles inside the column. Allow the beads to settle in the column and the buffer to slowly drain by gravity flow.
- Wash the column thoroughly with 10 ml of cold IgG washing buffer after all of the brain lysate has flowed through the settled IgG column. Repeat the wash 2x. Note: never let the bead dry in the air.
- Perform TEV cleavage
- After the third wash, wash the column again with 10 ml TEV cleavage buffer. This step prepares the IgG bead that sequestrates the bait complex for TEV cleavage.
- Right before the last drop of TEV buffer is about to drip out, put a cap at the bottom of the column to block the flow, add 1.3 ml TEV buffer containing 130 units of TEV enzyme to the column, and then securely cap the top of the column. Make sure the column is sealed well at both ends.
- Rotate the column at 18 ºC for 2 hr to allow the TEV enzyme to cleave the peptide at the TEV site and release the protein complex while leaving behind the protein A domain peptide bound to the IgG sepharose beads.
- Perform Calmodulin bead affinity purification
- Prepare the Calmodulin beads while the IgG beads are incubated with the TEV enzyme. Wash 200 μl Calmodulin beads in a 15 ml Falcon tube 3x, each time with 10 ml of cold Calmodulin binding buffer. For each wash, gently rock the tube for 2 min on a nutator, and then spin down the beads at 1,000 x g for 2 min. At the end of the third wash, take out all the buffer and leave only the beads in the tube.
- At the end of the TEV incubation (step 3.2.3), return the IgG column back to the cold room and set it straight up. Let the beads settle for 10 min.
- Remove the top cap and then the bottom cap, and then collect the 1.3 ml TEV cleavage product in a 15 ml Falcon tube. Let the buffer drain completely. Add an additional 200 µl TEV buffer to the column to push out the dead volume of the column, collect the flow-out in the same tube.
- Add 4.5 ml of Calmodulin binding buffer and 4.5 µl 1 M CaCl2 to the 1.5 ml TEV cleavage product collected above. The CaCl2 serves to titrate the EDTA in the TEV buffer. Transfer the 6 ml mixture to the tube containing the Calmodulin beads and rotate the tube at 4 ºC on a nutator for 1 hr.
- Set up another clean and empty micro column with about 10 ml total volume in the cold room. Load the Calmodulin bead mixture to the column and allow it to drain by gravity.
- When all the solution has flowed through the settled Calmodulin column, wash the column two times, each with 10 ml of cold Calmodulin binding buffer. Note: avoid disturbing the Calmodulin beads and try to keep the surface of the beads as flat as possible during the wash.
- Elute the bait complex from Calmodulin column.
Right after washing, elute the Calmodulin column with five fractions of 200 µl cold Calmodulin elution buffer. For each fraction, gently add 200 µl of elution buffer to the column and collect the eluate with a marked 1.5 ml Eppendorf tube. Repeat this 4x. - Analyze the protein complex by SDS-PAGE
- Take a small aliquot from each of the five fractions (about 30 µl) and add SDS loading buffer. Boil the samples for 5 min and load the samples side-by-side with protein molecular markers in a gradient (4-15%) SDS-PAGE gel.
- After the samples have fully resolved in the gel, stop the electrophoresis and stain the gel with any G-250-based sensitive colloidal Coomassie staining procedures such as 'blue silver' staining13. Silver staining is optional but not preferable because it is not fully compatible with the subsequent mass spectrometry analysis. Store the rest of the eluate in a -80 °C freezer for further analysis such as mass spectrometry for uncovering the molecular identities of the purified protein complex. See discussion.
Results
Here we demonstrate our effort in identifying Highwire-interacting proteins in the fly brain. Highwire (Hiw) and its vertebrate and invertebrate homologues are huge ubiquitin ligases that regulate the development and repair of the nervous system14. They share a number of highly conserved functional domains. However, their molecular actions are not entirely clear. Work done in worm, fly and mouse led to the current working model that Hiw functions as an E3 ligase and as a scaffolding protein to facilitate formation of a multi-subunit ubiquitination complex, which regulates time- and cell type-specific neuronal functions through the combination of interacting with different cofactors and targeting different ubiquitin substrates. To identify the Hiw-associated ubiquitination complex, we first generated a N-terminal tagged UAS-TAP-Hiw transgene that is fully functional in rescuing the hiw mutant phenotype15. About 10 g of adult fly heads that express TAP only or TAP-Hiw transgenic proteins were collected and subjected to TAP procedures side by side as described above. Final eluates from both purifications were analyzed by SDS-PAGE and then silver staining. Mass spectrometry identified a list of proteins in the TAP-Hiw sample only, including Drosophila FSN (DFsn, an F-box protein) and Rae1 (Figure 2). Subsequent genetic and biochemical analyses revealed that Hiw and DFsn work together as SCF-like E3 ubiquitin ligase complex to regulate synaptic structure and function16, and Rae1 associates with Hiw in vivo and restrains synaptic overgrowth17. This function of Rae1 is at least partially achieved by its ability to promote Hiw protein stability via protecting Hiw from the autophagic degradation, which reveals a novel mechanism that selectively controls Hiw protein abundance during synaptic development17.
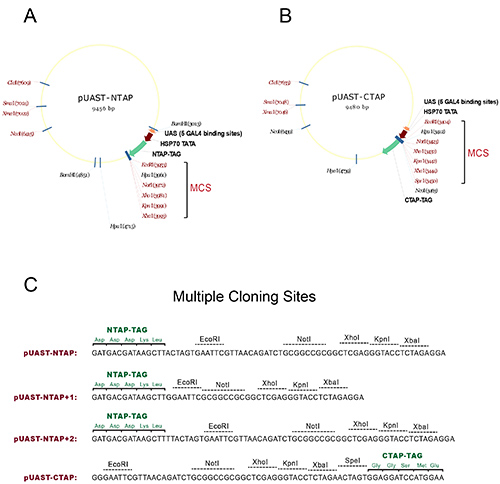
Figure 1. Vectors for generating TAP-tagged transgenic flies. (A) The map of the pUAST-NTAP construct. (B) The map of the pUAST-CTAP construct. The multiple cloning sites (MCS) in both constructs are marked with a bracket, where single-cut restriction sites are presented with red color. (C) Primary sequence showing the multiple cloning sites of the TAP vectors with the reading frame of the TAP tag. To facilitate subcloning, two more NTAP constructs are generated from the original pUAST-NTAP: pUAST-NTAP+1 and pUAST-NTAP+2. Together the three NTAP constructs covers all three possible reading frames to accommodate the use of the MCS. All vectors are constructed using the NTAP or CTAP fragments originally generated in Seraphin Lab1. Click here to view larger image.

Figure 2. Purification of Highwire-interacting proteins from fly brain by TAP purification. Adult heads from flies expressing TAP (BG380-GAL4; UAS-TAP) or TAP-Hiw (BG380-GAL4; UAS-TAP-Hiw) in the nervous system are collected, homogenized, and subjected to TAP purification, respectively. The final eluates were analyzed by one-dimensional SDS-PAGE gel followed by silver staining. The arrow head indicates the bait protein Hiw and the asterisk indicates a truncated TAP protein due to TEV cleavage. In the TAP-Hiw sample, a list of proteins are identified by mass spectrometry, including HSC-70, β-Tubulin, Rae1 and DFsn (indicated by arrows). This figure is modified from Figure 1a of Tian et al.17 Click here to view larger image.
| Buffer | Composition | Comments |
| Lysis buffer | 50 mM Tris-HCl pH 7.5 | NOTE: 1) Add DTT and the protease and proteasome inhibitors just before use. |
| 125 mM NaCl | 2) Showing here is a lysis buffer with 0.5% NP40. See Discussion for modifications on nonionic detergents. | |
| 5% Glycerol | ||
| 0.5% NP40 | ||
| 1.5 mM MgCl2 | ||
| 25 mM NaF | ||
| 0.2 mM DTT | ||
| (the following are protease and proteasome inhibitors) | ||
| 1 mM Na3VO4 | ||
| 0.05 mM MG-115 | ||
| 1 mM PMSF | ||
| Protease inhibitor mix (Sigma P8340) | ||
| Protease inhibitor cocktail (Roche 04693159001) | ||
| IgG washing buffer | 10 mM Tris-Cl pH 8.0 (0.5 ml of 2 M stock) | |
| 150 mM NaCl (3 ml of 5 M stock) | ||
| 0.1% NP40 (1.0 ml of 10% stock) | ||
| H20 to 100 ml final | ||
| TEV cleavage buffer | 10 mM Tris-Cl pH 8.0 (0.5 ml of 2 M stock) | NOTE: add DTT just before use. |
| 150 mM NaCl (3 ml of 5 M stock) | ||
| 0.1% NP40 (1.0 ml of 10% stock) | ||
| 0.5 mM EDTA (100 µl of 0.5 M stock) | ||
| 1 mM DTT (100 µl of 1 M stock) | ||
| H2O to 100 ml final | ||
| Calmodulin binding buffer | 10 mM β-mercaptoethanol (69.7 µl of stock) | |
| 10 mM Tris-Cl pH 8.0 (0.5 ml of 2 M stock) | ||
| 150 mM NaCl (3 ml of 5 M stock) | ||
| 1 mM Mg-acetate (100 µl of 1 M stock) | ||
| 1 mM imidazole (100 µl of 1 M stock) | ||
| 2 mM CaCl2 (200 µl of 1 M stock) | ||
| 0.1% NP40 (1 ml of 10% stock) | ||
| H2O to 100 ml final | ||
| Calmodulin elution buffer | 10 mM β-mercaptoethanol (69.7 µl of stock) | |
| 10 mM Tris-Cl pH 8.0 (0.5 ml of 2 M stock) | ||
| 150 mM NaCl (3 ml of 5 M stock) | ||
| 1 mM Mg-acetate (100 µl of 1 M stock) | ||
| 1 mM imidazole (100 µl of 1 M stock) | ||
| 10 mM EGTA (2 ml of 0.5 M stock) | ||
| 0.1% NP40 (1 ml of 10% stock) | ||
| H2O to 100 ml final |
Table 1. Composition of buffers.
Discussion
Tandem affinity purification (TAP) method offers a dual purification protocol that allows the isolation and enrichment of protein complexes through two independent affinity purification steps. The design of the TAP tag is not restricted to what is presented in this protocol, other protein binding domains and motifs are also applicable if buffer conditions are adjusted accordingly. A good example of other TAP tags is the GS-TAP tag, a combination of a G protein and a streptavidin-binding motif, designed by Giulio Superti-Furga's group aimed at purifying protein complex in cultured mammalian cell lines18. The GS-TAP was later adapted to study protein-protein interaction in Drosophila S2 cells and embryos19. A recent JoVE publication by Bailey et al. demonstrated how the GS-TAP procedure was performed with cell culture lysate20. Here we presented the use of TAP-tag in Drosophila neural tissues (adult heads) for large-scale proteomic screening. This protocol can be potentially adapted to other tissues that allow large scale sample collection, such as embryos. The adult bodies collected on the top sieve (step 2.3.2.3) may also be used for TAP, which can be a very challenging task. The lysis buffer must be modified and conditioned to inhibit the massive proteases present in the digestive tract at the most and the first purification procedure be significantly shortened in order to reduce the exposure time of the proteins to the proteases. Although different TAP tags require different handlings and buffer conditions for affinity purifications, the general principles for TAP are the same. Below we discuss matters that will affect the quality of TAP results.
The success of the TAP approach depends on generating the right transgene. First, the TAP tag itself must not change the overall conformation of the bait protein, which is critical to retain its ability to interact with its physiological binding partners. Thus, it is crucial to decide where to fuse the TAP tag to the gene of interest. The decision can be made based on previous studies of the protein, especially information about its structure or functional epitode-tagged transgenes. In case such information is unavailable, tagging the bait protein at N- or C-terminal in parallel is recommended. Then functional rescue experiments can be performed to determine which transgene should be used. Second, the expression levels of the transgenic protein should be close to that of the endogenous protein. This is particularly important as the Gal4/UAS system normally drive the expression of the transgene at different timing and at a much higher level compared to the endogenous proteins, which may either increase false positives or cause unexpected consequences that alter the profile of the protein interaction networks in the cells. For example, overexpressing scaffolding proteins may cause dominant negative effects and overexpressing proteins that possess enzymatic activities, such as kinases, often times cause gain-of-function effects, both of which may in turn affect the ability of their endogenous binding partners to interact with them. Thus, the GAL4/UAS system should be avoided when the timing and level of the expression of the gene of interest is critical for the preservation of the endogenous interactions that only present under normal conditions. Instead, the expression of the TAP-tagged transgene should be controlled under its own promoter. This can be achieved either by replacing the UAS sequence with the endogenous promoter sequence, or by fusing the TAP sequence in frame with the exonal sequence in a genomic DNA fragment that contains the entire loci of the gene of interest21. In some cases, the TAP experiment can be performed in a loss of function mutant background of the gene of interest to reduce competition from the endogenous protein for incorporating into multiprotein complexes. For this instance, a protein null background, if available, provides the ideal condition to completely eliminate the endogenous protein. Using the genetic mutant background will always increase the chance to produce results with higher quality.
Solubility of the bait protein is another crucial factor for success. Nonionic detergents are normally used in the lysis buffer to dissolve the plasma membrane and retain the protein complex, soluble and intact, in a status that is close to the physiological environment. However depending on whether or not the proteins of interest are membrane-integrated or -associated proteins, the composition and concentration of nonionic detergents could be crucial to solubilizing the bait protein. 0.1-0.3% NP40 is often adequate to solubilize cytosolic proteins. For membrane proteins, a combination of NP40 (0.1-1%), NaDOC (0.1-1%), and Triton-X 100 (0.05-0.5%) is sometimes required to solubilize and enrich enough proteins for the TAP experiment. However, since a stringent lysis buffer tend to disrupt weaker protein-protein interactions, a balance needs to be reached in which enough soluble bait proteins are present in the sample while at the same time the majority of protein-protein interactions are preserved. A general principle is that always try to use the fewest types of detergents with the lowest concentration.
To facilitate troubleshooting, it is highly recommended to save a small aliquot of sample from each step of the two purifications. Subsequent SDS-PAGE and protein staining/ Western analysis on the aliquots allow the levels of the bait protein and the sequential enrichment of certain protein species to be monitored. For example, if a sudden reduction of the bait protein was detected in aliquot of the flow-out after TEV cleavage (step 3.3.3), it is likely that the bait protein were lost during the IgG bead washing (step 3.1.4). A possible cause might be the inadequate detergent composition of the IgG washing buffer. There is a sharp drop on the composition and concentration of the detergents in the IgG washing buffer (0.1% NP-40 only) compared to the lysis buffer. The bait protein may be sensitive to these changes and disassociate from the IgG moiety on the beads. Adjusting the IgG washing towards lysis buffer may help solve the problem. Alternatively, although less likely, the TEV cleavage (step 3.2) may not have worked sufficiently to release the bait-containing complex from the IgG beads. The steps worth a second inspection are the equilibration of IgG beads with the TEV buffer (step 3.2.1) and the enzyme activity, mishandling may deaden the enzyme.
Keep in mind that even with the two-step purification, there will still be false positives and proteins that are pulled down nonspecifically present in the final TAP-purified complex. One way to identify those nonspecific interactions, at least partially, is to include a control TAP using the TAP-only transgene in parallel with TAP-tagged-transgene in the TAP purification experiments. Proteins identified in the TAP-only procedure should be removed from the list identified in TAP-transgene purification.
A successful TAP will purify a mixture of proteins that are potentially associated with the protein of interest in vivo. The next step is the molecular identification of these proteins, and Mass Spectrometry is commonly used for this purpose. Depending on the results from the SDS-PAGE and the experiment's needs, the molecular identities in the purified protein complex can be determined in the following two ways: 1) each individual protein band can be cut out from the gel and subjected to a low-complexity LC-MS/MS; or 2) the elute fraction that contains the peak protein content (normally the second elution) can be directly subjected to moderate-complexity LC-MS/MS. Such a shot-gun proteomic approach will potentially identify all the proteins in the complex, depending on the dynamic range of the MS equipment22.
In summary, we present a method to identify protein-protein interactions in neural tissues of a genetic model organism - the fruit fly. There are a number of advantages of applying the TAP method to the fly: 1) fruit flies have a short life cycle, so it is relatively quick and easy to generate transgenic flies and to obtain tissues in large amount; both are crucial for the TAP approach; 2) the fly genome is fully annotated and contains 70% of human disease-causing genes; and 3) most importantly, it is easy to functionally validate and characterize the candidate interacting proteins in flies. There are comprehensive resources available in the fly research community for the characterization of a given gene, such as transgenic-based RNAi collection useful to study tissue-specific loss-of-function of a majority of the genes, mutant and deficiency alleles for most of the gene loci, as well as cDNA clones and transgenes useful for gain-of-function studies. We believe that the fly TAP method will be a valuable addition to the tool box used in fly labs. In future perspective, this method can be combined with fly behavioral paradigms and human-disease models to screen the change in protein-protein interaction network in response to specific conditions and stimuli.
Disclosures
The authors have nothing to disclose.
Acknowledgements
We thank EUROSARF for sending us yeast TAP expression plasmids. We are also grateful for editorial help from Ryan Labadens. This work was supported by a NIH/NINDS grant (R01NS070962) to C.W.
Materials
| Name | Company | Catalog Number | Comments |
| U.S.A. standard test sieve No. 25 | Fisher Scientific | 04-881-18 | |
| U.S.A. standard test sieve No. 40 | Fisher Scientific | 04-881-21 | |
| Kontes Dounce Tissue Grinders 15 ml | Kimble Chase | 885300-0015 | |
| IgG sepharose beads | Pharmacia | 17-0969-01 | |
| Econo-column 0.7 cm x 20 cm | Bio-Rad | 737-4721 | |
| Econo-column 0.5 cm x 15 cm | Bio-Rad | 737-4716 | |
| Calmodulin beads | Stratagene | 214303 | |
| Coors Mortar and Pestle | CoorsTek | 60311 | |
| AcTEV Protease | Invitrogen | 12575-015 | |
| Protease Inhibitor Cocktail | Roche | 11836153001 | |
| Protease Inhibitor Mix | Sigma | P8340 |
References
- Rigaut, G., et al. A generic protein purification method for protein complex characterization and proteome exploration. Nat. Biotechnol. 17, 1030-1032 (1999).
- Collins, S. R., et al. Toward a comprehensive atlas of the physical interactome of Saccharomyces cerevisiae. Mol. Cell. Proteomics. 6, 439-450 (1074).
- Gavin, A. C., et al. Proteome survey reveals modularity of the yeast cell machinery. Nature. 440, 631-636 (2006).
- Krogan, N. J., et al. Global landscape of protein complexes in the yeast Saccharomyces cerevisiae. Nature. 440, 637-643 (2006).
- Forler, D., et al. An efficient protein complex purification method for functional proteomics in higher eukaryotes. Nat. Biotechnol. 21, 89-92 (2003).
- Veraksa, A., Bauer, A., Artavanis-Tsakonas, S. Analyzing protein complexes in Drosophila with tandem affinity purification-mass spectrometry. Dev. Dyn. 232, 827-834 (2005).
- Li, Y. The tandem affinity purification technology: an overview. Biotechnol. Lett. 33, 1487-1499 (2011).
- Volkel, P., Le Faou, P., Angrand, P. O. Interaction proteomics: characterization of protein complexes using tandem affinity purification-mass spectrometry. Biochem. Soc. Trans. 38, 883-887 (2010).
- Xu, X., et al. The tandem affinity purification method: an efficient system for protein complex purification and protein interaction identification. Protein Expr. Purif. 72, 149-156 (2010).
- Brand, A. H., Perrimon, N. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development. 118, 401-415 (1993).
- Bachmann, A., Knust, E. The use of P-element transposons to generate transgenic flies. Methods Mol. Biol. 420, 61-77 (2008).
- Puig, O., et al. The tandem affinity purification (TAP) method: a general procedure of protein complex purification. Methods. 24, 218-229 (2001).
- Candiano, G., et al. Blue silver: a very sensitive colloidal Coomassie G-250 staining for proteome analysis. Electrophoresis. 25, 1327-1333 (2004).
- Tian, X., Wu, C. The role of ubiquitin-mediated pathways in regulating synaptic development, axonal degeneration and regeneration: insights from fly and worm. J. Physiol. , (2013).
- Wu, C., Wairkar, Y. P., Collins, C. A., DiAntonio, A. Highwire function at the Drosophila neuromuscular junction: spatial, structural, and temporal requirements. J. Neurosci. 25, 9557-9566 (2005).
- Wu, C., Daniels, R. W., DiAntonio, A. DFsn collaborates with Highwire to down-regulate the Wallenda/DLK kinase and restrain synaptic terminal growth. Neural Dev. 2, 16 (2007).
- Tian, X., Li, J., Valakh, V., DiAntonio, A., Wu, C. Drosophila Rae1 controls the abundance of the ubiquitin ligase Highwire in post-mitotic neurons. Nat. Neurosci. 14, 1267-1275 (2011).
- Burckstummer, T., et al. An efficient tandem affinity purification procedure for interaction proteomics in mammalian cells. Nat. Methods. 3, 1013-1019 (2006).
- Kyriakakis, P., Tipping, M., Abed, L., Veraksa, A. Tandem affinity purification in Drosophila: the advantages of the GS-TAP system. Fly. 2, 229-235 (2008).
- Bailey, D., Urena, L., Thorne, L., Goodfellow, I. Identification of protein interacting partners using tandem affinity purification. J. Vis. Exp. (60), e3643 (2012).
- Wu, Y., et al. A Drosophila model for Angelman syndrome. Proc. Natl. Acad. Sci. U.S.A. 105, 12399-12404 (2008).
- Liao, L., McClatchy, D. B., Yates, J. R. Shotgun proteomics in neuroscience. Neuron. 63, 12-26 (2009).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved