Method Article
Spatio-Temporal Manipulation of Small GTPase Activity at Subcellular Level and on Timescale of Seconds in Living Cells
In This Article
Summary
A method for spatio-temporal control of small GTPase activity by light is described. This method is based on rapamycin-induced FKBP-FRB heterodimerization and photo-caging systems. Optimization of light-irradiation enables the spatio-temporally controlled activation of small GTPases at the subcellular level.
Abstract
Dynamic regulation of the Rho family of small guanosine triphosphatases (GTPases) with great spatiotemporal precision is essential for various cellular functions and events1, 2. Their spatiotemporally dynamic nature has been revealed by visualization of their activity and localization in real time3. In order to gain deeper understanding of their roles in diverse cellular functions at the molecular level, the next step should be perturbation of protein activities at a precise subcellular location and timing.
To achieve this goal, we have developed a method for light-induced, spatio-temporally controlled activation of small GTPases by combining two techniques: (1) rapamycin-induced FKBP-FRB heterodimerization and (2) a photo-caging method of rapamycin. With the use of rapamycin-mediated FKBP-FRB heterodimerization, we have developed a method for rapidly inducible activation or inactivation of small GTPases including Rac4, Cdc424, RhoA4 and Ras5, in which rapamycin induces translocation of FKBP-fused GTPases, or their activators, to the plasma membrane where FRB is anchored. For coupling with this heterodimerization system, we have also developed a photo-caging system of rapamycin analogs. A photo-caged compound is a small molecule whose activity is suppressed with a photocleavable protecting group known as a caging group. To suppress heterodimerization activity completely, we designed a caged rapamycin that is tethered to a macromolecule such that the resulting large complex cannot cross the plasma membrane, leading to virtually no background activity as a chemical dimerizer inside cells6. Figure 1 illustrates a scheme of our system. With the combination of these two systems, we locally recruited a Rac activator to the plasma membrane on a timescale of seconds and achieved light-induced Rac activation at the subcellular level6.
Protocol
1. Transfection of Plasmid DNA
- Add plasmid DNAs, 0.5 μg membrane-tethered FRB (hereafter referred to as LDR) and 0.5 μg FKBP-Tiam1 (T-cell lymphoma invasion and metastasis-inducing protein 1: Rac activator) tagged with fluorescent protein to 37.5 μl dH2O. Add 1 μl FuGENE HD.
- Vortex and incubate at room temperature for 20 minutes. Meanwhile, proceed to steps 1.3-1.8.
- Wash each well of an 8-well chamber with 50 μl poly-D-Lysine.
- Wash the center of each well with dH2O.
- Trypsinize 80-85% confluent NIH-3T3 cells and dilute to 10 ml culture medium (DMEM with 10% FBS).
- Remove 375 μl of suspension and centrifuge at 1750 rpm for 3 minutes.
- Aspirate the media, being careful not to disturb the pellet.
- Add 750 μl of DMEM w/ 10% FBS and resuspend.
- Add cell suspension to dH2O/DNA/FuGENE HD solution and mix gently.
- Add 250 μl of cell/DNA suspension to each well (will fill up to 3 wells).
- Incubate the transfected cells at 37 °C and 5% CO2 for 15-18 hr.
2. Preparation of cRb-A Solution
- Synthesize caged rapamycin-biotin adduct (cRb). See "Synthetic scheme of cRb" for details. Briefly, caging and biotin moieties are prepared separately. The caging moiety is then conjugated to rapamycin (LC lab), the product subsequently coupled to the biotin moiety by a means of click reaction7 to afford cRb.
- Prepare a 1 μM stock solution of cRb in DMSO.
- Dissolve 1mg avidin in 250 μl of PBS in a 0.5 ml Eppendorf tube.
- Add 1 μl of cRb stock solution to dissolved avidin. Mix by inverting several times.
- Incubate at room temperature for 15 min to yield cRb-avidin conjugate (cRb-A) solution.
- Exclude unbound small molecules and purify cRb-A using a size-exclusion column.
3. Microscopy and Light Illumination at Subcellular Level
- Serum starve transfected cells in DMEM without FBS for 12 hours.
- Gently aspirate the media off the cells and add 250 μl of the prepared 400 nM cRb-A solution (from step 2.6).
- To visualize protein dynamics in living cells at subcellular resolution before and after light treatment, spinning disk microscopy was used (Figure 2a). UV illumination was achieved by coupling optics for UV LED light (365 nm) with epi-fluorescence light (Figure 2b).
- MetaMorph software was used to capture fluorescence images of transfected cells every 15 seconds.
- Define the coordinates and size of an illumination spot by using a glass plate coated with fluorescent dyes excited by UV light. The size can be varied by applying objective lens with different magnifications and by selecting optical fibers with different core size.
- After determining background levels of membrane ruffling, irradiate UV light at the periphery of cells. The following two steps are to contrast preceding localized effects.
- Globally irradiate cells with 365 nm light provided by a mercury lamp.
- Dissolve uncaged, thus original rapamycin in DMSO and then in PBS (400 nM final), and add the solution to the imaging chamber to induce global effects.
4. Representative Results
An example of representative results is shown in Figure 3. UV light irradiation at a small region near a cellular edge rapidly induced formation of localized ruffles only in and near the irradiated area. To confirm that this was indeed local activity, we then globally irradiated cells with UV, or added rapamycin to the bulk media, both of which induced global ruffle formation throughout the membrane. For a quantitative analysis, we divided target cells into four quadrants, and counted the frequency of ruffle formation in each quadrant upon local as well as following global stimulation under various conditions including controls (Figure 4). It is only this combination of UV irradiation, cRb-A, and appropriate FKBP-FRB constructs that induces local ruffling.

Figure 1. Schematic of spatially confined protein dimerization using caged rapamycin-avidin conjugate (cRb-A) and UV light. UV irradiation cleaves the linker between rapamycin and biotin, resulting in the release of chemical dimerizer (HE-Rapa, C40-O-(2-hydroxyethyl) rapamycin) and its by-product. The released dimerizer then diffuses into cells and induces dimerization between FKBP-POI (protein of interest) and plasma membrane anchored FRB only in the proximity of the irradiated region (blue circle). Without the UV irradiation, FKBP and FRB do not associate (red cross), hence producing no effect. (Copyright@ACS2011) 6
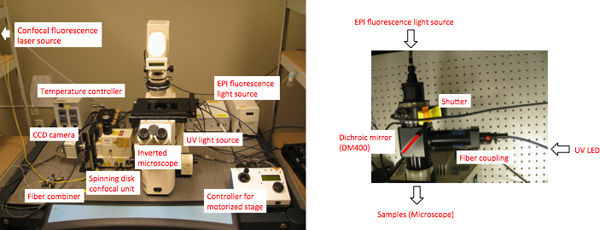
Figure 2. Picture of the customized confocal fluorescence microscope. (a) Frontal view of the confocal microscope (inverted Axiovert 200, Zeiss). (b) Close-up, top view of a coupling unit for EPI fluorescence and UV illumination.
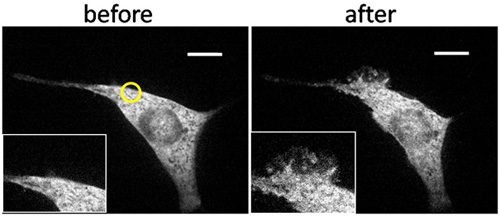
Figure 3. Spatially confined ruffle formation in a transfected NIH3T3 cell induced by localized UV irradiation. Confocal fluorescence images of a NIH3T3 fibroblast cell that is transfected with YFP-tagged FKBP-Tiam1 (Rac activator) and LDR (membrane-anchored FRB) underwent irradiation in a confined area near its edge with LED light at 365 nm for 15 sec starting at 210 sec in the presence of cRb-A conjugate in the bulk media. This treatment was followed by global illumination with mercury lamp for 1 sec at 1063 sec and global addition of rapamycin final 400 nM at 1560 sec. The cell showed almost no ruffling activity at the beginning (a) but showed spatially confined ruffling after localized illumination (yellow circle) (b). The cell showed global ruffle formation after global illumination and addition of rapamycin(c-d), indicating that the local ruffle formation was caused by spatially confined activation of Rac. Scale bar: 10 μm. (Copyright@ACS2011) 6
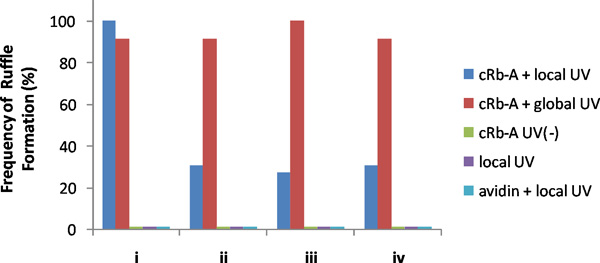
Figure 4. Frequency of ruffle formation in quadrants of transfected NIH3T3 cells. Local UV irradiation with cRb-A and following global UV irradiation were performed on 13 cells. cRb-A addition (no UV irradiation), local UV irradiation with or without avidin were implemented on 13, 5 and 6 cells, respectively. None of the observed cells showed ruffle formation with cRb-A without UV irradiation, or local UV irradiation with or without avidin in bulk media. We collected data from cells with low background ruffle formation but which had the capacity of rapamycin-induced ruffle formation, which we checked after each experiment by confirming global ruffle formation as a result of addition of rapamycin (final 400 nM). These results showed that cRb-A and local UV irradiation are necessary for local ruffle formation in the cells. (Copyright@ACS2011) 6
Abbreviations:
FKBP: FK506-binding protein
FRB: FKBP-rapamycin-binding protein
Tiam1: T-cell lymphoma invasion and metastasis-inducing protein 1 (Rac GEF)
LDR: Lyn-DSAG linker-FRB (Lyn: N-terminal 11 amino acids of Lyn kinase)
HE-Rapa: C40-O-(2-hydroxyethyl) rapamycin
cRb: caged rapamycin with biotin moiety
cRb-A: cRb conjugated to avidin
Discussion
We described a technique that employs a novel caged compound together with the FKBP-FRB heterodimerization system in order to manipulate Rho GTPase activity at a precise subcellular location on a time scale of seconds.
There are three limitations of the present approach. Firstly, because the method is based on preventing or allowing diffusion of dimerizer across the plasma membrane, the target cellular location needs to be plasma membrane or its vicinity. Further optimization of the chemical structure of cRb may allow the compound to enter cells without inducing dimerization until light irradiation. With such a dimerizer, we could in theory manipulate activity of signaling molecules anywhere inside cells. Secondly, molecular diffusion of chemical dimerizers, FKBP-FRB dimerization complex, or activated endogenous signaling molecules can greatly affect precise confinement of the intended effects. This effect is particularly pronounced at later time points and is dependent on parameters associated with UV irradiation (such as beam width, energy, and beam mode (continuous vs. pulsed)) and the diffusion coefficients of the compounds involved. Further empirical optimization of these parameters enables more stringent confinement of signal activities. Thirdly, an UV light exhibits non-trivial phototoxicity to cells. One easy way to ameliorate this problem is to use a longer wavelength such as 405 nm to uncage nitrobenzyl caging groups. Two-photon illumination for uncaging allows illumination at an even longer wavelength as well as at extremely localized region of cells. Recently, other researchers have reported an elegant approach to activate Rac at a subcellular location using light-sensitive plant proteins8-11. So far, its application is limited to only a few proteins. Compared to their strategies, our current technique is more readily adaptable to expand the number of target proteins without extensive and time-consuming re-engineering. We and others have already developed a variety of dimerization probes that can manipulate the activity of diverse signaling molecules at the plasma membrane5, 6, 12-14. Spatiotemporally dynamic cellular events are now directly testable.
Disclosures
No conflicts of interest declared.
Acknowledgements
This study was supported by NIH research funding (DK090868 and GM092930 to T.I.). There is a pending patent on a caged rapamycin analog.
References
- Etienne-Manneville, S., Hall, A. Rho GTPases in cell biology. Nature. 420, 629-635 (2002).
- Takai, Y., Sasaki, T., Matozaki, T. Small GTP-binding proteins. Physiol. Rev. 81, 153-208 (2001).
- Kiyokawa, E., Aoki, K., Nakamura, T., Matsuda, M. Spatiotemporal regulation of small GTPases as revealed by probes based on the principle of Forster Resonance Energy Transfer (FRET): Implications for signaling and pharmacology. Annu. Rev. Pharmacol. Toxicol. 51, 337-358 (2011).
- Inoue, T., Heo, W. D., Grimley, J. S., Wandless, T. J., Meyer, T. An inducible translocation strategy to rapidly activate and inhibit small GTPase signaling pathways. Nat. Methods. 2, 415-418 (2005).
- Komatsu, T. Organelle-specific, rapid induction of molecular activities and membrane tethering. Nat. Methods. 7, 206-208 (2010).
- Umeda, N., Ueno, T., Pohlmeyer, C., Nagano, T., Inoue, T. A photocleavable rapamycin conjugate for spatiotemporal control of small GTPase activity. J. Am .Chem. Soc. 133, 12-14 (2011).
- Kolb, H. C., Finn, M. G., Sharpless, K. B. Click Chemistry: Diverse Chemical Function from a Few Good Reactions. Angew. Chem. Int. Ed. Engl. 40, 2004-2021 (2001).
- Kennedy, M. J. Rapid blue-light-mediated induction of protein interactions in living cells. Nat. Methods. 7, 973-975 (2010).
- Levskaya, A., Weiner, O. D., Lim, W. A., Voigt, C. A. Spatiotemporal control of cell signalling using a light-switchable protein interaction. Nature. 461, 997-1001 (2009).
- Wu, Y. I. A genetically encoded photoactivatable Rac controls the motility of living cells. Nature. 461, 104-108 (2009).
- Yazawa, M., Sadaghiani, A. M., Hsueh, B., Dolmetsch, R. E. Induction of protein-protein interactions in live cells using light. Nat. Biotechnol. 27, 941-945 (2009).
- Fegan, A., White, B., Carlson, J. C., Wagner, C. R. Chemically controlled protein assembly: techniques and applications. Chem. Rev. 110, 3315-3336 (2010).
- Suh, B. C., Inoue, T., Meyer, T., Hille, B. Rapid chemically induced changes of PtdIns(4,5)P2 gate KCNQ ion channels. Science. 314, 1454-1457 (2006).
- Varnai, P., Thyagarajan, B., Rohacs, T., Balla, T. Rapidly inducible changes in phosphatidylinositol 4,5-bisphosphate levels influence multiple regulatory functions of the lipid in intact living cells. J. Cell. Biol. 175, 377-382 (2006).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionExplore More Articles
This article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved