Method Article
エレクトロポレーションによりマウス胎児蝸牛外植の文化と遺伝子導入
要約
We present a method that describes isolation and culture of cochlear explants from embryonic mouse inner ear. We also demonstrate a method for gene transfer into cochlear explants via square-wave electroporation. The in vitro explant culture coupled with gene transfer technique enables researchers to study the effects of altering gene expression during development.
要約
Auditory hair cells located within the mouse organ of Corti detect and transmit sound information to the central nervous system. The mechanosensory hair cells are aligned in one row of inner hair cells and three rows of outer hair cells that extend along the basal to apical axis of the cochlea. The explant culture technique described here provides an efficient method to isolate and maintain cochlear explants from the embryonic mouse inner ear. Also, the morphology and molecular characteristics of sensory hair cells and nonsensory supporting cells within the cochlear explant cultures resemble those observed in vivo and can be studied within its intrinsic cellular environment. The cochlear explants can serve as important experimental tools for the identification and characterization of molecular and genetic pathways that are involved in cellular specification and patterning. Although transgenic mouse models provide an effective approach for gene expression studies, a considerable number of mouse mutants die during embryonic development thereby hindering the analysis and interpretation of developmental phenotypes. The organ of Corti from mutant mice that die before birth can be cultured so that their in vitro development and responses to different factors can be analyzed. Additionally, we describe a technique for electroporating embryonic cochlear explants ex vivo which can be used to downregulate or overexpress specific gene(s) and analyze their potential endogenous function and test whether specific gene product is necessary or sufficient in a given context to influence mammalian cochlear development1-8.
概要
The mammalian organ of Corti is comprised of a mosaic of specialized cell types, including two types of mechanosensory hair cells as well as at least four types of nonsensory supporting cells making it an ideal model system to study normal cellular processes like proliferation, fate specification, differentiation and patterning. In addition, the normal development of these different cell types is essential for normal hearing function. Hence, it is crucial to understand the factors, both molecular and cellular, that regulate their development. However, the small size of the mouse cochlea as well as its inaccessibility poses a particular challenge for gene expression studies. Moreover, most of the cell fate specification and patterning events occur during embryonic time periods and are mostly completed before birth. Therefore, identification and characterization of signaling events during embryonic time periods is essential to gain insight into the molecular basis of cochlear morphogenesis.
Here, we demonstrate a method to culture intact cochlea in vitro from embryonic mouse inner ears. The rationale behind the use of this technique is that cultured cochleae maintain their molecular and morphological characteristics thereby providing a valuable model for investigating potential candidate genes and exploring the mechanisms involved in cochlear morphogenesis. Although transgenic mice can be used for gene expression studies, an in vitro system is often needed for monitoring specific gene functions. Moreover, cochlear cultures can be established from transgenic mouse embryos so that their in vitro development and response to various soluble factors and antagonists can be studied. Although embryos at day 13 (E13) are used in this protocol, cultures from E12 or E14 to early postnatal inner ears can give similar results.
We also present a gene transfer technique in cultured embryonic cochlear explants using square wave electroporation. Following the isolation of the cochlear explants, electroporation can be used to express DNA plasmids of gene(s) of interest in individual cells within the cochlear duct. This technique serves as a complementary approach to studies utilizing transgenic mice to gain insight into the molecular pathways underlying cellular phenotype. Using this method of gene transfer, a variety of epithelial cell types within the embryonic cochlea are transfected, thereby enabling loss-and gain-of-function analyses at the single-cell level. In addition, electrophysiological studies can also be performed in cochlear explant cultures8. This method of in vitro electroporation is relatively simple and straightforward, combined with minimal damage to the tissue, has resulted in a rapid expansion of this technique.
プロトコル
注:生きた動物を使用するすべてのプロトコルを見直し、施設内動物管理使用委員会(IACUC)によって承認され、実験動物の管理と使用のために公式に承認された方法に従わなければなりませんしなければなりません。すべての解剖清浄な層流ベンチで滅菌技術を使用して行われるべきである。手袋とマスクは、必要に応じて、この手順の間に着用してください。
マウス胎児インナーイヤー1.解剖
- コルチ植片培養のオルガンのためのセットアップ:
- 約30分間、UV光をオンにすることによって、層流組織培養フードを滅菌し、使用前に70%エタノールで表面を消毒する。また、オートクレーブを介して、または、使用前に少なくとも20分間、70%エタノールに浸漬することによって、胚の解剖のための細かい解剖ツールとシルガードコーティングされた料理など、すべての解剖器具を滅菌する。 70%エタノールをオフに注ぎ、料理や楽器がきれいな層流CLEAで空気乾燥することができますn個のベンチ使用前。
- 準備無菌ハンクス平衡塩溶液(HBSS)/(4-(2-ヒドロキシエチル)-1-ピペラジンエタンスルホン酸)、HEPES 10×HBSSおよび0.5%の10%を添加し、約7.2にpHを調整し、フィルター滅菌することにより(HEPES)混合4℃での最終溶液とストア。より良い作業中は組織を維持するために冷やしたHBSS / HEPES溶液中のすべての解剖を行ってください。
- 基底膜マトリックスで被覆ガラスボトムディッシュの滅菌15mlのポリプロピレンチューブ中の基底膜マトリックスの300μlのアリコートを冷(4℃)滅菌ダルベッコ改変イーグル培地(DMEM)を5ml加えることによって。内容をボルテックスで混合し、それは皿の中央にも存在する全体の文化を覆うように、各培養皿の中心に基底膜マトリックス-DMEM混合物の150μlを添加する。使用前に少なくとも45分間37℃のインキュベーター中で料理や店舗をカバーしています。
- 目にHBSSを冷やし4〜5ミリリットルを注ぐREE無菌ポリスチレンペトリ皿やフード内に閉じたまま。
- DMEMの9ミリリットル、N2サプリメント100μlの、10グラム/ mlのシプロとFBSの1ミリリットルを追加することにより、滅菌15ミリリットルポリプロピレンチューブに培養液を準備します。
- 胚の単離:
注:胚のマウスから蝸牛外植体を培養するこの手順では、収穫内耳日胚(E)から13頭骨E1 9として検討されて受精後の日と。プロトコルは、E12からE18に始まる胚性マウス内耳と一緒に使用することができる。- 頸椎脱臼または適切な承認されたプロトコルによってCO 2と犠牲動物と時限妊娠マウスを安楽死させる。組織培養室で、無菌性を維持するために、離れた層流の組織培養フードから安楽死を実施する。
- 上に向けて腹部をペーパータオルに安楽死させたマウスを置き、70%エタノールで腹部を消毒する。
- 皮膚をつかんによって腹腔を開き別の手を使用してハサミで正中線に沿って湾曲した一方の手で鉗子とカット表皮と筋肉と。両側の2つの垂直なカットを作成し、慎重に子宮を引き出す鉗子で二国間の子宮角を持ち上げて、ハサミを使って結合組織の下からそれを切り離す。
- チルド解剖溶液(HBSS / HEPESの混合物)と層流クリーンベンチへの転送を含むペトリ皿に胚チェーンを配置します。
- 胎盤から慎重に胚を取り出し、解剖液を含む滅菌ペトリ皿の1にそれらを配置します。解が血まみれになった場合、新たなペトリ皿に移動します。
注:この時点で、胚はタイラーステージングガイドを使用して上演することができます。 - 清潔な鉗子やハサミで首領域でつまんで胚を斬首し、チルド解剖溶液を含む新鮮なペトリ皿に頭を置く。
- 内耳の解剖:
- 置きO冷たい解剖を含む滅菌シルガード皿の中のNE胚ヘッド
- ソリューション。 〜1.6倍の倍率で解剖顕微鏡の下で働く、Minutienの周りにピンまたは眼の領域( 図1A)に近くに配置することによって、胚の頭部を固定する。
- 滅菌ピンセット二組を使用すると、慎重に皮膚を除去し、正中線( 図1B)に沿って背側の頭蓋骨を開く。頭蓋腔から脳を取り出し、側頭骨内にある内側の耳は、この段階で識別することができます( 図1C、D)。通常、内側の耳の周りに存在する血管のライニングは、この段階( 図1D)で内耳を識別するための目印として使用することができる。
- 組織の下に鉗子を配置し、冷解剖溶液を含む新しい皿に頭蓋骨転送のベースから内耳を単離することによって側頭骨から内耳を分析( 図1E、F)。
オルガンコルチ植片の培養の2世代
注:開発のこの段階では、組織は軟骨であり、容易に鉗子を用いて解剖することができる。
- オリエント内耳腹側は、( 図2A)を上に向け、静かに内耳( 図2B)の前庭部分を通ってMinutienピンの鋭い端を挿入することにより、内耳の安定化されるように。
- 超微細鉗子を使用すると、楕円形の窓の近くに鉗子の一端を使用して切開を行うことによって、上にある軟骨を開き、カットし、慎重に蝸牛( 図2C)から軟骨を取り除く。
注:これは、その上の軟骨が、時にはそれが下にこすることによって、基礎となる蝸牛管から軟骨を解放するために、閉じた鉗子を使用すると便利である場合には、蝸牛管と融合されているように鉗子が軟骨にあまりにも深く挿入されていないことを確認することが重要ですS軟骨のurface。開発のこの段階では、蝸牛のらせんの長さは、ターンの唯一の四分の三である。 - 次に、ベースまたは非常に頂点のいずれかで、蝸牛管の好ましい領域で鉗子を配置することによって感覚上皮を公開、そして優しく蝸牛( 図2D)の屋根を引き出す。
注:それは分析を妨害感覚上皮をマスクすることができるように蝸牛の屋根が完全に除去しなければならない。
注:それは感覚上皮を引き裂くことは容易であるように、この手順を静かに行う必要があります。 - 蝸牛外植片のベースが一様に平らになるように、最後のステップとして、慎重にさらさ感覚上皮から下にある結合組織を除去。
- ピンセットを用いて、内耳の前庭部分から解剖蝸牛を分離します。これは、( 図2E)の前またはステップ2.4の後に行うことができる。
- 地下membraに解剖蝸牛感覚上皮を転送よく1.5ミリメートル無菌すくう( 図2F)を使用して、NEマトリックスでコーティングされた文化。
- オリエントは、上皮の内腔表面を持つ蝸牛外植片が上に向け、慎重に組織をフラット化し、静かに皿に追加することによって、新鮮な培養培地150μlでそれを置き換えるために基底膜マトリックス-DMEM溶液を吸引。注意が各植片がコーティングされたガラスカバースリップによく付着し、培養液中に浮遊していないことを確認するために注意する必要があります。蝸牛外植片の損傷を防ぐために、ガラスウェルに貼付しながら、鉗子の先端がアップ指摘されていることを確認してください。
- 穏やかに所望の期間、 インビトロでの 、通常3-6日間(DIV)、5%CO 2、37℃で組織培養インキュベーター中で無菌の150mm培養皿、所定の位置に培養皿に移す。 1 DIVの後、蝸牛外植片を培養皿によく付着していることを確認するために解剖顕微鏡下での文化を調べます。
- イン後時間の所望の長さのために、in vitroで cubationは、培養物を免疫組織化学のために処理される。
注:植片培養は通常の発達段階、P0と同等である6 DIVインキュベートする。 インビトロでのインキュベーション後、培養物を、in situハイブリダイゼーション 、および/ またはウェスタンブロットにおいて 、免疫組織化学( 図3B)、RT-PCRのような更なる下流の適用のために処理することができる。
3.エレクトロ媒介遺伝子導入
- エレクトロポレーションのためのセットアップ:
- 約20分間、70%エタノール中でオートクレーブ処理または浸漬ずつ100ミリメートルのSylgard被覆ガラス皿を滅菌し、使用前にクリーンベンチ内で空気乾燥させ。
- 適切な最大またはミディプレップキットを使用して、選択した発現ベクターからDNAを準備します。このプロトコルで使用される発現ベクターは、たpIRES2-Atoh1.EGFPとpCLIG-NeuroD1.EGFPです。 DNAの最終濃度は、無菌のDNA中に少なくともを1μg/μlのあるべき/RNaseフリー水。
- 胚性蝸牛外植片にDNAをエレクトロポレーション:
- 新鮮な100ミリメートルのシルガードコートディッシュにプラスミドDNA溶液10μlを加え、DNA溶液に(ステップ2.5から得た)完全に解剖蝸牛外植片を移す。
- それは皿の面に垂直だように、上向きに上皮の管腔表面では、わずかに蝸牛を傾ける。
- 感覚上皮と蝸牛のベースに隣接して配置された正パドル(アノード)に向かって負のパドル(カソード)との蝸牛の両側に電極パドルを置きます。
注:DNAが負に帯電しているので、それが負から正極に、感覚上皮に隣接するプローブのカソード端を配置することによって移行し、 DNAは、主に感覚上皮の表面を通過します。 - エレクトロを使用して、トンで24 mVでの9~10パルス、フットペダルスイッチと30ミリ秒のパルス持続時間を提供彼エレクトロ、その後にエレクトロ蝸牛に暖かい培養培地100μlを追加。
- 繰り返しますが、すべての蝸牛外植のために、目的のすべての遺伝子のDNAに対する3.2.1-4ステップと、(ステップ2.7)をめっきするための基底膜マトリックスコートディッシュにエレクトロポ蝸牛を移す。その後、免疫細胞化学のために処理される時間の所望の長さのために37℃、5%CO 2の加湿インキュベーター中で培養すべてのエレクトロポ蝸牛。
注:CD1マウス(約8〜12匹)の全体のごみから蝸牛は、孤立顕微解剖、エレクトロポレーションおよび<4時間でめっきすることができる。
4.分析蝸牛外植片の文化
注:培養物は、通常の発達段階、P0と同等である6 DIVインキュベートする。 インビトロでのインキュベーション後、培養物を固定し、免疫細胞化学のために処理した。
- 株式会社の所望の長さの後にubation、培地を除去し、すぐにリン酸緩衝生理食塩水(PBS)中の蝸牛外植片をすすぎ、室温で15分間(PBS中で調製)を4%パラホルムアルデヒド中でインキュベートする。
- PBSを加えることによって固定液を洗浄し、15分間PBSで3回リンス。
- 10%ヤギ血清を含むPBS-Tでブロッキングする前に、30分間、PBS-T(PBS + 0.5%のTween)を用いて透過性。
- 4℃で一晩、1%ヤギ血清を含むPBS-T中で一次抗体を含む一次抗体溶液中でインキュベートする。
- 適切なアレクサ結合二次抗体を加える前に、各PBS-Tの15分を使用して室温で蝸牛を3回洗浄します。
- 蝸牛外植片、ガラスカバースリップ上で培養しているので、それは皿からカバースリップを分離し、スライド上に取り付けることにより、個別に処理することができます。これは、室温で少なくとも1時間OS30溶媒中で培養皿を浸漬することによって達成することができる。無菌のカミソリの刃を使用して、dからカバースリップを切り離すISHとスライド上にマウントし、蛍光顕微鏡下で可視化する。
結果
We describe a method to isolate cochlea from embryonic inner ears and micro-dissect to expose the sensory epithelium. Once dissected, it may be plated and cultured as an intact cochlear duct (Figure 3) and analyzed by immunohistochemistry. The cultured cochlear explants provide a useful assay to examine the effect of variety of soluble factors and pharmacological drugs on cochlear development. Following dissection of cochlear explants, electroporation technique can be used to misexpress genes of interest and examine their effect on cochlear morphogenesis. The results presented here demonstrate that forced expression of basic helix-loop-helix (bHLH) transcription factor, Atoh1 into cultured cochlear explants established from E13 inner ears leads to ectopic hair cell formation. In contrast, forced expression of another bHLH transcription factor, NeuroD1 in prosensory cells and nonsensory epithelial cells within GER leads to the formation of ectopic neurons.
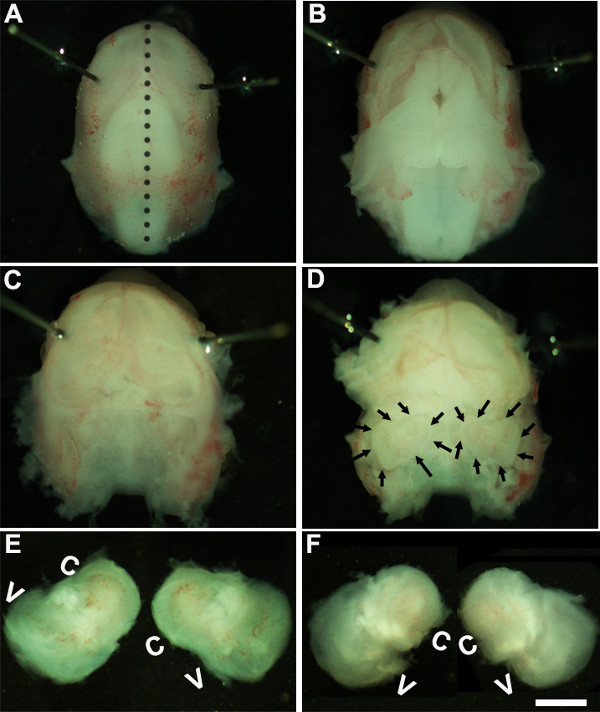
Figure 1: Dissection of the embryonic cochlea. Dissection steps involved in isolating developing cochleae from E13 mouse head as discussed in Part 1. Dotted line in (A) indicates dorsal midline and the borders of inner ears are indicated by arrows in (D). In (E), C indicates cochlea and V, vestibule. Scale bar: 1.0 mm.
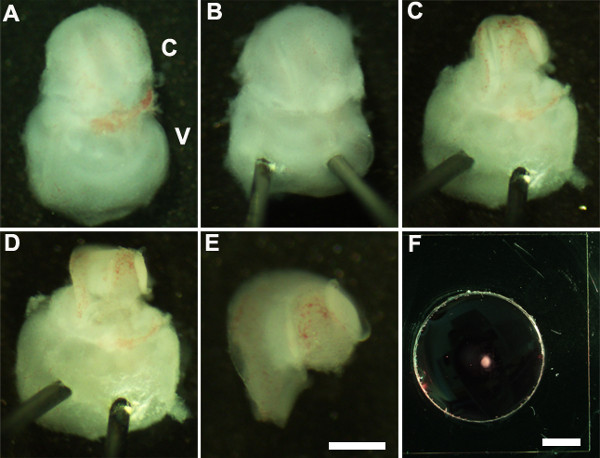
Figure 2: Generation of embryonic cochlear explant cultures. Panels (A-F) illustrate key steps in the dissection of the cochlea down to the sensory epithelium as described in Part 2. C, cochlea; V, vestibule. Scale bar: A-E, 100 m; F, 10 mm.
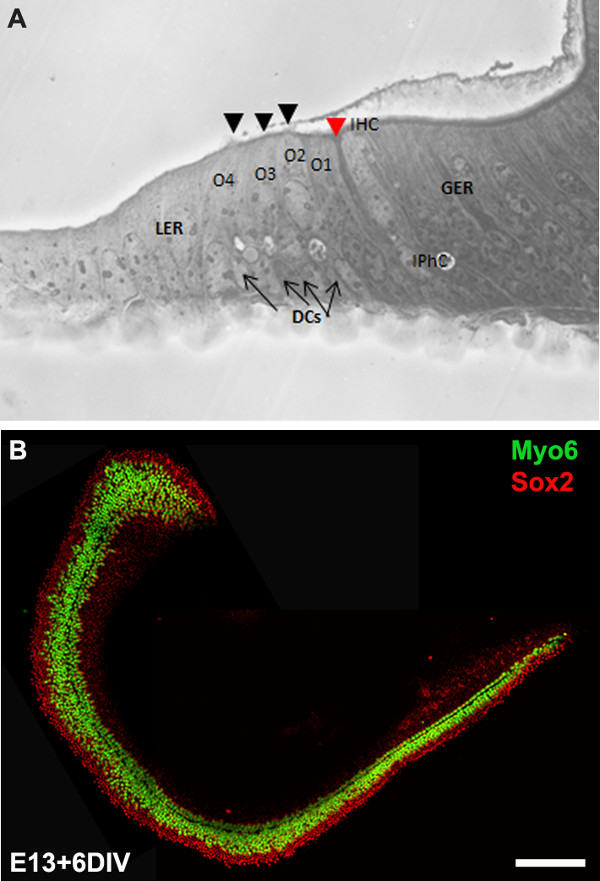
Figure 3: The development of the organ of Corti in cochlear cultures. The formation of hair cells and supporting cells occurs normally in cultured explants. Cross-sectional view of E13 cochlear explant incubated for 6 DIV (equivalent to P0) (B) and top-down view of a whole-mount cochlear explant immuno-stained with Myo7a in green and Sox2 in red demonstrates the presence of one inner and 3-4 rows of outer hair cells and surrounding support cells. Arrows point to Deiters’ cells and black arrowheads indicate the presence of stereociliary bundles within the cochlear explant. Arrowhead in red indicates the depression formed by the prominent pillar cell projection. IHC, inner hair cell; O1,2,3, three outer hair cells, DCs, Deiters’ cells; IPhC, inner phalangeal cell; GER, greater epithelial ridge which includes nonsensory epithelial cells present on the modiolar side of the epithelium; LER, lesser epithelial ridge, includes nonsensory epithelial cells present on strial edge of the sensory epithelium. Scale bar: B, 100 µm.

Figure 4: Electroporation of Atoh1.EGFP (A-C) and NeuroD1 into the organ of Corti explant culture. Embryonic (E) day 13 cochlear explants were established from WT CD1 mouse pups and electroporated with Atoh1.EGFP (A-C), NeuroD1-EGFP (D-F) reporter constructs as described and immunolabeled with hair cell-specific marker anti-Myo7a or neuronal marker, TuJ1 (β-tubulin III) in red. Electroporated cells which can be visualized by EGFP expression are seen throughout the GER, LER and sensory epithelium (SE) cells. The NeuroD1 transfected cochlear epithelial cells after 5 DIV acquire neuronal phenotype with dendritic processes, most of which are positive for TuJ1 while Atoh1 transfected cells are positive for Myo7a expression. SE, sensory epithelium; GER, greater epithelial ridge. Scale bar: A-C, 20 µm; D-F, 50 µm. Please click here to view a larger version of this figure.
ディスカッション
、感覚非感覚と螺旋神経節ニューロンを含むマウス内耳の膜迷路内のすべての細胞はすべてE8 10-14の周りに、外胚葉の後脳に隣接して位置placodally由来の耳胞に由来している。 E11で、耳胞の腹側領域は蝸牛管を形成するように延びており、開発が進むにつれて、となる蝸牛内の上皮細胞、ならびに内耳胞の他の領域群は、その後に生じさせるprosensoryパッチとして指定機械感覚有毛細胞および非感覚支持細胞の異なるタイプ。現像蝸牛内、内有毛細胞および外有毛細胞の3つの行の一つの行にE15.5の周り及びE17により同定することができる、パターニング内有毛細胞、柱細胞および外有毛細胞の3つの行の単一の列を有する本質的に完了する。蝸牛管は、最初E17を通してDIFのすべてを成長を開始E11からまたがる時間の期間ではferent細胞運命決定とパターニングは有毛細胞と支持細胞の正常な補数との印象的な携帯パターンを生成するために開発上皮内で発生する。有毛細胞および/または支持細胞の損失は、聴覚障害の主要な原因である。これらの細胞型は、胚発生の間に、かなりコンパクトな期間中にのみ生成されるので、再生戦略に重要な洞察をもたらすはずであるこれらの細胞型のそれぞれを特定の分子的および遺伝的経路を理解することが重要である。
蝸牛培養およびエレクトロポレーション技術は、発生中のマウスにおける遺伝子発現を操作するために開発されてきた。この動画では、一次外植片およびコルチ培養胚マウス器官への遺伝子送達のためのエレクトロポレーション法を生成するための技法を培養することを実証した。このようにして調製した一次外植片は、 インビトロで 7〜10日間維持することができる。蝸牛外植CAnは運命仕様、コミットメント、分化、およびパターニングなどの発生過程を調節するメカニズムを理解することを可能にする薬理学的アプローチによって遺伝子発現を操作するために使用される。さらに、この方法は、E12を過ぎて生存しない突然変異体マウス胎児の発達の表現型の解析を容易にします。
方形波エレクトロポレーション媒介遺伝子導入手順は、感覚有毛細胞、支持細胞、およびエクスビボ運命を視覚化するために、GERとLER領域内の細胞における遺伝子発現を操作するための機構を提供する。この手順を使用して、我々はダウンレギュレートまたは異所的にそうでなければ、野生型バックグラウンドでprosensory細胞、有毛細胞および/または支持細胞の特定の遺伝子を発現し、運命の指定及び分化に及ぼす特異的な効果を分析することができる。これは発展途上蝸牛内の特定の遺伝子の機能を理解することが可能となります。例えば、のように図4、GERまたはLER内の細胞内のAtoh1 2,4またはNeuroD1の8の強制発現は、それぞれ異所性有毛細胞と神経細胞の形成につながる。また、この技術は、複数の候補遺伝子6,7エレクトロポ感覚有毛細胞と支持細胞の形成、分化および組織に対するその効果を調べることができます。アデノウイルスベクターを含む遺伝子導入技術は、広範な発現を提供し、正常内耳15,16で使用されてきた。しかしながら、この技術は、多くの場合、時間がかかるウイルスを生成し、精製するための技術に依存する。
cochear文化は早くもE12として確立することができますが、E12.5 / E13よりも若い蝸牛外植片のエレクトロポレーションは、分析のために、それが面倒なこと組織に損傷が発生します。さらに、発現ベクターのプロモーターは、細胞型はドゥ蝸牛内にトランスフェクトされたかを判断し使用CT。感覚上皮内の細胞におけるトランスフェクションの効率化におけるCMV初期エンハンサー/ニワトリβアクチンプロモーターの使用により、たとえば、発現ベクターを含有するヒトサイトメガロウイルスプロモーターは、Kollikers '器官における強固なトランスフェクションをもたらす。胚性蝸牛外植片のエレクトロポレーション中に発生した一般的な問題は、過剰な細胞死および/または組織と貧弱なトランスフェクション効率の損傷である。電極およびDNA濃度の適切な間隔は、最小限の損傷および高いトランスフェクション効率を得る際に重要な役割を果たしている。要約すると、これらの技術は、機能の獲得または喪失戦略的および薬理学的操作を介して遺伝子発現を操作し、大幅にパターニングおよび細胞の運命決定に影響を与える信号の分析において補助するために我々の能力を強化する。
開示事項
No conflicts of interest declared.
謝辞
We would like to acknowledge Dr. Bradley Schulte for comments on this protocol. This work was supported by National Institutes of Health grant R00 (5R00DC010220). This project was performed in a renovated laboratory space supported by Grant C06RR014516.
資料
| Name | Company | Catalog Number | Comments |
| HBSS | Gibco | 14065-056 | |
| HEPES | Gibco | 15630-080 | |
| Dulbecco’s Modified Medium | Gibco | 12430-054 | |
| Fetal bovine serum | Gibco | 10082 | |
| N-2 Supplement (100x) | Gibco | 17502-048 | |
| Ciprofloxacin hydrochloride | Cellgro | 61-277-RF | |
| Glass Dish 60 mm | Kimble Chase | 23062-6015/23064-6015 | |
| Glass Dish 100 mm | Kimble Chase | 23064-10015/23062-10015 | |
| Minutien pins | Fine Science Tools | 26002-15 | |
| Dumont #5 forceps | Fine Science Tools | 11251-10 | |
| Pulse generator | Protech International Inc | CUY21Vivo-SQ | |
| Glass bottom culture dishes | MatTek | P35G-0-10-C | |
| Matrigel matrix | BD Biosciences | 356237 | |
| Culture dish, 60 x 15 mm | Becton Dickinson | 353037 | |
| Tissue culture dishes | Greiner Bio-one | 639160 | |
| Phosphate buffered saline | Gibco | 10010-023 | |
| OS-30 | Dow Corning | 4021768 | |
| Fluoromount | Southern Biotech | 0100-01 | |
| Conical tubes, 15 ml | Greiner Bio-one | 188261 | |
| Myosin 6 | Proteus Biosciences Inc | 25-6791 | |
| Myosin 7a | Proteus Biosciences Inc | 25-6790 | |
| TuJ1 | Sigma | T2200 |
参考文献
- Sobkowicz, H. M., Bereman, B., Rose, J. E. Organotypic development of the organ of Corti in culture. J Neurocytol. 4 (5), 543-572 (1975).
- Zheng, J. L., Gao, W. Q. Overexpression of Math1 induces robust production of extra hair cells in postnatal rat inner ears. Nat Neurosci. 3 (6), 580-586 (2000).
- Zheng, J. L., Shou, J., Guillemot, F., Kageyama, R., Gao, W. Q. Hes1 is a negative regulator of inner ear hair cell differentiation. Development. 127 (21), 4551-4560 (2000).
- Woods, C., Montcouquiol, M., Kelley, M. W. Math1 regulates development of the sensory epithelium in the mammalian cochlea. Nat Neurosci. 7 (12), 1310-1318 (2004).
- Jones, J. M., Montcouquiol, M., Dabdoub, A., Woods, C., Kelley, M. W. Inhibitors of differentiation and DNA binding (Ids) regulate Math1 and hair cell formation during the development of the organ of Corti. J Neurosci. 26 (2), 550-558 (2006).
- Dabdoub, A., et al. Sox2 signaling in prosensory domain specification and subsequent hair cell differentiation in the developing cochlea. Proc. Natl. Acad. Sci. U.S.A. 105 (47), 18396-18401 (2008).
- Doetzlhofer, A., Basch, M. L., Ohyama, T., Gessler, M., Groves, A. K., Segil, N. Hey2 regulation by FGF provides a Notch-independent mechanism of maintaining pillar cell fate in the organ of Corti. Dev Cell. 16 (1), 58-69 (2009).
- Puligilla, C., Dabdoub, A., Brenowitz, S. D., Kelley, M. W. Sox2 induces neuronal formation in the developing cochlea. J Neurosci. 20 (2), 714-722 (2010).
- Kaufman, M. H. . The Atlas of Mouse Development. , (1995).
- Sher, A. E. The embryonic and postnatal development of the inner ear of the mouse. Acta Otolaryngol Suppl. 285, 1-77 (1971).
- Torres, M., Giraldez, F. The development of the vertebrate inner ear. Mech Dev. 71 (1-2), 5-21 (1998).
- Brown, S. T., Martin, K., Groves, A. K. Molecular basis of inner ear induction. Curr Top Dev Biol. 57, 115-119 (2003).
- Puligilla, C., Kelley, M. W. Building the world’s best hearing aid; regulation of cell fate in the cochlea. Curr Opin Genet Dev. 19 (4), 368-373 (2009).
- Wu, D. K., Kelley, M. W. Molecular mechanisms of inner ear development. Cold Spring Harb Perspect Biol. 4 (8), a008409 (2012).
- Holt, J. R. Viral-mediated gene transfer to study the molecular physiology of the mammalian inner ear. Audiol Neurootol. 7 (3), 157-160 (2002).
- Stone, I. M., Lurie, D. I., Kelley, M. W., Poulsen, D. J. Adeno-associated virus-mediated gene transfer to hair cells and support cells of the murine cochlea. Mol Ther. 11 (6), 843-848 (2005).
転載および許可
このJoVE論文のテキスト又は図を再利用するための許可を申請します
許可を申請さらに記事を探す
This article has been published
Video Coming Soon
Copyright © 2023 MyJoVE Corporation. All rights reserved