Method Article
Fluorescence-Guided Matrix-assisted Laser Desorption/Ionization with Laser-Induced Postionization Mass Spectrometry of Individual Rat Neural Cells
* These authors contributed equally
In This Article
Summary
This protocol outlines the use of microMS for fluorescence-guided, single-cell MALDI-2 mass spectrometry, enabling enhanced molecular profiling of primary rat neuronal cells.
Abstract
Single-cell measurements are critical to understanding the rich spatiochemical heterogeneity of the brain. Matrix-assisted laser/desorption ionization (MALDI) mass spectrometry (MS) is capable of label-free, high-throughput characterization of endogenous molecules in individual cells. The recent advances in the development of MALDI mass spectrometers with laser-induced post-ionization (MALDI-2) provide greatly enhanced sensitivity of detection for a variety of lipids and other small molecules. However, MS imaging of large samples with MALDI-2 at cellular resolution is prohibitively slow for most applications. In this protocol, primary cells are isolated and dispersed onto conductive slides. Relative cell locations are determined by whole-slide fluorescence microscopy, followed by accurate coregistration of the microscopy coordinates to the stage coordinates of the MALDI-2 mass spectrometer. Targeted MS analysis of only cell locations provides high-throughput, single-cell measurements with high analyte coverage and reduced data size as compared to MS imaging of the entire sample. We describe the critical steps necessary for single-cell preparation, whole-slide fluorescence imaging, matrix application, and MALDI-2 mass spectrometry.
Introduction
Lipids and metabolites are fundamental to cellular function and serve as essential components of membranes, energy sources, and signaling molecules1,2. However, their composition and abundance can vary significantly between individual cells, reflecting the differences in cell types and developmental and functional states3,4,5. Analyzing these differences is crucial for understanding biological variability and identifying distinct cell subpopulations. Single-cell measurement techniques, such as RNA sequencing, provide useful cell-specific transcript profiles6. However, these transcript-level measurements do not directly reflect the actual cellular amounts of lipids and metabolites, as gene expression does not always correlate with the actual abundance of these analytes. Specialized methods for direct measurements of lipids and metabolites are, therefore, required for comprehensive analysis of the chemical composition of single cells and their populations.
Matrix-assisted laser desorption/ionization (MALDI) mass spectrometry imaging (MSI) is a tool of choice for label-free spatial mapping of endogenous biomolecules in situ7,8. Typically, with MALDI, a UV laser is used to ablate material from a thin sample layer co-crystallized with an organic matrix, forming a plume of ions and neutral molecules. The formed ions are then separated by an MS analyzer and detected within a mass spectrometer. Given the accurate positioning of modern mass spectrometer stages, the laser can be positioned to target specific sample regions or rastered across regions to generate molecular images by MSI. MSI at cellular or subcellular spatial resolution (< 10 µm), achieved with a focused laser beam and accurate stage movement, can be used to obtain chemical information about individual cells9,10. However, MSI measurements at this scale are inefficient, especially in the case of samples with low targeted cell densities, due to the amount of time spent imaging empty regions between cells. Further, the detectability of many analytes is limited due to the small volume sampled. To overcome these challenges, we have developed an image-guided MALDI MS approach for high-throughput single-cell analysis11,12. In this approach, the locations of dispersed cells are programmatically determined from whole-slide fluorescence images and used to guide the mass spectrometer stage to positions where individual cells with specific parameters (e.g., size and shape) are located and are then analyzed via irradiation with the MALDI laser. Previous work using this targeted approach has been used for characterizing lipids, peptides, and other biomolecules in heterogeneous cell populations5,13.
Given the mass-limited nature of single cells, the number of analytes detected in such samples is generally less than that observed directly from tissue. Therefore, to increase analyte coverage in single-cell MS analysis, increasing the analyte detection sensitivity is critical. One recently developed approach that aids in overcoming this detection challenge is MALDI with laser post-ionization (MALDI-2), which has been shown to enhance sensitivity for a broad range of analytes9,14,15,16. As a result, MALDI-2 generates more comprehensive single-cell datasets and provides deeper molecular coverage in mass-limited samples, such as isolated cells.
The goal of this method is to obtain lipid measurements from thousands of individual cells. To this end, we describe a workflow that enables high-throughput single-cell MALDI-2 mass spectrometry and is generally extendable to any probe-based mass spectrometry approach with precise stage control17. In this workflow, tissue from the brain region(s) of interest is dissected, and individual cells are obtained from the tissue after a papain dissociation procedure. The cells are then labeled with a nuclear stain and are dispersed onto conductive glass slides etched with fiducial markers, where they are allowed to adhere. Next, whole-slide images are taken using fluorescence microscopy. Matrix is deposited by sublimation, generating a repeatable homogenous crystal layer and high signal-to-noise for single-cell MS analysis. Using the open-source software microMS11, the relative coordinates of cell locations from the microscopy image are mapped and aligned with mass spectrometer stage coordinates by a point-set registration using fiducial markers etched onto the glass slides where the cells were deposited. Lastly, using this information, precise, targeted MS spectra are acquired from each individual cell, allowing for thousands of cells to be profiled in a single run (<1 h)12,13.
Protocol
All animal experiments in this study were done in accordance with the animal use protocol approved by the Illinois Institutional Animal Care and Use Committee (23228) with strict adherence to both national and ARRIVE standards for the ethical treatment and care of animals.
1. Preparation of materials and solutions
- Prepare modified Gey's balanced salt solution (mGBSS) with 1.5 mM CaCl2, 4.9 mM KCl, 0.2 mM KH2PO4, 11 mM MgCl2, 0.3 mM MgSO4, 138 mM NaCl, 27.7 mM NaHCO3, 0.8 mM Na2HPO4, and 25 mM HEPES. Adjust pH to 7.2 using 3 M NaOH. It is recommended to prepare 1 L of mGBSS and store at 4 °C until use.
- Prepare 50 mL syringes by filling them with the prepared mGBSS solution and place them on ice for the transcardial perfusion. Prepare 1 syringe for each rat dissected.
- Prepare aliquots of papain solution following the Worthington Papain Dissociation System protocol. This system contains four vials as described below, with vial 4 not used for this protocol:
Vial 1: sterile Earle's balanced salt solution (EBSS) with bicarbonate and phenol red
Vial 2: papain with L-cysteine and EDTA
Vial 3: deoxyribonuclease I (DNase)
Vial 4: ovomucoid protease inhibitor with bovine serum albumin- Add 5 mL of EBSS (vial 1) to the papain vial (vial 2) and place it in a 37 °C water bath for 10 min to dissolve the papain.
- Add 500 µL of EBSS (vial 1) into the DNase vial (vial 3) and mix gently. Add 250 µL of the diluted DNase vial (vial 3) into the papain vial (vial 2). This preparation contains a final concentration of 20 units/mL papain, 0.005% DNase, 0.5 mM EDTA, and 1 mM L-cysteine.
- Freeze 250 µL aliquots of this prepared papain solution for subsequent dissociations (makes ~20 aliquots). Supplement this solution with the following antibiotics: 100 units/mL penicillin G, 100 µg/mL streptomycin, and 100 µg/mL gentamicin. This can be modified for the user's application.
- Prepare a 150 mM ammonium acetate solution and store it at 4 °C. Prepare a 33% glycerol solution by diluting glycerol into the prepared mGBSS solution.
- Etch fiducials onto indium tin oxide (ITO) coated microscopy slides using either an engraving pen with a tungsten carbide or diamond tip or a CO2 laser engraver. On the conductive side of the ITO glass slide, etch 20-40 X-shape fiducial markers along the periphery of the slide to enable cross-platform (e.g., microscope and mass spectrometer) spatial registration.
NOTE: Take care not to etch in one connected line surrounding the sample or its areas as this disrupts surface conductivity between the area and a glass slide adapter.
2. Preparation of primary neural cells
NOTE: Rat hippocampal tissue is dissected, dissociated into individual cells with papain, and deposited onto conductive glass slides at low density. The isolation of cells in this manner enables high-throughput single cell mass spectrometry of endogenous lipids.
- Prior to animal dissection, thaw an aliquot of the prepared papain solution and place it into a container with an inlet and outlet tube allowing for CO2 to displace the air inside the container. Place this system into a water bath set to 37 °C and continue CO2 flow until the solution is the proper pH, as indicated by the color, using the pH color chart included in the Worthington Papain Dissociation System package. The target pH is between 7.2 and 7.4.
NOTE: Do not bubble CO2 into the enzymatic solution, as this will result in denaturation of the DNase and loss of biological activity. Use a 50 mL centrifuge tube with two tubes glued into the lid, allowing CO2 to fill the tube from the bottom and escape from the top outlet tube. - Euthanize rat(s) in accordance with IACUC protocols following institutional, local, and federal guidelines. Here, 2- to 3-month-old male Sprague-Dawley rats are used and sacrificed by CO2 asphyxiation.
- Transcardially perfuse the rat with ice cold (4 °C) mGBSS solution to remove blood from the vascular system and to quickly chill the animal's body. One example of this procedure can be found in the protocol by We et al.18.
- Surgically isolate the brain from the rat, making sure not to damage the brain region of interest. A previously published overview of this isolation provides more details on some of the individual steps19.
- Use a tissue chopper or tissue cube to cut a cross-section (~2 mm thick) of the brain to expose the region (hippocampus, cortex or striatum) to sample.
- Use a 2 mm biopsy punch to sample the brain region of interest and deposit the tissue chunk into the vial of papain solution.
- Incubate this tissue and papain solution at 37 °C under constant agitation (e.g., by an electric rocker) for 30-90 min.
NOTE: Enzymatic treatment duration should be optimized based on brain region, animal strain, age, and desired cell output (e.g., neurons and astrocytes with terminals versus maximum yield of cells without terminals). For rat hippocampal tissue, 60-90 min is typical. Shorter treatments may preserve some terminals but leave many cells in undissociated clusters, while longer treatments can improve yield but increase cell damage, requiring assessment using live/dead assays (e.g., LIVE/DEAD viability/cytotoxicity kit). For comparative studies, treatments must be consistent across sample types. - Place a vial of tissue and papain onto ice to cool and suppress enzymatic activity. Triturate the tissue using a cut pipette tip attached to a P100 adjustable volume pipette until most of the tissue is visually broken up. Do not form air bubbles during dissociation to avoid excessive cell damage.
NOTE: Vigorous trituration results in a high yield of cells; however, they do not retain most of their processes. Gentle trituration provides more processes on cells but results in more undissociated cells. Different-sized pipette tips and other methods can also be used and optimized for ideal dissociation results20. - Centrifuge the dissociated cells at 300 x g for 5 min at room temperature to pellet the cells. Remove the supernatant and resuspend the pellet in 1 mL of mGBSS. Add in Hoechst 33342 nuclear stain to a final concentration of 1 µg/mL.
- Pipette 100-500 µL of the cell suspension onto etched ITO glass slides and allow to sit for roughly 5 min or longer to allow for cells to sediment and adhere to the slide.
- The dilution of the cell suspension will need to be empirically determined. Using an optical microscope, add a droplet of the cells onto the slide to determine rough density and dilute from there to ensure that there is enough distance between cells (i.e., sufficiently larger than the MALDI beam size). This dilution can be performed on the first slide, and then the dilution can be adjusted as necessary for subsequent slides.
- Gently aspirate the cell suspension off the slide using a vacuum system containing (located in order starting from the vacuum line): 1) Tubing with an in-line gas HEPA filter, 2) Tubing fitted onto a 50 mL filtering flask, 3) Tubing connected to a second filtering flask inlet with an attached pipette tip (e.g., 100 μL). If storing the plated cells for future use, replace the aspirated suspension with 33% glycerol solution for 1-2 min and remove it.
NOTE: This step will ensure cell stability. Dispensing glycerol solution from one side while gently aspirating from the other side works is optimal for maintaining cellular structure. Always visually monitor all steps described here using an inverted microscope during the preparation of the first sample. - Apply 100-500 µL of ammonium acetate to the deposited regions, let sit for about 1 min, then aspirate to clean the slides. Repeat as necessary to ensure the slides are free of any salt or glycerol.
3. Microscopy
NOTE: To determine the location of deposited cells, each slide is imaged by brightfield/fluorescence microscopy. The fluorescence channel allows Hoechst-stained cells to be accurately located, while brightfield imaging provides morphological information. Any microscope capable of tiled image acquisition is suitable for this process.
- If not done previously, rinse slides with 2-3 mL of 150 mM ammonium acetate. This step is critical to remove glycerol and salt crystals, which can interfere with microscopy observation and MALDI matrix application. Dry slides under a gentle stream of N2 or allow to air dry.
- Load slide into the microscope stage. Focus the microscope using at least 10 support points distributed evenly across the slide.
- Using filters suitable for DAPI (excitation: 335-383 nm, emission: 420-470 nm) and brightfield imaging, obtain a tiled fluorescence image at 5x-10x magnification over the entire slide, ensuring that the fiducial markers (created in step 1.5) are captured.
NOTE: A 5x magnification is usually sufficient, especially when many slides need to be imaged. To resolve finer cellular morphology, 10x or 20x objectives can be used at the cost of quadratically longer imaging time and increase of file sizes. - Stitch the tiled image using microscopy software, such as ZEN (Zeiss). Accurate stitching is critical, as any errors in this step will propagate to the final registration step, resulting in inaccurate targeting. To check, ensure that vertically adjacent tiles are not offset. Additionally, consider acquiring tiled images with a large tile overlap (20%-30%).
- Process and export each image as a bigTIFF file in the microscopy software. If the region imaged is relatively small and results in a final image size of less than 2 GB, use a standard TIFF file format instead.
4. Matrix application
NOTE: Consistent and proper MALDI matrix application is critical to obtaining quality single-cell data. While sublimation using a commercial apparatus is used here, matrix application can also be performed using a robotic sprayer12, airbrush21, or homemade sublimation apparatus22. We have found that single-cell preparations require less matrix than thin tissue sections typically used for mass spectrometry imaging. To reduce batch effects, it is recommended to apply the matrix to all slides under study during one session and to deposit cells from different groups (e.g., brain region or treatment vs. control) onto the same slide whenever possible. Matrix selection is crucial for both traditional MALDI and MALDI-2 single-cell workflows. For single-cell MALDI, DHB23, 9-AA24, and CHCA25 have been successfully used. In MALDI-2, we and others have observed significant signal enhancement with DHAP9, while matrices such as NEDC16 and CHCA26 have also been applied effectively.
- Dissolve 20 mg of 2,5-dihydroxyacetophenone (DHAP) in 1.5 mL of acetone. Place slides in the holder of the sublimation chamber and place them in the sublimation apparatus.
- Pipet the dissolved matrix solution onto the ceramic wafer and allow the acetone to evaporate. Close the sublimation chamber to seal it.
- Fill the coolant chamber with an ice-water slush and place it on top of the chamber.
- Turn on the vacuum and allow the system to equilibrate for 5 min. The pressure in the system should be less than 40 mbar.
- Begin sublimation by heating the chamber to 200 °C for 5 min.
- Remove the ice-water bath, turn the temperature to 25 °C, and place a heatsink on top of the chamber. Allow the system to warm up to room temperature for 5 min to avoid condensation.
NOTE: The container holding the ice-water bath can be emptied and filled with hot water to serve as the heat sink. - Slowly vent the system, open the chamber, and remove the slides.
5. Single-Cell MALDI MS
NOTE: Single-cell MS data is obtained on a MALDI-2 timsTOF instrument (timsTOF flex) using the open-source microMS package to detect cells and guide the mass spectrometer. This requires that the optical image pixel locations of the targeted cells be translated to the physical coordinates of the mass spectrometer stage.
- Open microMS and decimate the bigTIFF microscopy images using the Image Group option, as there are both brightfield and fluorescence channels.
- Using the Blob Options tool, identify the optimal parameters to select for the targeted cells. Adjust the following parameters: the max and min blob size to select how small or large of blobs to find; the threshold setting, which determines how to threshold the fluorescence channel for blob detection; and the circularity, which determines how circular the identified cells need to be for consideration. For the data presented here, we used the following parameter values: max circularity: none; min circularity: 0.6; threshold: 75; min size: 20; max size: none.
- Use the Blob find option to find blobs and the popup window to save the blob list under a desired name.
- Use the Distance filter tool to select a specific distance that each cell needs to be away from each other (for this data, using 5x fluorescence microscopy, we used a distance filter of 35 pixels). This is to ensure that cells are far enough away from each other to perform single-cell analysis. This number is in pixel units, so know the pixel to µm conversion for the microscope to determine the ideal number. Use the size of the laser probe diameter plus around 20-30 µm to account for errors in the registration.
- Load the slides into the instrument using the MTP Slide Adapter II. Return to the computer with microMS, and using a remote desktop application, access the mass spectrometer computer.
- If this is the first time the instrument is being used, verify the instrument's X-Y position. To do this, go to Tools and Instrument Settings. The popup window will display a set of coordinates with their X and Y positions. Select Each of These Specific Points on the Slide Adapter II geometry on the instrument and update the X and Y positions in the microMS window.
- Using the camera and stage controls of the mass spectrometer, navigate to an easily identifiable location on the slide and copy the instrument coordinates. In microMS, find the corresponding location in the microscope image, right-click on that position, and type the coordinates into the popup window text location. Round all coordinates to the nearest integer, and separate X and Y coordinates with space (i.e., 39595 -23232).
- Repeat step 5.7. to register at least 12 fiducial markers across the entire slide. After three registration points have been added, one of the circles will turn red, indicating that it is the most off position from the registration. If this occurs, delete the current registration point using shift + right-click and try again.
- Under File, go to Save and then Registration to save the registration (.msreg) file.
- With the blobs displayed on the slide, save the instrument position (.xeo) file by going to File, then Save, and then Instrument Positions. Using remote desktop software, transfer this .xeo file to the instrument computer.
- Copy the text from the custom .xeo file generated into the MTP Slide Adapter II .xeo file on the instrument and save it. This will update this geometry file with the cells' locations.
- Click the Automation tab and select New… to start an automatic run. Drag across the displayed sample region to select the cells, and right-click to add them to the analysis list. Save the automation run and click Start Automatic Run to begin the acquisition. The length of the run depends on a variety of factors, including number of cells, laser shots, and laser frequency, but in general, roughly 5-10 cells can be analyzed in MALDI mode (10 kHz laser repetition rate), and 1-5 cells can be analyzed in MALDI-2 mode (1k Hz laser repetition rate) every second.
6. Data processing
NOTE: Existing commercial software packages are not well suited for analyzing high-throughput single-cell mass spectrometry data. While individual spectra can be visualized, extracting meaningful biological insights requires specialized tools. To address this, we provide freely available software that facilitates single-cell MALDI-2 MS data analysis. Our updated workflow facilitates the direct conversion of single-cell data into the open-source imzML format27, allowing compatibility with SCiLS MVS and other vendor software. For more advanced data analysis, the complete script also includes functionality for lipid annotation, clustering, and other visualization tools enabled by Matplotlib (version 3.7.3). Parsing and reading the raw data is facilitated by the pyTDFSDK library, a set of functions encompassed in the TIMSCONVERT workflow28.
- Initialize the custom Python library SC_MALDI2_Analysis by copying the file provided in Supplementary File 1 into the same working path as Jupyter Notebook and then press Shift + Enter on the keyboard on the first code cell. This will allow us to call these functions later and use them to process the data.
- Copy the path to the collected data (Bruker .d files). We recommend naming the folders and names of each file descriptively such that we can parse these file paths later to establish metadata for each sample. Run the Importing the Bruker .d Files and save to a list code cell. This will output the total number of .d files loaded.
- Run the code cell titled Processing the raw data and saving to imzML. This will read each Bruker .d file provided, perform peak picking on the data, then save 20 ppm of data around each peak to an .imzML file written inside of the .d file.
- To find the saved data, open the location of the first .d file and the imzML and other metadata will be saved into this path. At this stage, the saved imzML file can be imported into vendor software, such as SCiLS Lab MVS. For more advanced data analysis, continue following the script.
- Load the saved imzML data by running the Loading in the previously saved imzML file code cell. This loads the saved data into the variable data.
- Run the Binning the data and saving data matrix cell to bin all of the data along a common m/z axis, remove features that are present in low abundance (<0.1%), and remove values less than noise (100 relative intensity). This function then rebins the data by averaging each peak m/z value within each bin. The resulting data matrix is saved as datacube_array, the m/z bins associated with the matrix are saved as valid_mz_bins, and a count of each bin is saved as count.
- In order to annotate the data, run the Exporting peaks for LIPID MAPS annotation cell. This will copy all of the m/z peaks within your data matrix to your clipboard. Paste this into an empty text file and save it to upload it on LIPID MAPS.
- Open lipidmaps.org, then select MS Data Bulk Search and Search LMSD to search for biologically relevant lipid species. Upload the peak list by choosing the text file and select the appropriate adducts (e.g., [M+H]+, [M+Na]+, and [M+K]+). Select the mass tolerance appropriate for your mass spectrometer (we chose +/- 0.01 m/z), select all lipid classes of interest, and hit Search. Download the .tsv file.
- Load in the spectra metadata by running the Loading in the single cell paths for metadata cells. Alter the split code here based on the file path structures.
- Preprocess the data by running the Data Preprocessing and Lipid Annotation cell. This will use the lipidMAPS tsv file to annotate the dataset to 10 ppm error, eliminate odd chain lipids from the lipidMAPS results, normalize the remaining data, and remove features or cells that don't pass a specific threshold. This will save the new processed dataset as data2 and the updated spectra metadata as updated_cell_types.
- Analysis of the data is performed by scanpy. Run the UMAP and Clustering Analysis for Single Cells cell to select data of interest (here we selected only MALDI-2, M2, data), scale the dataset, and load it into a scanpy AnnData object for easy analyses.
NOTE: Here, Leiden clustering and dimensionality reduction (UMAP) were performed on the dataset, both the original data classes and the clusters on the UMAP were visualized, and some of the most statistically significant features among the clusters were summarized using the rank_genes_groups_dotplot function from scanpy.
Results
An overview of the workflow for fluorescence-guided single-cell MALDI-2 MS is shown in Figure 1. First, the tissue dissected from targeted brain regions (Figure 1A) is dissociated into single cells and deposited onto conductive ITO-coated microscopy slides (Figure 1B). The locations of cells are determined by whole-slide fluorescence imaging (Figure 1C), followed by MALDI matrix application (Figure 1D), microMS-assisted MALDI-2 MS analysis (Figure 1E), and data analysis (Figure 1F). Using this workflow, tens to hundreds of lipids can be putatively identified within individual cells with a mass accuracy of less than 10 ppm (using a MALDI-2 timsTOF).
Mass spectrometry imaging can be performed on a small region of interest to assess the quality of the cell preparation and matrix application. A representative result of MALDI-2 imaging of dispersed cells is shown in Figure 2A. From this image, the user can assess the extent of analyte spreading by comparison to a photomicrograph of the same region. Based on the amount of spreading, the analyst can adjust the size of the laser beam field size and the distance filter to ensure true single cell acquisitions. In single-cell MSI, a small laser size and raster width will be used to obtain cellular resolution (1-5 µm), whereas a larger laser size can be used for microMS, increasing the signal intensity and number of detectable lipids relative to imaging. Representative results of the signal enhancement obtained with MALDI-2 are shown in Figure 2B. Enhancement of cholesterol, PE, and PC species is observed in agreement with prior literature14. Generally, we obtain the best MALDI-2 signal enhancement when using a relatively high laser power (>50%) and a smaller number of shots (around 10 to 200). Some optimization is necessary, as too low of laser power will not provide enhancement, while too much laser energy will cause excessive analyte fragmentation29. For single-cell analysis, spatial registration is a critical step to ensure accurate targeting of individual cells. To validate the registration, a small set of test points (~5) can be generated around the slide by creating blobs at known spots and exporting the instrument coordinates via microMS. Verifying that these test spots correctly position the instrument stage when selected ensures that subsequent cell targeting will be accurate.
MALDI MS enables profiling cellular heterogeneity both between and within individual brain regions (Figure 3). By analyzing variations in lipid profiles, dimensionality reduction techniques effectively separate cells into distinct clusters, highlighting the diversity of lipid compositions across the dataset (Figure 3A). Further, the application of clustering techniques, such as Leiden clustering, shows the presence of unique subpopulations of cells within specific brain regions, including the striatum and cortex (Figure 3B). Additionally, by comparing the lipid profiles of each cluster to each other (Figure 3C), specific lipids can be used as markers to define cluster-specific identities. Several lipid species are significantly up- or down-regulated in specific clusters, suggesting distinct functional roles or states, while others are shared across clusters but vary in their relative signal intensity. To ensure robust downstream data analysis, it is essential to achieve reliable lipid detection in the majority of cells and assess potential batch effects. Figure 3D presents representative spectra from six individual cortical cells dispersed across separate ITO-coated slides. While individual lipid profiles exhibit heterogeneity, lipids are consistently detected across all acquisitions. Figure 3E displays a UMAP of cortical cells from different slides, prepared and analyzed under identical conditions. The substantial overlap of cells between slides indicates that batch effects do not significantly confound biological interpretations. If necessary, batch correction algorithms such as ComBat30 can be applied to mitigate any residual effects. In summary, single-cell MALDI-2 is an advancing technique for lipid biomarker identification, offering critical insights into cellular subpopulations and enhancing our understanding of regional and functional diversity in the brain.
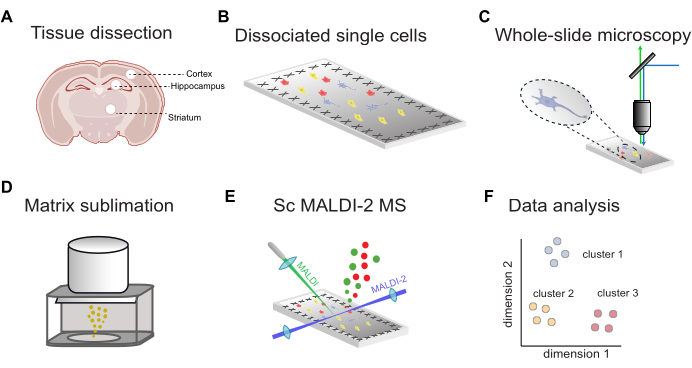
Figure 1: General workflow for scMALDI-2 MS. (A) Manual tissue dissection and isolation of brain regions. (B) Papain dissociation of tissue into single cells and cell deposition onto conductive ITO-coated slides. (C) Whole-slide fluorescence microscopy via nuclear staining (DAPI/Hoechst). (D) MALDI matrix (DHAP) sublimation onto sample slides. (E) Image-guided single-cell MALDI-2 MS measurements. (F) Comprehensive data analysis, including dimensionality reduction, clustering, and statistical analysis. Please click here to view a larger version of this figure.
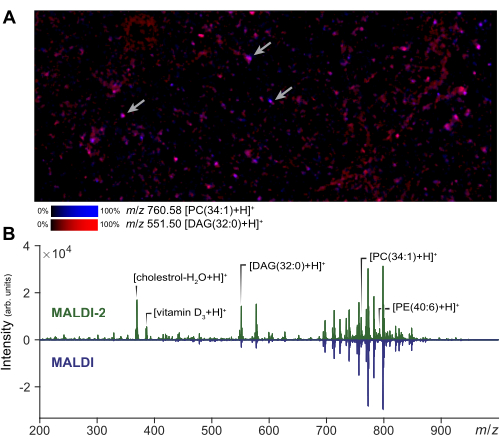
Figure 2: MALDI-2 MS optimization and imaging for single cell analysis. (A) MALDI-2 MSI imaging of dispersed populations of primary cells. The arrows indicate a few individual cells. Performing MSI and a small region allows the analyst to optimize method parameters and assess sample quality. (B) Comparison of results obtained using MALDI-2 and MALDI MS acquisitions. The signal enhancement (MALDI 2 vs MALDI) of various lipids, including cholesterol, DAG (diacylglycerols), PC (phosphatidylcholine), and PE (phosphatidylethanolamine), is highlighting by showing the MALDI-2 spectrum (top) and MALDI spectrum (bottom). Please click here to view a larger version of this figure.
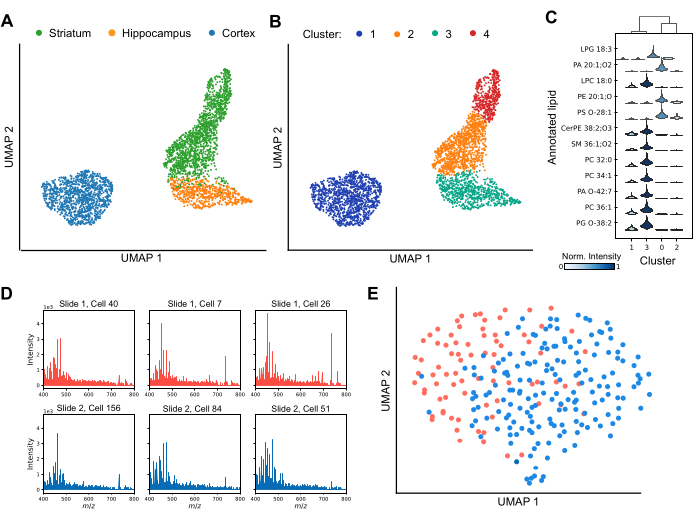
Figure 3: Single-cell lipid profiling within three rodent brain regions by MALDI-2 MS. (A) uniform manifold approximation and projection (UMAP) analysis of the analyzed cells colored by brain region of origin. (B) UMAP analysis of the same cells after undergoing Leiden clustering, where clusters correspond to subpopulations of cells with similar lipid compositions. The colors here show different Leiden clusters of data. (C) A violin plot showing the difference in the intensity distributions for different lipids between clusters. (D) Spectra from six representative individual cortical cells were analyzed from two separate slides. (E) UMAP analysis of cortical cells analyzed from two separate slides. Please click here to view a larger version of this figure.
Supplementary File 1: SC-MALDI2_Analysis.py. This file contains a set of functions developed for processing single-cell MALDI-2 mass spectrometry data. It includes methods for reading raw mass spectrometry data, performing binning, and conducting annotation and downstream analyses. The set of cells to load and perform all data preprocessing and analyses are provided in Supplementary File 2. Please click here to download this File.
Supplementary File 2: JOVE Analysis.ipynb. This file contains a Jupyter notebook that contains a set of cells to load in the custom Python functions from SC-MALDI2_Analysis.py and perform all data preprocessing and analyses mentioned. Please click here to download this File.
Discussion
High-throughput, image-guided single-cell MALDI MS is a valuable tool for understanding chemical heterogeneity on a single-cell scale. The addition of laser-induced post-ionization (MALDI-2) provides deeper analyte coverage, which is critical for mass- and volume-limited samples such as isolated mammalian cells.
While the overwhelming majority of published single-cell lipid and metabolite MS workflows use cultured cells, our approach is applied to relatively quickly isolated primary cells. This distinction is critical, as cultured cells show a demonstrable change in their metabolite and lipid content in just a few days of culturing23,31. Enzymatic cell isolation, in most cases, leads to the loss of fine cellular structures such as axons, dendrites, and astrocytic processes. Therefore, the observed analyte signals are obtained primarily from the cell soma. In spite of this, successful classification by cell type4 and brain region is achieved12. This suggests that many of the lipids that are unique to distinct cells are located in the soma.
Small differences in the MALDI matrix application method can have profound effects on the resultant analyte signal. We have presented a workflow based on reproducible matrix sublimation with a commercial apparatus, which generates a homogeneous matrix layer, high signal-to-noise, and limited analyte spreading. However, matrix application using a robotic sprayer or artist's airbrush, among other approaches, can be used. Regardless of the approach, a lower matrix density should be applied to single-cell preparations as compared to that used in standard tissue MSI in order to achieve the optical matrix-to-analyte ratio. The matrix density applied here is approximately half of that used for tissue MSI. Of course, this does imply that the amount of matrix per cell is higher than in MSI, which helps to contribute to the enhanced analyte extraction and detection in the isolated cell approach used here.
For samples that require significant optimization, performing MSI on a small ROI of single cell dissociates, as demonstrated in Figure 2A, can be performed as a part of the optimization process. This step can also allow the analyst to assess the extent of analyte spreading in solvent-based matrix application approaches.
Successful image-guided MALDI of single cells requires high-quality fluorescence images, reproducible matrix application, and precise stage control. These experiments are more demanding than standard MSI workflows, which tolerate minor registration errors and variability in matrix and sample conditions due to larger imaging areas and higher analyte counts. Despite these challenges, we have successfully targeted single organelles using traditional MALDI.
Accurate stitching of tiled fluorescence images is essential, as small errors can compound and affect stage control, making registration accuracy critical. Batch effects also present a major challenge, with signal variations observed across the same slide, between slides, and across preparations. Proper normalization strategies, including the use of internal standards and interquartile normalization, help mitigate these issues. Additionally, batch correction algorithms such as ComBat30 can be used to reduce technical variability and enhance true biological differences.
Obtaining confident molecular annotations from single cells remains a challenge. Often, the amount of material and sensitivity of detection is insufficient for tandem MS from all but the most abundant species. For those that are not detectable enough for tandem MS directly from individual cells, alternative approaches to create an analyte library can be applied. For instance, on-tissue tandem MS data can be obtained from thin cryosections from the same brain region and animals used to generate cellular populations. Lipid extraction from tissue followed by LC-MS can also be employed. As sample preparation strategies and MS technology continue to improve, more and more relevant structural information can be obtained directly from single cells. In the future, we anticipate this workflow will be extended to obtain tandem MS data from single cells, eliminating the need for ancillary LC-MS/MS experiments. We further envision that this approach could be extended to numerous samples on the microscale in biology and beyond, including unique cell types, mammalian organelles, powders, and microplastics.
Disclosures
The authors have no competing interests to disclose.
Acknowledgements
S.W.C acknowledges support provided by the Peixin He and Xiaoming Chen PhD4 Fellowship and the University of Illinois Block Grant Fellowship. This work was also supported by the National Institute on Drug Abuse under award No. P30DA018310, the National Institute on Aging under Award No. R01AG078797, and by the Office of The Director, of the National Institutes of Health under Award Number S10OD032242.
Materials
| Name | Company | Catalog Number | Comments |
| 2',5'-dihydroxyacetophenone | Sigma Aldrich | D107603 | DHAP, 97% purity |
| Ammonium acetate | Sigma Aldrich | 238074 | ACS reagent, ≥97% |
| Axio M2 Imager | Zeiss | N/A | N/A |
| Biopsy punch, 2 mm | Fisher Scientific | 12-460-399 | integra miltex standard biopsy punch, 2mm |
| Calcium chloride | Sigma Aldrich | C4901 | anhydrous, powder ≥97% |
| Eppendorf Centrifuge | Sigma Aldrich | EP5405000441 | centrifuge 5425 with rotor FA-24x2 |
| Gentamicin | Sigma Aldrich | G1272 | liquid, BioReagent |
| Glass etching pen | Sigma Aldrich | Z225568 | carbide time, pkg of 1 |
| Glycerol | Sigma Aldrich | G7893 | ACS reagent, ≥99.5% |
| HEPES buffer | Sigma Aldrich | H3375 | ≥99.5% (titration) |
| Hoechst 33258 Solution | Sigma Aldrich | 94403 | 1 mg/mL in H2O, ≥98.0% (HPLC) |
| In line HEPA Filter | Sigma Aldrich | WHA67225001 | VACU-GUARD 60 mm disc, 0.45 PFTE housing |
| ITO-Coated Microscopy Slides | Delta Technologies | CG-90IN-S115 | 70-100Ω resistance |
| Magnesium chloride | Sigma Aldrich | M8266 | anhydrous, ≥98% |
| Magnesium sulfate | Sigma Aldrich | 208094 | anhydrous, ≥97% |
| Microcentrifuge tubes | Sigma Aldrich | HS4323K | tube capacity 1.5 mL, pack of 500 |
| Papain dissociation system | Worthington Biochemical | LK003150 | one box, 5 single use vials |
| Penicillin-Streptomycin | Sigma Aldrich | P4458 | liquid, BioReagent |
| Potassium chloride | Sigma Aldrich | 529552 | Molecular biology grade |
| Potassium phosphate monobasic | Sigma Aldrich | P5379 | Reagent Plus |
| Sodium biocarbonate | Sigma Aldrich | S6014 | ACS reagent, ≥99.7% |
| Sodium chloride | Sigma Aldrich | S9888 | ACS reagent, ≥99% |
| Sodium hydroxide | Sigma Aldrich | 221465 | ACS reagent, ≥97%, pellets |
| Sodium phosphate dibasic | Sigma Aldrich | S9763 | ACS reagent, ≥99% |
| Sublimate | HTX | N/A | N/A |
| timsTOF FleX MALDI-2 | Bruker | N/A | microGRID enabled |
| Vacuum tubing | Thermo Scientific | 8701-0080 | Nalgene Non-phthalate PVC Tubing |
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved