Method Article
Fluorescence Assays for the Study of Mycobacterium tuberculosis Interaction with the Immune Receptor SLAMF1
In This Article
Summary
This study provides a protocol for evaluating the interaction of Mycobacterium tuberculosis with the SLAMF1 microbial sensor. The assays were conducted on human monocyte-derived macrophages using flow cytometry and fluorescence microscopy. The described tools are relevant for studying interactions between pathogens and immunoreceptors.
Abstract
The evaluation of direct interaction between pathogens and immune receptors usually involves sophisticated techniques or implies the use of transgenic strains and genetically engineered cells. Here, an alternative method to detect biochemical interaction between the macrophage microbial sensor SLAMF1 and Mycobacterium tuberculosis is described. Two technical approaches employing flow cytometry and fluorescence microscopy were developed. Total cell protein extracts from human macrophages were generated, then incubated with whole cells of M. tuberculosis (WCMtb) or M. tuberculosis antigens (Mtb Ags) overnight at 4 °C and finally cross-linked using formaldehyde/glycine/ethylene glycol bis (succinimidyl succinate) treatment. SLAMF1 interaction with WCMtb by flow cytometry was detected with a PE-specific anti-SLAMF1 antibody. The existence of interaction by fluorescence microscopy was performed by attaching Rhodamine-PE stained Mtb Ags to poly-D-lysine coated slides, which were incubated with the total protein extract from monocyte-derived macrophages. After cross-linking treatment, SLAMF1 was visualized using primary (anti-SLAMF1) and secondary (Alexa Fluor 488) antibodies. The assays provided a strong biochemical tool to measure pathogen-immunoreceptor interactions, overcoming the difficulties associated with transgenic cell lines and protein gene expression modulation experiments.
Introduction
Mycobacterium tuberculosis, the Tuberculosis-causative pathogen identified 142 years ago, remains a global challenge, currently infecting at least a quarter of the world's population1. Transmitted through airborne droplets from infected people, M. tuberculosis reaches alveolar macrophages in the lungs where it can survive for long periods in a latent state2,3. Not only is the local macrophagic response activated, but in recent years, it has been described that peripheral monocytes can be recruited to the respiratory tract and differentiate into alveolar macrophages, generating an even more robust response against M. tuberculosis than their counterpart of fetal origin2,4.
Macrophages are fundamental players of innate immunity. Upon M. tuberculosis ingestion, macrophages display numerous microbicidal functions, such as the secretion of proinflammatory cytokines, the fusion of the phagolysosome, and the activation of other immune cells in order to kill M. tuberculosis2,3. However, the complex architectural structure of this pathogen provides effectors (e.g., proteins and lipids) capable of regulating macrophage metabolism and functions and, in consequence, the inflammatory process3. This manipulation of macrophage responses, through the secretion of virulence factors or the exploitation of host factors, leads to different escape strategies exerted by M. tuberculosis. Some key evasion mechanisms include induction of anti-inflammatory cytokines, inhibition of phagolysosome maturation and acidification, oxidative stress alterations, autophagy disruption, and deficiencies during antigen processing and presentation3,5.
A tightly regulated interaction between macrophages and M. tuberculosis is crucial for the development of a proper immune response. Therefore, studying these synapses is key to identifying immunoprotective or immunopathogenic mechanisms induced by host-pathogen crosstalk, as well as identifying potential therapeutic targets. Many receptors mediate the recognition and/or internalization of M. tuberculosis, including TLRs6,7,8, NLRs7,8, complement receptors6,8, C-type lectin receptors6,8, and scavenger receptors6,8. Accumulating data indicate that both surface-bound and intracellular PRRs play an important role during infection, recognizing opsonized and non-opsonized M. tuberculosis.
Zihad and Sifat et al. have recently reviewed the participation of the PRRs in M. tuberculosis-induced responses by innate cells9. In particular, most members of the TLR family have been implicated in the interaction with M. tuberculosis ligands6,7. Surface TLR2 recognizes mycobacterial antigens such as acylated lipoproteins, 19-kDa lipoprotein, LprA lipoprotein, LprG lipoprotein, 30-kDa antigen, 38-kDa antigen, MymA, proline-proline-glutamic acid (PPE)-57, LAM, LM, PIM, heat shock protein 60, signature protein Rv1509 and the secreted protein ESAT-67. Membrane TLR4 interacts with heat shock proteins, 38-kDa antigen, RpfE, Rv0652, Rv0335c, Rv2659c, Rv1738, Rv2627c, Rv2628, GrpE, and HBHA. On the other hand, ligands for endosomal TLRs (TLR 3, 7, 8, and 9) include dsRNA, tRNA, ssRNA, phagosomal RNA, and dsDNA of M. tuberculosis. In addition, TLR2 has an active role in initiating immune responses when acting in conjunction with TLR1 and TLR6, TLR4, and TLR96,7. NOD2 and NLRP3 are the most well-characterized cytoplasmic NLRs in Tuberculosis. Although their specific ligands remain under study, these receptors are activated by muramyl dipeptide or ESAT-6, respectively7,8. C-type lectin receptors are classically involved in M. tuberculosis endocytosis. While Dectin-2 acts as a direct PRR for ManLAM of M. tuberculosis cell wall, the Dectin-1 ligand has not been discovered yet8. Mincle and MCL both recognize the glycolipid trehalose-6,6′-dimycolate (TDM), also called the cord factor7. DCAR interacts with the mycobacterial glycolipids phosphatidyl-myo-inositol mannosides (PIMs)7. CR3 recognizes mycobacterial LAM and PIM, DC-SIGN ligates ManLAM, MR recognizes a number of M. tuberculosis components, including ManLAM, PIM, LM, 38-kDa glycoprotein, 19-kDa antigen, and other mannosylated proteins while DCIR ligand is still unidentified.
Soluble CLRs include SP-A, which recognizes ManLam, LM, 60-kDa glycoprotein, and glycoprotein Apa; SP-D interacting with LAM, LM, and PILAM; and MBL, which specializes in ManLAM recognition6,7. Scavenger receptors are also phagocytic PRRs. SR-A and MARCO have TDM as their ligand, SR-B1 recognizes ESAT-6, and CD36 binds ManLAM and LM7,8. Additionally, Dectin-1, Mincle, and MARCO can also combine with TLR2 or TLR4 to trigger signals after detecting M. tuberculosis PAMPs6,7. CD14 is a surface receptor that has the ability to internalize nonopsonized bacteria and also recognizes the heat shock protein Chaperonin 60.1. In particular, CD14 functions as a co-receptor along with MARCO and TLR26. AIM2 is a cytosolic receptor that can sense ssDNA upon M. tuberculosis escapes from the phagosome8. Finally, AhR is a ligand-activated transcription factor that binds pigmented virulence factor naphthoquinone phthiocol from M. tuberculosis6.
Many of the interactions described above have been postulated and not strictly demonstrated. Even the ligands for certain receptors remain unknown, which reinforces the need to better understand the field of M. tuberculosis immunorecognition. In this context, the costimulatory molecule SLAMF1 (Signaling Lymphocytic Activation Molecule) has been recently described as a M. tuberculosis receptor by Barbero et al.10. By acting not only as a signaling molecule but also as a M. tuberculosis sensor, SLAMF1 has a particularly intriguing role in Tuberculosis. SLAMF1 can induce the activation of immune cells by modulating protective functions such as the production of IFN-γ by T cells through Erk/CREB phosphorylation11,12,13, autophagy in neutrophils14, and bacterial clearance in macrophages15.
Receptors-ligand interactions have been studied over the years using techniques such as ELISA, Surface Plasmon Resonance (SPR), Isothermal Titration Calorimetry (ITC), Fluorescence Polarization (FP), X-ray Crystallography, Nuclear Magnetic Resonance (NMR) Spectroscopy, Microscale Thermophoresis (MST), Resonance Energy Transfer (e.g., BRET or FRET) Confocal Microscopy, Electron Microscopy, Cryo-Electron Microscopy (Cryo-EM) and Atomic Force Microscopy (AFM)16,17,18,19,20,21,22,23,24,25,26,27,28. Some of these approaches imply the use of reporter genes, labeled recombinant proteins or chimeric molecules, knockout, knockdown, or overexpression models. Alternatively, computational tools can predict receptor-ligand interactions and binding sites and are often used in combination with biological approaches to gain a comprehensive understanding of the interactions29,30,31. Here, two alternatives to detect biochemical interaction and also a section on how to fluorescently label bacteria are described. A protocol that allows the study of receptor-pathogen interactions in vitro is presented, particularly evaluating the SLAMF1-M. tuberculosis engagement through flow cytometry and fluorescence microscopy, two usually available and routinely used techniques.
Protocol
All procedures involving human monocyte-derived macrophages were performed in accordance with the Helsinki Declaration (2013) and in agreement with the Ethics Committee of UNNOBA (COENOBA). Written informed consent was obtained prior to sample collection. The male/female group distribution was 13/6, and the median age was 32 years, with an interquartile range (IQR) of 18-75 years. The presence of previous pathologies, comorbidities, or a positive diagnosis for Tuberculosis were defined as exclusion criteria. The details of the reagents and equipment are listed in the Table of Materials.
1. Monocyte-derived macrophage culture and stimulation
- Blood draw
- Obtain 60 mL of blood from healthy donors by venipuncture in a heparinized syringe.
NOTE: Blood extraction must be performed by a biochemist or phlebotomy technician. - Discard the needle in the sharps disposal container and any material that has been in contact with donors' blood in the pathological waste container.
- Obtain 60 mL of blood from healthy donors by venipuncture in a heparinized syringe.
- Monocyte isolation and macrophage generation
- Carefully transfer the blood to 50 mL tubes and dilute it by half with saline solution.
- Perform centrifugation over a density gradient media to obtain peripheral blood mononuclear cells (PBMCs).
- Collect the PBMCs from the whitish halo.
- Perform two washes with saline solution at 400 x g for 10 min and a final wash at 200 x g for 15 min at 20 °C to eliminate platelets.
- Determine the number of PBMCs in a cell counting chamber and perform CD14 positive magnetic selection with CD14 beads.
NOTE: During magnetic selection, carefully follow the manufacturer's instructions. Additionally, one can check the purity of isolated monocytes using flow cytometry. - Resuspend the isolated cells in RPMI 1640 and determine the number of monocytes in a cell-counting chamber.
- Seed 500 µL of 1 × 106/ mL CD14 positive selected monocytes per well in a 24-well cell culture plate in serum absence to promote adherence.
- After 2 h, remove non-adherent cells by washing them with pre-warmed RPMI 1640.
- To differentiate macrophages, culture the adherent monocytes for an additional 16-18 h (overnight) in 1 mL of complete media (RPMI 1640 medium supplemented with L-glutamine, 10% fetal bovine serum (FBS), 100 U/mL of penicillin, and 100 µg/mL of streptomycin).
- The next day, change the culture medium. Remove the medium, wash with 1 mL of 1x PBS, and add 1 mL of complete media.
- Stimulate the macrophages with sonicated M. tuberculosis (M. tuberculosis antigens (Ags)) for 24 h. Use 10 µL of M. tuberculosis Ags (10 µL = 1 × 106 bacteria) per 1 × 106 monocyte-derived macrophages.
NOTE: 10 µL of M. tuberculosis Ags (Mtb Ags) = 1 × 106 bacteria, quantified by O.D. 600nm in spectrophotometer considering an O.D. of 1 as 1 x 108 bacteria/mL. Use BSL2 biosafety cabinets and an incubator at 37 °C in an atmosphere with 5% CO2 for all the culture steps.
- Corroboration of SLAMF1 expression
- Collect the macrophages from a single well. Place the culture plate on a cold surface. Detach the cells using up and down movements by adding 1 mL of cold FACS (1x PBS + 2% FBS) twice consecutively. Use a cytometry round-bottom tube for the collection.
- Centrifuge the cells at 500 x g for 5 min at 4 °C. Discard the supernatant by dumping it.
- Stain the cells with a fluorophore-coupled anti-human SLAMF1 antibody for 30 min at 4 °C in the dark. Vortex after adding 1.25 µL of the antibody.
- Wash the cells to eliminate the excess of antibodies using FACS, resuspend the pellet in FACS, and acquire the sample using a flow cytometer.
NOTE: 1.25 µL of anti-SLAMF1 antibody corresponds to 0.0625 µg that is used to label 0.5 x 106 macrophages. However, the titration of the antibody is strongly recommended, as well as performing the corresponding isotype or Fluorescence Minus One (FMO) control.
2. Total cell protein extract preparation
- Lysis buffer preparation
- Prepare Radio Immunoprecipitation Assay (RIPA) buffer with NaCl 150 mM, Tris 10 mM, EDTA 5 mM, SDS 1%, Triton X-100 1% and Sodium Deoxycholate 1%.
- Supplement RIPA buffer with 1 mM phenylmethylsulfonyl fluoride (PMSF) and protease inhibitors such as Pepstatin A and Leupeptin.
- Cell collection and protein isolation
- Discard complete RPMI from the plate wells using a P1000 pipette and harvest macrophages by up and down pipetting with cold 1x PBS.
- Transfer the cells to 1.5 mL microcentrifuge tubes. Collect one well per microtube.
- Centrifuge at 500 x g for 5 min at 4 °C. Discard the supernatant.
- Add 1 mL of cold PBS and repeat steps 2.2.2 and 2.2.3 collecting the cells in the same microcentrifuge tube.
- Resuspend the cell pellets in 100 µL of 1x PBS and pool four tubes. Repeat step 2.2.3. Each tube will contain 2 x 106 macrophages.
- Add 100 µL of supplemented RIPA buffer and incubate the suspension on ice for 1 h, vortexing every 10 min.
- Centrifuge at 14,000 x g for 15 min. Collect the supernatant and conserve until used at -20 °C.
3. M. tuberculosis -SLAMF1 interaction by flow cytometry
- Proteins-bacteria cross-linking
- Incubate 1 × 106 whole inactivated M. tuberculosis cells (WCMtb, M. tuberculosis inactivated by gamma irradiation) with protein extract from 1 x 106 macrophages (50 µL) overnight at 4 °C in a rotating microtube holder.
NOTE: 10 µL of WCMtb = 1 × 106 bacteria, quantified by O.D. 600nm in spectrophotometer considering an O.D. of 1 as 1 x 108 bacteria/mL. - The following day, add 500 µL of 1% formaldehyde diluted in 1x PBS. Incubate for 15 min under agitation (shaker) at room temperature (RT).
- Add 25 µL of 0.125 M glycine diluted in water. Incubate for 5 min under agitation (shaker) at RT.
- Wash twice with 1 mL of 1x PBS at 14,000 x g for 5 min. Discard the supernatant between washing steps.
- Resuspend the pellet and incubate the suspension in 500 µL of 2 mM of ethylene glycol bis (succinimidyl succinate) (EGS) crosslinker diluted in a 1:1 mixture of glacial acetic acid:water for 1 h at RT.
NOTE: The preparation of the reagents at the time of use is highly recommended. To prepare the EGS, glacial acetic acid, and water must be mixed in equal proportions and heated to 70 °C. If the solution is not hot, the EGS is not completely resuspended.
- Incubate 1 × 106 whole inactivated M. tuberculosis cells (WCMtb, M. tuberculosis inactivated by gamma irradiation) with protein extract from 1 x 106 macrophages (50 µL) overnight at 4 °C in a rotating microtube holder.
- SLAMF1 staining
- Repeat step 3.1.4 using 500 µL of 1x PBS in the first wash and 1 mL in the second.
- Stain the protein-bacteria complex for 30 min at 4 °C in the dark with an anti-human SLAMF1 antibody (as in step 1.3.3) by adding the antibody to the microtube and vortexing. Use the amount of antibody suitable for 1 x 106 cells (2.5 µL or 0.125 µg).
- Wash the cells to eliminate the excess of antibodies using FACS, resuspend the pellet in FACS, and acquire the sample in a flow cytometer (as in step 1.3.4).
NOTE: Perform the corresponding isotype or FMO control.
4. M. tuberculosis antigens labeling
- Resuspend Rhodamine B in ethanol at a stock concentration of 5 mg/mL (1000x). Store at -20 °C until use.
- Add 1 µL of Rhodamine B per 100 µL of M. tuberculosis Ags in a 1.5 mL microcentrifuge tube.
- Incubate for 45 min at RT under continuous agitation (vortex).
- Wash at least 3 times at 14,000 x g for 5 min at 24 °C using 1 mL of 1x PBS until the supernatant is transparent. The pellet (Mtb-R Ags) will look light pink.
- Resuspend the Mtb-R Ags in 100 µL of 1x PBS to restore the initial volume.
NOTE: Working under sterile conditions is recommended. Ensure that the staining is performed immediately before performing experiments.
5. M. tuberculosis -SLAMF1 interaction by fluorescence microscopy
- Bacteria-protein cross-linking
- Introduce 12 mm round coverslips into 24-well culture plates (1 coverslip per well) using round-nosed surgical tweezers.
NOTE: Clean the coverslips thoroughly with 70% ethanol. If desired, they can be autoclaved or sterilized under UV light for 30 min. - Incubate the coverslip with 400 µL of 10 µg/mL Poly-D-Lysine overnight at 4 °C. Cover the plate with aluminum foil to protect it from light.
- The next day, remove the plate from the refrigerator and wash the coverslip twice with 1 mL of water.
- Let the coverslip dry.
- Dilute 20 µL (2 x 106) of Mtb-R Ags in 180 µL of 1x PBS. The final 200 µL is enough to completely cover a coverslip.
- Incubate the coverslip with Mtb-R Ags for 1 h at 37 °C in a cell incubator.
- Discard the remaining liquid and wash twice with 1mL of 1x PBS.
NOTE: In this step, the correct adhesion of Mtb-R to the coverslip can be checked with a fluorescence microscope using the filters corresponding to the red channel. - Add 400 µL of blocking buffer (10% FBS in 1x PBS) for 30 min at RT in agitation (shaker).
- Wash twice with 400 µL of blocking buffer.
- Incubate with 100 µL of protein extract diluted in 300 µL of 1x PBS for 2 h at RT in agitation (shaker).
- Add 400 µL of 1% formaldehyde diluted in 1x PBS. Incubate for 15 min under agitation (shaker) at RT.
- Add 500 µL of glycine 0.125 M diluted in water. Incubate for 5 min under agitation (shaker) at RT.
- Wash twice with 1 mL of 1x PBS.
- Add 400 µL of 2 mM EGS for 1 h at RT in agitation (shaker).
- Repeat step 5.1.13
NOTE: The preparation of the reagents at the time of use is highly recommended.
- Introduce 12 mm round coverslips into 24-well culture plates (1 coverslip per well) using round-nosed surgical tweezers.
- SLAMF1 staining
- Wrap the lid of the culture plate in paraffin film.
- Add a drop of 60 µL of previously titrated anti-SLAMF1 primary antibody (1:75 dilution) on the paraffin film-covered lid.
- Carefully remove the coverslip from the plate using curved fine-tip surgical tweezers and needles.
NOTE: To facilitate coverslips removal, the needle can be bent at an approximate 45° angle. - Invert the coverslip over the drop containing the antibody. Incubate for 30 min at RT in the dark.
- Lift the coverslip and wash twice with the blocking buffer.
- Invert the coverslip over a new drop containing the secondary antibody (1:200 dilution). Incubate for 30 min at RT in the dark.
- Repeat step 5.2.5.
- Remove the excess liquid and mount the coverslip on a drop of mounting liquid placed on a glass slide.
- Observe under the fluorescence microscope using adequate filters.
NOTE: Working under sterile conditions is recommended. Dilute the antibodies in the blocking buffer. Handle the coverslip carefully to avoid scratches that may hinder observation under the microscope. A mounting fluid suitable for fluorescence is highly recommended.
Results
In this work, a protocol that allows the evaluation of M. tuberculosis interaction with the immune receptor SLAMF1 in human macrophages is provided (Figure 1). To this end, peripheral blood from healthy donors was obtained. Then, PBMCs were separated by centrifugation over the density gradient media, and the monocytes were isolated by magnetic positive selection (≥95% purity, Figure 2A,B). The isolated monocytes were adhered to plastic culture plates for 2 h to obtain monocyte-derived macrophages and then cultured ON. Afterward, macrophages were stimulated with sonicated M. tuberculosis (Mtb Ags) to induce SLAMF1 surface expression as reported before10, and surface SLAMF1 levels were confirmed by flow cytometry (Figure 2C). The macrophages were finally lysed using RIPA lysis buffer combined with vortexing and incubation on ice to facilitate cell lysis. The total protein extract generated was used in the interaction assays.
In the first set of experimental procedures, flow cytometry was employed to study SLAMF1 interaction with whole cells of M. tuberculosis (WCMtb) (Figure 3). First, the physical interaction between SLAMF1 (content in the protein extract) and WCMtb was promoted by incubating them together ON in continuous rotation at 4 °C. Cross-linking treatment was then performed and, by using a specific anti-SLAMF1 antibody, the SLAMF1 receptor that was bound to WCMtb was detected. In this assay, WCMtb alone was used to set the voltages corresponding to the size and granularity of M. tuberculosis in the flow cytometer, which was the population of interest in which to detect SLAMF1 binding.
In the second experimental approach, fluorescence microscopy was employed to detect SLAMF1-M. tuberculosis interaction (Figure 4). This assay, in which sonicated M. tuberculosis (Mtb Ags) was used, complements the previous one. Rhodamine B-stained Mtb Ags (Mtb-R Ags) were used to facilitate fluorescence detection in the channel/filter corresponding to the PE fluorochrome. Mtb-R Ags were attached to poly-D-lysine coated coverslips, and the successful staining was checked by microscope observation (Figure 4A). Thereafter, the slides coated with Mtb-R Ags were incubated with the protein extracts containing SLAMF1. After cross-linking treatment, SLAMF1 was detected by using a primary specific anti-SLAMF1 antibody followed by a secondary antibody coupled to a compatible fluorochrome to be co-observed with PE. After obtaining individual fluorescent microscopy images in the two channels for Mtb-R Ags and SLAMF1, the interaction was corroborated by image merge (Figure 4B).
Performing these approaches demonstrated the existence of an interaction between SLAMF1 and M. tuberculosis. The crosstalk of SLAMF1 with both whole and sonicated bacteria was detected, which gave strength to the hypothesis that points to SLAMF1 as a new innate human macrophages' receptor for M. tuberculosis10.

Figure 1: Workflow to detect SLAMF1-M. tuberculosis interaction. Peripheral blood was obtained from healthy donors by venipuncture and centrifuged over a density gradient media to isolate peripheral blood mononuclear cells (PBMCs). After determining the number of cells, the PBMCs were subjected to positive magnetic selection to purify CD14pos monocytes. Monocytes were then adhered to culture plates for 2 h in the absence of fetal bovine serum and then cultured overnight in supplemented RPMI (resting step) to obtain monocyte-derived macrophages. Afterward, macrophages were stimulated with sonicated M. tuberculosis (Mtb Ags) for 24 h to induce SLAMF1 surface expression. SLAMF1 levels were tested by flow cytometry using a human anti-SLAMF1 specific antibody in a fraction of the macrophage population. The remaining macrophages were used to obtain total protein extracts. Cell lysis was performed by combining vortexing and incubations on ice in a lysis buffer. Finally, the interaction between SLAMF1 and M. tuberculosis was evaluated by flow cytometry and fluorescence microscopy after cross-linking macrophage proteins with Mtb Ags or whole cells of M. tuberculosis (WCMtb). Please click here to view a larger version of this figure.

Figure 2: Experimental and technical controls. (A) CD14pos monocytes were isolated from PBMCs by positive magnetic selection. The resulting positive (monocytes, blue) and negative (red) cell fractions were evaluated by flow cytometry (SSC-A vs. FSC-A) to confirm a successful selection. (B) The purity of the positive fraction was corroborated by flow cytometry analyzing cell granularity and size (SSC-A vs. FSC-A). (C) Monocyte-derived macrophages were selected by SSC-A vs. FSC-A and then gated to exclude doublets by a double singlets-strategy (FSC-A vs. FSC-H followed by SSC-A vs. SSC-H). SLAMF1 surface expression was evaluated in single cells. In all cases (A-C), representative plots or histograms are shown. Please click here to view a larger version of this figure.
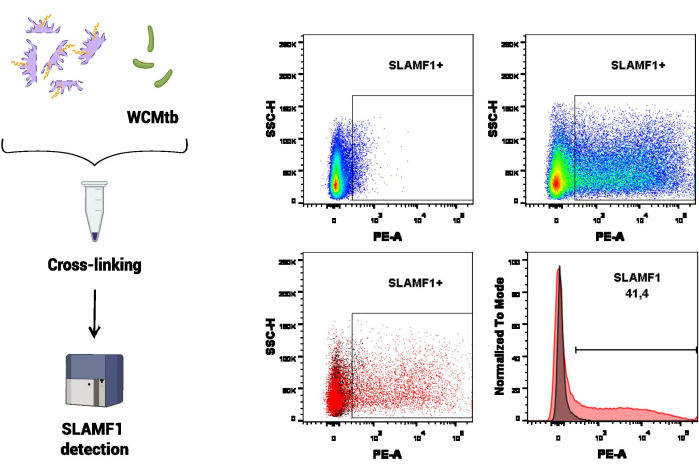
Figure 3: SLAMF1-M. tuberculosis interaction by flow cytometry. Cross-linking between macrophage proteins and whole cells of M. tuberculosis (WCMtb) was performed by treatment with formaldehyde/glycine/ethylene glycol bis, as described in the protocol section. Then, the interaction was evaluated by flow cytometry by detecting a positive SLAMF1 (PE) signal after selecting the population of interest (SSC-A vs. FSC-A). The upper panel shows a positive signal for SLAMF1, comparing the fluorescence minus one (FMO) control (left) with the positive staining (right). The low panel shows the overlay (left, dot plot and right, histogram) between the SLAMF1 signal and FMO control. Please click here to view a larger version of this figure.

Figure 4: SLAMF1-M. tuberculosis interaction by fluorescence microscopy. (A) Mtb Ags were stained with Rhodamine B (Mtb-R Ags) and attached to a round coverslip. The correct adhesion and fluorescence of Mtb-R Ags were corroborated by fluorescence microscopy using filters corresponding to the red channel. (B) Cross-linking between macrophage proteins and Mtb-R Ags was performed by treatment with formaldehyde/glycine/ethylene glycol bis as described in the protocol section by adding the protein extract over the attached Mtb-R Ags. The interaction was evaluated by fluorescence microscopy using a human anti-SLAMF1 antibody followed by Alexa Fluor 488-labeled secondary antibody. The upper micrographs show SLAMF1 (left, green) and Mtb-R Ags (right, red) positive signals. The low micrograph evidences the interaction in yellow (merge of both channels). The scale bars correspond to 10 µm. Please click here to view a larger version of this figure.
Discussion
This study provides a useful guide for studying the biochemical interaction between M. tuberculosis and microbial sensors expressed in human macrophages, a key cell type involved in the host response during Tuberculosis. The provided protocols will be relevant to decipher molecules that play a role in the entry of M. tuberculosis into phagocytes.
Characterizing bio-molecular interactions, such as that between pathogens and immunoreceptors, is crucial to understanding both immunoprotective mechanisms and evasion strategies elicited by M. tuberculosis. Many times, demonstrating a direct interaction of the receptor with its bacterial ligand can be complex and may demand sophisticated techniques or systems lacking versatility. Driessen et al. studied the role of phosphatidylinositol mannosides in the interaction between Mycobacteria and DC-SIGN using mutant deficient strains of M. bovis BCG32. Although they managed to study these interactions, the authors did not find differences for the mutant strains and discussed that the creation of mutant bacteria that show reduced binding would be an enormous task32. Other authors carried out excellent and laborious work to demonstrate the interaction between TLR2 and some PE/PPE proteins of M. tuberculosis33,34. These studies required the use of knockout mice for the receptor, transfection of cells, and purification of the aforementioned proteins or certain protein domains33,34. In addition, the function of the receptors is often inferred, but the interaction is not reliably demonstrated. An example is the soluble receptor PTX3, for which haplotypes associated with disease outcome have been found, but whose specific interaction with M. tuberculosis has not been strictly studied35. In relation to these shortcomings, our method allows for evaluating the interaction of the target receptor under study with M. tuberculosis in a simple way and with accessible techniques of easy interpretation.
Regarding the previous points, and particularly for SLAMF1, the interactions with OmpC and OmpF from E. coli36 and Omp25 from B. abortus37 have been previously evaluated. Degos et al.37 used COS-7 cells transfected with a plasmid encoding only the extracellular domain of SLAMF1 to then purify SLAMF1 and analyze the interaction with Omp25 by Western blot. They also performed experiments using SLAMF1-/- mice and wild-type B. abortus or an Omp25-defective mutant. Berger et al.36 work also showed compelling evidence for a direct interaction between SLAMF1 and E. coli Omp and S. typhimurium SseB-. They used different approaches, including SLAMF1-/- mice, RAW264.7 macrophages encoding a SLAMF1mCherry construct, and also an amplification assay using SLAMF1-transfected Jurkat T cells. They developed a sensitive signal amplification assay with a luciferase reporter using 1 x 108 E. coli. In this regard, one strength of the biochemical interaction assays presented in this report is that the interaction was actually found by using wild type cells and the same ratio of cells:bacteria used for functional evaluations in a previous work10, without the need to overexpress or abrogate SLAMF1 expression.
The provided protocol entails some critical steps. One of them is the corroboration of the SLAMF1 expression in human macrophages before performing the interaction assays. This is crucial to avoid obtaining a false negative result, which would actually be due to a lack of receptor expression. Here, SLAMF1 levels were checked using flow cytometry since it is a rapid technique that allows obtaining this data in less than 2 h. However, other techniques, such as microscopy or Western blot, could be used. We do not recommend methodologies such as real-time PCR that identify mRNA levels when the ultimate goal is to study a receptor at the protein level. Another fundamental step is the cross-linking stage. The use of crosslinkers seeks to stabilize protein-protein interactions. During the assay based on flow cytometry, we were not able to detect a signal for SLAMF1 without applying the cross-linking step. In the case of the microscopy approach, we have observed interaction without the use of cross-linking treatment but with very low sensitivity. Therefore, the cross-linking step is highly recommended in all cases.
The described protocol provides evidence of interaction between SLAMF1 and M. tuberculosis antigens accessible on the surface of the pathogen, helping to demonstrate that SLAMF1 recognizes a molecular signature present in M. tuberculosis. However, this model shows some limitations. The weak point is that this protocol does not allow us to identify which specific M. tuberculosis antigen is the one that interacts with SLAMF1. Despite this, the protocol could be modified to achieve this end, for example, using purified antigens from the bacteria. Another limitation is the need to know which stimulus induces the expression of SLAMF1 in macrophages. This could imply a complication if the chosen receptor is not expressed in basal conditions or for which the expression pattern is unknown. Finally, these approaches do not discriminate whether SLAMF1 requires other molecules to interact with M. tuberculosis. In this case, immunoprecipitation assays could be performed, or more antibodies could be used in the methodological scheme.
The methodology proposed in this report can be easily adapted to the study of other immune receptors, of the same receptor in other cell types in which it is expressed, or to evaluate interaction with other bacteria or strains of different mycobacteria. Moreover, here, a short and simple protocol is provided to fluorescently stain sonicated M. tuberculosis, a labeling that could be applied to other bacteria and even live strains. However, this procedure focuses on SLAMF1-M. tuberculosis crosstalk, other potential uses include understanding the outcome of a blocked, delayed, or inappropriate interaction, studying evasion mechanisms, or revealing potential molecular targets involved in the recognition of pathogens. Similarly, this protocol could be applied to the study of novel therapeutic strategies and immunotherapies to understand the coevolution of M. tuberculosis and macrophages or other host cells and could also be used in different fields that seek to understand ligand-receptor dynamics.
Disclosures
The authors declare no conflicts of interest.
Acknowledgements
This work was supported by Universidad Nacional del Noroeste de la Provincia de Buenos Aires (grant numbers SIB 0618/2019, SIB 2582/2012 to V.P.), Agencia Nacional de Promoción Científica y Tecnológica (ANPCyT, grant numbers PICT-2012-2459 and PICT A 2017-1896 to V.P. and PICT-2021-I-INVI-00584 to A.B.); Florencio Fiorini Foundation; and Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET, grant number PIO 15720150100010CO to V.P.). We thank Natalia Menite and Gastón Villafañe for the technical support. We acknowledge Dr. Paula Barrionuevo and Dr. Luciana Balboa for the scientific discussion during the publication that gave rise to the development of the assays presented in this work. We finally thank Dr. Estermann for his advice on cross-linking protocols, Lic. Moroni for his advice on working with Poly-Lysine and Lic. Moriconi for his help with schematic figures.
Materials
| Name | Company | Catalog Number | Comments |
| Alexa Fluor 488 secondary antibody | Invitrogen | A21121 | For fluorescence microscopy |
| anti-SLAMF1 FITC antibody | eBioscience | 11-1509-42 | For flow cytometry |
| anti-SLAMF1 PE antibody | BioLegend | 306308 | For flow cytometry |
| anti-SLAMF1 primary antibody | BioLegend | 306302 | For fluorescence microscopy |
| Aqua-Poly/Mount | Polysciences | 18606-20 | Mounting media |
| CD14 MicroBeads | Miltenyi Biotec | 130-097-052 | For monocytes isolation |
| Coverslips 12mm | HDA | - | For interaction assay by microscopy |
| EGS | ThermoFisher Scientific | 21565 | For crosslinking treatment |
| FACSCanto II | BD Biosciences | 338960 | Flow cytometer with BD FACSDiva software |
| Fetal Bovine Serum | Natocor | - | Inactivated and irradiated, for macrophages culture |
| Ficoll-Paque PLUS | Cytiva | 17144003 | For PBMCs separation |
| Fiji/ImageJ | Open Source software | - | For micrographs analysis |
| FlowJo 7.6.2 | Tree Star | - | For flow cytometry analysis |
| Formaldehyde | Merck | K47740803613 | For crosslinking treatment |
| Glass slides | Glass Klass | - | For interaction assay by microscopy |
| Glycine | Sigma | G8898 | For crosslinking treatment |
| Imager.A2 | Carl Zeiss | 430005-9901-000 | Fluorescence microscope with Colibri 7 illumination module |
| iMark | BIO-RAD | 1681130 | Microplate absorbance reader |
| L-glutamine | Sigma Aldrich | 49419 | For macrophages culture |
| M. tuberculosis, strain H37Rv, gamma-irradiated whole cells | BEI Resources, NIAID, NIH | NR-14819 | For interaction assay |
| M. tuberculosis, strain H37Rv, whole cell lysate | BEI Resources, NIAID, NIH | NR-14822 | For macrophages stimulation and interaction assay |
| Neofuge 13R | Heal Force | Neofuge 13R | High Speed Refrigerated Centrifuge for protein extraction |
| Penicillin/Streptomycin | Gibco | 15140122 | For macrophages culture |
| PMSF | ThermoFisher Scientific | 36978 | For proteins isolation |
| Poly-D-Lysine | Sigma Aldrich | A-003-M | For coverslips treatment |
| Protease Inhibitor Cocktail | Sigma Aldrich | P8340 | For proteins isolation |
| Rhodamine B | Sigma Aldrich | 21955 | For M. tuberculosis staining |
| RPMI 1640 | Gibco | 11875093 | For macrophages culture |
| Sorvall ST 16/16R centrifuge | ThermoFisher Scientific | 75004240 | For PBMCs and monocytes isolation |
References
- Global Tuberculosis Report 2023. , World Health Organization. Available at: https://www.who.int/tb/publications/global_report/en/ (2023).
- Ahmad, F., et al. Macrophage: A cell with many faces and functions in tuberculosis. Front Immunol. 13, 882130(2022).
- Bo, H., et al. Mycobacterium tuberculosis-macrophage interaction: Molecular updates. Front Cell Infect Microbiol. 13, 1187205(2023).
- Papp, A. C., et al. AmpliSeq transcriptome analysis of human alveolar and monocyte-derived macrophages over time in response to Mycobacterium tuberculosis infection. PLoS One. 13 (5), e0198069(2018).
- Zhai, W., Wu, F., Zhang, Y., Fu, Y., Liu, Z. The immune escape mechanisms of Mycobacterium tuberculosis. Int J Mol Sci. 20 (2), 340(2019).
- Van Crevel, R., Ottenhoff, T. H. M., Van der Meer, J. W. M. Innate immunity to Mycobacterium tuberculosis. Clin Microbiol Rev. 15 (2), 294-309 (2002).
- Mortaz, E., et al. Interaction of pattern recognition receptors with Mycobacterium tuberculosis. J Clin Immunol. 35 (1), 1-10 (2015).
- Stamm, C. E., Collins, A. C., Shiloh, M. U. Sensing of Mycobacterium tuberculosis and consequences to both host and bacillus. Immunol Rev. 264 (1), 204-219 (2015).
- Zihad, S. M. N. K., et al. Role of pattern recognition receptors in sensing Mycobacterium tuberculosis. Heliyon. 9 (10), e20636(2023).
- Barbero, A. M., et al. SLAMF1 signaling induces Mycobacterium tuberculosis uptake leading to endolysosomal maturation in human macrophages. J Leukoc Biol. 109 (8), 257-273 (2021).
- Pasquinelli, V., et al. Expression of signaling lymphocytic activation molecule-associated protein interrupts IFN-γ production in human tuberculosis. J Immunol. 172 (2), 1177-1185 (2004).
- Pasquinelli, V., et al. Phosphorylation of mitogen-activated protein kinases contributes to interferon-γ production in response to Mycobacterium tuberculosis. J Infect Dis. 207 (2), 340-350 (2012).
- Pasquinelli, V., et al. IFN-γ production during active tuberculosis is regulated by mechanisms that involve IL-17, SLAM, and CREB. J Infect Dis. 199 (5), 661-665 (2009).
- Pellegrini, J. M., et al. Neutrophil autophagy during human active tuberculosis is modulated by SLAMF1. Autophagy. 17 (2), 423-426 (2021).
- Song, T., Dong, C., Xiong, S. Signaling lymphocyte-activation molecule SLAMF1 augments mycobacteria BCG-induced inflammatory response and facilitates bacterial clearance. Int J Med Microbiol. 305 (6), 572-580 (2015).
- Eble, J. A. Titration ELISA as a method to determine the dissociation constant of receptor-ligand interaction. J Vis Exp. (132), e57032(2018).
- Patching, S. G. Surface plasmon resonance spectroscopy for characterization of membrane protein-ligand interactions and its potential for drug discovery. Biochim Biophys Acta Biomembr. 1838 (1), 43-55 (2014).
- Draczkowski, P., Matosiuk, D., Jozwiak, K. Isothermal titration calorimetry in membrane protein research. J Pharm Biomed Anal. 87, 296-304 (2014).
- Smith, D. S., Eremin, S. A. Fluorescence polarization immunoassays and related methods for simple, high-throughput screening of small molecules. Anal Bioanal Chem. 391 (5), 1499-1507 (2008).
- García-Nafría, J., Tate, C. G. Cryo-electron microscopy: Moving beyond X-ray crystal structures for drug receptors and drug development. Annu Rev Pharmacol Toxicol. 60, 51-71 (2020).
- Asami, J., Shimizu, T. Structural and functional understanding of the toll-like receptors. Protein Sci. 30 (4), 761-772 (2021).
- Phạm, T. T. T., Rainey, J. K. On-cell nuclear magnetic resonance spectroscopy to probe cell surface interactions. Biochem Cell Biol. 99 (6), 683-692 (2021).
- El Deeb, S., et al. Microscale thermophoresis as a powerful growing analytical technique for the investigation of biomolecular interaction and the determination of binding parameters. Methods Appl Fluoresc. 10 (4), 045001(2022).
- El Khamlichi, C., et al. Bioluminescence resonance energy transfer as a method to study protein-protein interactions: Application to G protein-coupled receptor biology. Molecules. 24 (3), 505(2019).
- Schneider, P., Willen, L., Smulski, C. R. Tools and techniques to study ligand-receptor interactions and receptor activation by TNF superfamily members. Methods Enzymol. 545, 103-125 (2014).
- Park, A. M. W., Schirmer, P. S. H. Atomic force microscopy: A multifaceted tool to study membrane proteins and their interactions with ligands. Biochim Biophys Acta Biomembr. 1838 (1), 74-89 (2014).
- Zalejski, J., Sun, J., Sharma, A. Unravelling the mystery inside cells by using single-molecule fluorescence imaging. J Imaging. 9 (9), 183(2023).
- Zheng, S., Zou, M., Shao, Y., Wu, H., Wang, X. Two-dimensional measurements of receptor-ligand interactions. Front Mol Biosci. 10, 1075587(2023).
- Zlotnikov, I. D., Kudryashova, E. V. Computer simulation of the receptor-ligand interactions of mannose receptor CD206 in comparison with the lectin concanavalin A model. Biochemistry (Moscow). 87 (1), 54-69 (2022).
- Fu, Y., Zhao, J., Chen, Z. Insights into the molecular mechanisms of protein-ligand interactions by molecular docking and molecular dynamics simulation: A case of oligopeptide binding protein. Comput Math Methods Med. 2018, 7604567(2018).
- Farina, B., et al. A novel approach for studying receptor-ligand interactions on living cells' surface by using NUS/T1ρ-NMR methodologies combined with computational techniques: The RGDechi15D-αvβ5 integrin complex. Comput Struct Biotechnol J. 19, 3303-3318 (2021).
- Driessen, N. N., et al. Role of phosphatidylinositol mannosides in the interaction between mycobacteria and DC-SIGN. Infect Immun. 77 (10), 4538-4547 (2009).
- Bansal, K., et al. Src homology 3-interacting domain of Rv1917c of Mycobacterium tuberculosis induces selective maturation of human dendritic cells by regulating PI3K-MAPK-NF-κB signaling and drives Th2 immune responses. J Biol Chem. 285 (47), 36511-36522 (2010).
- Xu, Y., et al. PPE57 induces activation of macrophages and drives Th1-type immune responses through TLR2. J Mol Med. 93 (6), 645-662 (2015).
- Olesen, R., et al. DC-SIGN (CD209), pentraxin 3 and vitamin D receptor gene variants associate with pulmonary tuberculosis risk in West Africans. Genes Immun. 8, 456-467 (2007).
- Berger, S. B., et al. SLAM is a microbial sensor that regulates bacterial phagosome functions in macrophages. Nat Immunol. 11 (10), 920-927 (2010).
- Degos, C., et al. Omp25-dependent engagement of SLAMF1 by Brucella abortus in dendritic cells limits acute inflammation and favours bacterial persistence in vivo. Cell Microbiol. 22 (4), e13156(2020).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved