Method Article
Diaphragmatic Ultrasound in Adults: Image Acquisition and Interpretation
In This Article
Summary
Point-of-care ultrasound (POCUS) is an essential technique for screening for diaphragmatic dysfunction due to its portability, non-invasiveness, and real-time imaging capabilities. Although current diaphragmatic POCUS protocols exist, they suffer from poor interoperator reliability and lack consensus guidelines. Here we describe a technique that is reproducible and simple to perform.
Abstract
Diaphragm dysfunction is a widely recognized concern across numerous medical specialties and clinical settings. Timely and accurate assessment of the diaphragm is vital not only in critically ill patients, where it has a role in weaning from mechanical ventilation and respiratory outcomes, but also in the perioperative arena as a diagnostic tool to detect phrenic nerve function. Diaphragmatic assessment has traditionally utilized fluoroscopy and nerve studies that are time-consuming, costly, and non-portable. Point-of-care ultrasound (POCUS) overcomes these barriers and can be used as a tool for non-invasive screening of diaphragm function. However, POCUS for diaphragmatic dysfunction currently suffers from several issues such as a lack of consensus guidelines, a multiplicity of protocols, and poor interoperator reliability among existing protocols, most notably with the assessment of dome of diaphragm excursion and diaphragmatic thickening. To address these issues, this manuscript reviews the available literature on diaphragmatic POCUS and identifies an image acquisition technique that is both simple to perform and has high interoperator reliability. This technique first describes a qualitative evaluation of diaphragm excursion, followed by a quantitative assessment of the excursion of the zone of apposition. The technique is described stepwise along with all the following: patient positioning, transducer selection, probe placement, image optimization, and interpretation.
Introduction
Diagnostic ultrasound can be separated into two divisions: consultative and point-of-care. Consultative ultrasound incorporates an exam performed by a distinct specialist team, whereas POCUS is both performed and interpreted by the clinician caring for the patient in real time1.
Over the past few decades, diagnostic POCUS has emerged as a transformative tool in modern medicine, with applications rapidly expanding across specialties. These POCUS applications are driven by ultrasound's noninvasive nature, portability, and real-time imaging capabilities. Further, within diagnostic POCUS, the applications that have achieved the highest uptake in clinical medicine tend to have both reasonably high accuracy compared to a gold standard and high interobserver reliability2,3. For instance, POCUS of the lung is well established to narrow the differential diagnosis of respiratory insufficiency and has clear evidence-based guidelines supporting its standardized use4. However, while POCUS of the lung is well established, there remains an unmet need to develop a reproducible sonographic assessment of the diaphragm.
Such a non-invasive diaphragmatic assessment protocol would benefit multiple specialties and clinical situations, including but not limited to, critical care, pulmonology, perioperative care (including both general-purpose anesthesia and subspecialty regional anesthesia contexts), and neurology. In the intensive care unit, diaphragmatic dysfunction is a common concern, often arising from multiple underlying pathologies such as neuromuscular diseases, critical illness myopathy, trauma, and malnutrition5. Critically ill patients are often at high risk for both impaired contraction of the diaphragm and under-recognition of this phenomenon6. Further, diagnosing diaphragmatic dysfunction early is important, as not only can it assist in ventilation management strategies, but also dysfunction may be an early indicator of infection and sepsis7,8. In addition, prolonged intubation can lead to significant morbidity, mortality, and increased healthcare expenses2. In these scenarios, a noninvasive, portable protocol for diaphragmic assessment could be useful to assess the appropriateness for weaning from mechanical ventilation, evaluate work of breathing, and predict the probability of extubation success versus failure6,8,9,10,11.
Within regional anesthesia, diaphragmatic POCUS could have value in screening for diaphragmatic paresis related to transient phrenic nerve dysfunction from brachial plexus blocks. Although tolerated well by healthy patients, phrenic nerve palsies can lead to respiratory distress in patients with limited pulmonary reserve. Furthermore, in the perioperative arena, POCUS of the diaphragm can serve as a diagnostic tool for patients in the preoperative, intraoperative, and postoperative settings. For example, diaphragmatic POCUS could be used to detect phrenic nerve damage arising from a wide range of procedures, including but not limited to, coronary artery bypass grafting with internal mammary artery harvesting, atrial fibrillation ablation, and cervical or thoracic surgeries3,12.
Finally, within the specialty of neurology, POCUS could facilitate the assessment of diaphragmatic function in neurologic diseases such as Myasthenia gravis, Duchene's muscular dystrophy, amyotrophic lateral sclerosis, and cerebrovascular accidents13.
Accurate assessment of the diaphragm is essential due to its vital role in reparatory function. Oxygenation and ventilation depend on the generation of negative intrathoracic pressure created by the diaphragm, a dome-shaped muscle separating the abdomen and thorax that is composed of several muscular and tendinous membranes14,15. The diaphragm has at least two major components that can be distinguished on ultrasound: the dome of the diaphragm (DoD) and the zone of apposition (ZOA). The DoD is the central tendinous portion that exhibits a hyperechoic and curved appearance on ultrasound. The ZOA is the lateral portion of the diaphragm that attaches to the rib cage and consists of muscular fibers that run parallel and proximal to the inner surface of the chest wall3,15. The ZOA is thin (usually <1 cm in thickness), but it increases in thickness during inspiration as the diaphragm contracts. At the ZOA, the diaphragm has a characteristic appearance on ultrasound with three layers, including an anechoic muscular layer that is bounded externally by the superficial hyperechoic parietal pleural and internally by the deep hyperechoic peritoneum3,13.
Several noninvasive sonographic protocols have been proposed for diaphragmatic assessment, involving both qualitative and quantitative approaches. Qualitative visual assessment, the simplest approach, entails the evaluation of diaphragmatic motion bilaterally, during either tidal or vital capacity breathing, using two-dimensional ultrasound, also known as Brightness mode (B-mode). In contrast, quantitative protocols typically begin with B-mode and add one-dimensional ultrasound -- also known as Motion Mode (M-mode) -- to measure one of two things: the excursion of the dome of diaphragm (DoD) and/or Diaphragmatic thickening2,3,5,13. Measurement of DoD excursion is performed with a low-frequency transducer with the ultrasound beam directed through the posterior third of the hemidiaphragm at a perpendicular angle. M-mode is then utilized to measure excursion during vital capacity breathing.
Alternatively, the measurement of diaphragmatic thickening employs a high-frequency linear transducer in two steps. First, the high-frequency transducer is placed along the patient's flank overlying the diaphragm with B-mode to identify the zone of apposition (ZOA)3. Second, estimation of diaphragmatic thickening is performed using M-mode by measuring diaphragmatic thickness (in millimeters) from the visceral to parietal pleura and calculating the change in thickness by the following equation2,3,5,13:
Change in thickness = (Thickness at end inspiration - Thickness at end expiration) / Thickness at end expiration
However, quantitative methods (DoD excursion and diaphragmatic thickening) suffer from poor interoperator reliability. Interoperator reliability is low for the measurement of DoD excursion for several reasons. First, providers have difficulty finding a consistent angle of visualization of the excursion of the dome of the diaphragm3. Second, assessing on the left side is frequently challenging due to the small acoustic window through the spleen2,16. For example, studies have cited that identification of left-sided diaphragmatic excursion is not possible in up to 65-79% of cases17. Third, intraabdominal contents and patient positioning can influence the range of diaphragm excursion2.
Similarly, the measurement of diaphragmatic thickening has low interoperator reliability for at least two reasons. First, the natural thinness of the diaphragm causes millimeter errors in measurement to be consequential. Second, the diaphragm's variability in thickness across rib interspaces and by the patient's laterality further causes measurement dispersion2,3,17. In acknowledgment of these many limitations, in 2022, an expert consensus on diaphragm ultrasonography in critically ill patients concluded that current methods were not standardized and that many required a skilled sonographer18. They noted there was no agreement on cutoff values for diaphragm dysfunction based on thickening fraction and that measurement of thickening fraction is a difficult skill with a steep learning curve13,18. Furthermore, the use of multiple different sonographic protocols in literature has added to inherent challenges by making the comparison of studies difficult, leading to heterogeneity in research19.
To address these issues, this manuscript reviews the available literature on diaphragmatic POCUS and identifies an image acquisition technique that is both simple to perform and has been shown to have high interoperator reliability. This feasible yet effective protocol begins with a qualitative evaluation of diaphragmatic excursion, followed by a recently validated quantitative assessment of excursion of the cranial-most point of the ZOA17,19.
Protocol
All procedures performed in studies involving human participants were in accordance with the ethical standards of the Duke University Health System institutional research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all participants. Supplemental File 1 contains the most important still images from each video.
1. Phase 1: Qualitative assessment of diaphragmatic excursion (Visual screening for gross hemidiaphragmatic dysfunction)
- Machine setup and patient positioning
- Probe selection: Select a low-frequency (≤ 5 MHz) transducer (curvilinear or sector array [aka "phased-array"]19.
- Apply ultrasound coupling gel to the probe.
- Instrument settings: Select abdominal preset.
- Position the patient in the semirecumbent position.
- Scanning technique
- Right hemidiaphragm assessment
- Place the probe on the right flank, 5-7th intercostal space, mid-axillary line with the beam aligned with the coronal plane of the body and probe indicator pointing cranially (Figure 1 and Figure 2A).
- Adjust the probe positioning (slide, fan, rock as needed) until the view is centered on the diaphragm with the following structures also visible: sub-diaphragmatic organ (liver or spleen), diaphragm, spine, and supra-diaphragmatic space (i.e., pleural space)19 (Figure 2B,C).
- Ask the patient to take a slow vital capacity breath in and a slow breath out.
- Click Acquire (or equivalent) to capture a short clip during patient respiration.
- Visually assess diaphragmatic excursion as one of the following: Grossly intact (Video 1 and Video 2), Grossly absent (Video 3 and Video 4), or Indeterminate (Video 5 and Video 6).
- If indeterminate or further quantification is needed, proceed to section 2 (Phase 2) of the protocol.
- Left hemidiaphragm assessment: repeat steps 1.2.1.1-1.2.1.6 on the patient's left side.
- Right hemidiaphragm assessment
2. Phase 2: Quantitative assessment of ZOA excursion
- Machine setup and patient positioning
- Probe selection: Select a high-frequency (>10-13 MHz) linear transducer.
- Apply ultrasound coupling gel to the probe.
- Instrument settings: Select musculoskeletal (MSK) preset if available. If MSK preset is not available, select any preset and use the same preset for all high-frequency diaphragmatic scanning.
- Position the patient in the semirecumbent position (repeat step 1.1.4).
- Scanning technique
- Right hemidiaphragm assessment
- Place the probe at the mid-axillary line at the level of the eighth or ninth intercostal spaces, with the probe indicator pointing cephalad towards the patient's head (Figure 3 and Figure 4A).
- Angle the beam perpendicular to the chest wall and center the axis so that the rib interspace is centered in the screen with the cranial and caudal ribs visible on the edges of the screen (Figure 4B).
- Set the depth such that the pleural line or diaphragm is visible in the middle third of the screen.
NOTE: Typically, this means a depth of 3-5 cm but can be greater if there is additional subcutaneous tissue. - Set the gain such that the diaphragm/pleural line are visibly distinct from surrounding structures.
- Identify the pleural line on the screen.
- Measure the end-inspiratory location of the ZOA.
- Give the patient the following instructions: "Breathe in fully and then hold your breath in for 4 s. If you cannot tolerate 4 s, then please hold your breath for any amount of time you're comfortable."
- During the patient's breath hold, follow the pleural line caudally until the location is reached where the pleural line is visible in only a portion of the rib interspace, and the remaining interspace contains the diaphragm at a similar depth as the pleural line (Video 7 and Video 8).
NOTE: This rib interspace simultaneously containing pleura and diaphragm has been termed the Zone of Apposition (ZOA). - Adjust the probe positioning (slide, fan, rock as needed) until the view is centered on the ZOA with the following structures also visible: the subcutaneous tissue above and a rib on either size of the screen (Figure 4C).
- Using a non-permanent skin marker, draw a line on the patient that is perpendicular to the ultrasound transducer's long axis and bisects the probe to mark the interspace where the ZOA was found (Figure 5A). The marking should be aligned with the ZOA, at the transition between the pleura and diaphragm (Figure 4B,C).
- Ask the patient to exhale and then "breathe normally" (aka tidal breathing).
- If unsure that one has identified the ZOA, repeat steps 2.2.1.6.1-2.2.1.6.3 and examine the presumed diaphragm in this view to see its changes during the respiratory cycle.
NOTE: The true diaphragm should thicken during inspiration and decrease in thickness during expiration. - Repeat the measurement once (i.e., steps 2.2.1.6.1-2.2.1.6.5).
- Take the average of the two measurements and use this for the final value of the end-inspiratory location of the ZOA (Figure 5B).
- Measure the end-expiatory location of the ZOA.
- Give the patient the following instructions: "Breathe in fully, then breathe out all the way, and then hold your breath out for 4 s. If you cannot tolerate 4 s, then please hold your breath for any amount of time you're comfortable."
- Slide the probe cranially to find the end-expiratory location of the ZOA.
- Repeat steps 2.2.1.6.3-2.2.1.6.4.
- Ask the patient to "breathe normally."
- Repeat the measurement once (i.e., steps 2.2.1.7.1-2.2.1.7.4).
- Take the average of the two measurements and use this for the final value of the end-expiratory location of the ZOA.
- Measurement of ZOA excursion
- Measure the distance between both the averaged end-inspiratory and averaged end-expiratory skin markings in cm with a ruler. The distance between the two external skin marking represents maximal diaphragmatic excursion (Figure 5C).
- Left hemidiaphragm assessment
- Repeat all the sub-steps contained within step 2.2.1 on the left chest.
- Right hemidiaphragm assessment
Results
This diaphragmatic ultrasound protocol begins with the qualitative assessment of each hemithorax during a vital capacity breath to sort each hemidiaphragm into one of three categories: grossly intact excursion, grossly impaired excursion, or indeterminate. Examples of grossly normal vital capacity excursion of the right and left hemidiaphragms are shown in Video 1 and Video 2, respectively. Examples of grossly impaired vital capacity excursion of the right and left hemidiaphragms are shown in Video 3 and Video 4, respectively. Examples of qualitatively indeterminate vital capacity excursion of the right and left hemidiaphragms are shown in Video 5 and Video 6, respectively.
The qualitative assessment is likely to suffice to answer the question of whether there is gross hemidiaphragmatic dysfunction in most cases. However, if either the qualitative exam yields indeterminate results or the clinician needs more granular data about hemidiaphragmatic function (e.g., to quantify the effect of a regional nerve block on hemidiaphragmatic function as part of a research study), then this protocol calls for quantitative measurement of the excursion of the cranial-most portion of the ZOA. Currently, ZOA excursion normal values have a paucity of validation data.
However, some inferences can be drawn from normal values of a related measurement: dome of diaphragm (DoD) excursion. DoD excursion has been shown to have a variable range, depending on the study. For example, a narrative review by Boussuges et al. found that normal DoD excursion varies depending on sex and laterality3,13. Specifically, the normal range of DoD excursion for tidal breathing in men ranges from 1.4 cm to 2.3 cm on the right and from 1.7 cm to 2.4 cm on the left; for women, it ranges from 1.4 cm to 2.7 cm on the right and from 1.6 to 2.4 cm on the left. Similarly, for vital capacity breaths, normal DOD excursion in men ranges from 5.3 cm to 7.8 cm on the right to 5.4 cm to 7.8 cm on the left; for women it ranges from 4.7 cm to 8.0 on right and from 4.8 cm to 6.4 cm on the left3,13.
Notably, for the quantitative method of measuring ZOA excursion described in this manuscript, absolute normal values of ZOA excursion have yet to be firmly established. Further, published normal values for the DoD excursion method also should not be used to differentiate normal versus abnormal for the ZOA excursion method because a clear formula relating these two values has not been delineated. However, a major principle of diaphragmatic assessment is within-subject comparison: (a) left versus right (b), pre versus post intervention, and (c) monitoring change over time. Such comparisons are permitted by the ZOA excursion method even in the absence of published normal values for this technique.
For example, Kim et al. have proposed using the ZOA excursion measurement to sort patients into three categories of diaphragmatic dysfunction by comparing hemidiaphragmatic excursion pre versus post phrenic-nerve blunting intervention20: i) complete phrenic nerve dysfunction: either >75% decrease in ZOA excursion after intervention or paradoxical movement of the diaphragm; ii) partial phrenic nerve dysfunction: 25–75% decrease in ZOA excursion after intervention; iii) minor or no change in diaphragmatic function: 0–25% decrease in ZOA excursion after intervention.

Figure 1: Representation of the qualitative assessment protocol of gross diaphragm excursion. Note the anatomic illustration of the diaphragm (bright green line) overlying the liver (left) and spleen (right). To visualize the diaphragm for qualitative assessment, the curvilinear probe should be placed at the mid-axillary line with beam aligned with the coronal plane of the body and the patient in a semirecumbent position. Reprinted with permission from masterthemachines.com. Please click here to view a larger version of this figure.

Figure 2: Qualitative assessment of diaphragmatic excursion. (A) Proper probe positioning with the low-frequency transducer (curvilinear or sector array) placed on the right or left flank at the 5–7th intercostal space, mid-axillary line, and probe indicator pointing cranially. (B) Identification of the right hemidiaphragm in B-mode during qualitative assessment. The diaphragm is visible as a bright hyperechoic band overlying the liver. For an optimal view, the sub-diaphragmatic spine should be visible. (C) Identification of the left hemidiaphragm in B-mode during qualitative assessment. The spleen is seen here in place of the liver. Once the diaphragm is initially visualized, the motion of the diaphragm can then be observed to assess for gross hemidiaphragmatic dysfunction. Refer to Video 1, Video 2, Video 3, Video 4, Video 5, and Video 6 for examples or both right and left normal, abnormal, and indeterminate diaphragm excursion. Please click here to view a larger version of this figure.

Figure 3: Representation of the protocol for quantitative assessment of ZOA excursion. Note the anatomic illustration of the end-expiratory diaphragm as a dark blue line and the end-inspiratory diaphragm as a light blue line. To visualize the ZOA for quantitative assessment, the linear probe should be placed at the mid-axillary line with the beam aligned perpendicular to the torso and the patient in a semirecumbent position. Reprinted with permission from masterthemachines.com. Abbreviation: ZOA = zone of apposition. Please click here to view a larger version of this figure.
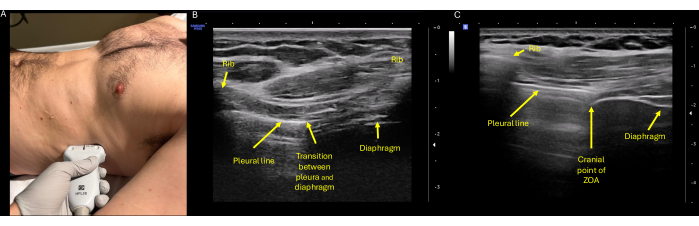
Figure 4: Ultrasound assessment of ZOA excursion. (A) Proper probe positioning with the high-frequency (linear) transducer placed on the right or left flank at the mid-axillary line at the level of the eighth or ninth intercostal spaces, with the probe indicator pointing cephalad towards the patient's head. (B) Identification of the ZOA, which is defined as the lateral portion of the diaphragm that attaches to the rib cage. On ultrasound it can be identified at the transition point of the diaphragm and pleura, shown here centered between two rib spaces. (C) The cephalad most aspect of the ZOA, again corresponding to the rib interspace that shows aerated lung and diaphragm concurrently. Here is the characteristic three-layered appearance of the diaphragm, with the superficial hyperechoic parietal pleura externally, the middle anechoic muscular layer, and the deep hyperechoic peritoneum. Abbreviation: ZOA = zone of apposition. Please click here to view a larger version of this figure.

Figure 5: Marking and measurement of ZOA excursion. (A) Once the ZOA is identified after full exhalation from a vital capacity breath, a perpendicular line is drawn from the center of the probe to mark the initial location of the end-expiratory ZOA. (B) Once the steps are repeated and the ZOA is again identified after exhalation from a second vital capacity breath, a new line is drawn from the center of the probe. This marks the second identified location of the end-expiratory ZOA. Subsequently, the distance between the two measurements is averaged, and the average line is used as the final value of the end-expiratory location of the ZOA. The same steps are repeated to obtain the end-inspiratory markings of the ZOA. The end-inspiratory and end-expiratory markings can be obtained in either order. (C) The distance between the averaged end-expiratory and averaged end-inspiratory skin markings is measured in centimeters with a ruler. This distance represents the quantified diaphragmatic excursion. Abbreviation: ZOA = zone of apposition. Please click here to view a larger version of this figure.
Figure 6: Probe maneuver to overcome rib shadowing. The linear probe can be rotated between two rib spaces for ZOA identification in the case of a rib obscuring the location of the ZOA. Abbreviation: ZOA = zone of apposition. Please click here to view a larger version of this figure.
Video 1: Coronal view obtained with a low-frequency transducer centered on the right hemidiaphragm showing qualitatively normal diaphragmatic excursion during vital capacity breathing. Please click here to download this video.
Video 2: Coronal view obtained with a low-frequency transducer centered on the left hemidiaphragm showing qualitatively normal diaphragmatic excursion during vital capacity breathing. Please click here to download this video.
Video 3: Coronal view obtained with a low-frequency transducer centered on the right hemidiaphragm showing gross absence of diaphragmatic excursion during attempted vital capacity breathing. Please click here to download this video.
Video 4: Coronal view obtained with a low-frequency transducer centered on the left hemidiaphragm showing gross absence of diaphragmatic excursion during attempted vital capacity breathing. Please click here to download this video.
Video 5: Coronal view obtained with a low-frequency transducer centered on the right hemidiaphragm showing a grossly submaximal (indeterminate) level of diaphragmatic excursion during attempted vital capacity breathing. Please click here to download this video.
Video 6: Coronal view obtained with a low-frequency transducer centered on the left hemidiaphragm showing a grossly submaximal (indeterminate) level of diaphragmatic excursion during attempted vital capacity breathing. Please click here to download this video.
Video 7: Coronal view obtained with a high-frequency linear transducer showing the normal motion of the cranial-most portion of the ZOA during tidal breathing. Abbreviation: ZOA = zone of apposition. Please click here to download this video.
Video 8: Coronal view obtained with a high-frequency linear transducer showing the transducer sliding caudal to follow the normal motion of the cranial-most portion of the ZOA during a vital capacity breath. Abbreviation: ZOA = zone of apposition. Please click here to download this video.
Video 9: Coronal view obtained with a high-frequency linear transducer showing how, at end-inspiration, the cranial-most portion of the ZOA may be hidden by a rib shadow. Abbreviation: ZOA = zone of apposition. Please click here to download this video.
Supplemental File 1: Reference to view the most important still images from each video. Please click here to download this file.
Discussion
POCUS offers clear advantages for diaphragmatic assessment, including portability, non-invasiveness and real-time imaging capabilities. These strengths can be taken advantage of with this feasible and accessible protocol and can be applied in a variety of clinical settings. This protocol begins with a qualitative assessment of diaphragmatic excursion to answer the question of whether gross hemi-diaphragmatic dysfunction is present. If the answer is unclear or if more specific information is needed, the second step of the protocol provides clarification via quantitative measurement of the excursion of the ZOA. These measurements can aid in clinical decision-making by utilizing within-subject comparison of laterality, pre vs. post intervention, or assessment of change over time.
Traditionally, diaphragmatic assessment has relied on methods such as chest X-ray, fluoroscopy, CT imaging, phrenic nerve conduction studies, and transdiaphragmatic pressure measurements. While these techniques provide valuable information, they are often time-intensive, costly, lack portability, and sometimes only offer static imaging2. Ultrasound offers advantages over these shortcomings. Notably, there are some advanced ultrasound approaches on the horizon for diaphragmatic assessment that involve two-dimensional speckle tracking imaging and shear wave elastography. These approaches assess tissue mechanical properties of the diaphragm and have experimental evidence supporting their validity3. However, speckle-tracking and shear wave elastography require advanced sonography skills and equipment that are rarely available to the common clinician.
Alternatively, many authors have advocated for the use of quantitative POCUS to assess diaphragmatic function. As explained in the introduction, the two techniques traditionally used for this purpose are measurement of DoD excursion and diaphragmatic thickening. These protocols are well-described and their clinical correlation continues to be researched3,6,13,21. Protocols simplifying methods of assessing diaphragm thickness and thickening fraction in both healthy and critically ill patients have recently been published, enabling these protocols to be accessible to clinicians with basic ultrasound knowledge21.
In recent years, evaluation of diaphragmatic function in critically ill patients has gained attention as its essential role in assessing respiratory outcomes has become increasingly evident. Patients requiring mechanical ventilation can develop diaphragm disuse atrophy and decreased contractile strength, which has been associated with increased mortality6,22. Diaphragm hypotrophy can present as early as 24 h within initiation of mechanical ventilation11. Thus, early recognition of diaphragm muscle dysfunction with non-invasive tools such as POCUS is vital. A study by Goligher et al. showed that in mechanically ventilated patients, POCUS assessment of the thickness of the right hemidiaphragm can be both feasible and reproducible23. However, they found that measurements of the thickness of the left hemidiaphragm and of the thickening fraction bilaterally were not reproducible. Both diaphragmatic thickening and DoD excursion are limited by poor interoperator reliability and measurement dispersion2,15,16.
Furthermore, expert consensus is lacking on which ultrasound mode is best to measure diaphragm thickness (B-mode vs. M-mode) and at which point of the respiratory cycle is optimal to perform the measurement18. In addition to operator challenges, patients themselves can also introduce confounding. In critically ill patients it is crucial to consider both the patient's pathology and physiology. For example, chronic diseases such as chronic obstructive pulmonary disease, interstitial lung disease, or neuromuscular disorders can cause decreased diaphragm mobility, whereas patients with congestive health failure and cystic fibrosis may have abnormally thickened diaphragms6,13. In addition, positive pressure ventilation may impact sonographic measurements by causing decreased diaphragm excursion and reduced thickening18.
Thus, there exists an unmet need for a simple, reproducible image acquisition protocol for diaphragmatic ultrasound that can be performed at the point of care. To address this unmet need, we have developed an evidence-based protocol that screens for diaphragmatic dysfunction in a two-step process: first qualitatively and then, if necessary, quantitatively with the latter step performed using a newly validated method of measuring the excursion of the cranial-most portion of the ZOA.
Qualitative assessment of diaphragm motion is a well-established technique that has been reported in the literature as early as the 1970s3. Qualitative evaluation in B-mode ultrasound requires no calculations and can often provide a binary answer (yes or no to whether there is grossly normal diaphragmic excursion). Variations of the qualitative approach have been tested in multiple studies and found to be useful for assessing gross asymmetry of hemidiaphragmatic function2,3,5,6,13,18,23.
In cases where an initial qualitative screening provides inconclusive data about the function of each hemidiaphragm, our proposed protocol moves on to a recently validated quantitative approach: measurement of the excursion of the cranial-most portion of the ZOA. This technique was first described in 2017 by El-Boghdadly and subsequently utilized by Kim et al. in 2019 to screen for diaphragmatic dysfunction after superior trunk nerve blocks19,20. More recently, this technique was tested and validated in 2023: Da Conceicao et al. compared this novel method to both measurement of DoD excursion and of diaphragmatic thickening fraction in 75 patients17. The authors found that measuring the excursion of the cranial-most portion of the ZOA excursion had a significantly higher success in measuring the excursion of both hemidiaphragms compared to the DoD method. The ZOA method had a 100% success rate bilaterally whereas the DoD method was only able to measure diaphragmatic excursion 98.7% on the right and 35% of the time on the left. While the thickening fraction method did demonstrate 100% success in being able to measure both diaphragms, the left and right thickening fraction values generated by this method neither correlated within patients nor correlated to the excursion measurements obtained using the DoD and ZOA methods. For the thickening fraction method, this lack of correlation both to other methods of assessing diaphragmatic function and between the left and right hemidiaphragms within subjects, raises concerns about the overall validity of this method as a diagnostic tool.
Since the ZOA excursion method remains new and unfamiliar to most providers, some troubleshooting suggestions may help to increase the probability of success. First, using a systematic approach, such as outlined in this protocol, is critical. Second, since initial identification of the ZOA may be challenging, it is important to ensure proper probe positioning and identify known anatomic landmarks. The probe should be between the anterior and mid-axillary line, and rib spaces should be centered on the screen. The ZOA can be identified by its characteristic three-layered appearance and is usually found at a depth of 1.5-3 cm (depending on the patient's BMI) and is often found below the costophrenic angle with inhalation2,16. If the ZOA is not identified, two possibilities are that it is hidden behind a rib shadow (Video 9) or is being continually obscured by the lung moving into the field19. The first possibility can be fixed by rotating the transducer obliquely to align with the intercostal spaces between the ribs (Figure 6). The second possibility can be addressed by sliding to a more caudal intercostal space.
Despite its numerous advantages, this diaphragmatic POCUS protocol has several limitations. First, the protocol requires visualization of the cranial-most portion of the ZOA, which may not be possible in the presence of diffuse subcutaneous emphysema, dressings, drains, tubes, or binders. Second, to date, the ZOA excursion method has only been tested against alternative approaches in a single study15. Although this single study demonstrated the relative superiority of ZOA excursion over two traditional quantitative approaches to diaphragmatic POCUS, the ZOA method has not been tested against a gold standard such as fluoroscopic assessment of diaphragmatic function. Such a comparison against a gold standard is needed to better define normal and abnormal values for the ZOA excursion approach. In addition, given its novelty, this protocol has not yet been correlated with clinical outcomes. However, until the ZOA method's normative values are firmly established, this technique can still be useful to quantify the extent of asymmetry in hemidiaphragmatic function in a variety of clinical situations where qualitative assessment alone is indeterminate. Third, this assessment provides generalized information of diaphragm function but lacks the specificity to provide information about disease states such as fibrosis or precise measures of contractility. Lastly, a basic level of POCUS knowledge is required to be able to successfully apply this protocol in clinical practice. However, these methods prove to be simpler than previous POCUS measures of diaphragm dysfunction (DoD excursion and diaphragmatic thickening) as they require fewer measurements and less familiarity with knobology. In addition, proficiency in this method can be quickly achieved with training. By following the outlined protocol and troubleshooting strategies, reproducible assessment of the diaphragm may become feasible for the average clinician using simple ultrasound equipment available at the point of care.
Disclosures
We have no relevant disclosures or conflicts of interest.
Acknowledgements
Thank you to Dr. Fintan Hughes for assisting with photography.
Materials
| Name | Company | Catalog Number | Comments |
| Medical Ruler | MediChoice | NA | We used Medichoice as that is what is readily available at our institution and it comes with the skin marker, however any medical ruler will work. The majority of skin markers come with a type of ruler or measurement system, but if not a separate ruler can be used. |
| Skin Marker | MediChoice | NA | We again used Medichoice as that is what is readily available at our institution and it comes with the ruler, however any standard skin marker will work. |
| Ultrasound Gel | Aquasonic | NA | Any standard gel will work. Sterile packs are not necessary but can be used on a case-by-case basis at the providers discretion. |
| Ultrasound Machine | Samsung and GE | NA | Any standard portable ultrasound machine will suffice. |
References
- Bronshteyn, Y. S., Blitz, J., Hashmi, N., Krishnan, S. Logistics of perioperative diagnostic point-of-care ultrasound: Nomenclature, scope of practice, training, credentialing/privileging, and billing. Int Anesthesiol Clin. 60 (3), 1-7 (2022).
- Osman Elew, A. N. E., Abd Alrahman, A. A. H., El Khayat, H. M. H. Diaphragmatic ultrasound: Review article. The Egyptian Journal of Hospital Medicine. 87 (1), 1006-1009 (2022).
- Boussuges, A., Rives, S., Finance, J., Bregeon, F. Assessment of diaphragmatic function by ultrasonography: Current approach and perspectives. World J Clin Cases. 8 (12), 2408-2424 (2020).
- Volpicelli, G., et al. International evidence-based recommendations for point-of-care lung ultrasound. Intensive Care Med. 38 (4), 577-591 (2012).
- Saad, M., et al. Ultrasonographic assessment of diaphragmatic function and its clinical application in the management of patients with acute respiratory failure. Diagnostics (Basel). 13 (3), 411 (2023).
- Santana, P. V., Cardenas, L. Z., Albuquerque, A. L. P. Diaphragm ultrasound in critically ill patients on mechanical ventilation-evolving concepts. Diagnostics (Basel). 13 (6), 1116 (2023).
- Petrof, B. J. Diaphragm weakness in the critically ill: Basic mechanisms reveal therapeutic opportunities. Chest. 154 (6), 1395-1403 (2018).
- Chu, S. E., et al. Point-of-care application of diaphragmatic ultrasonography in the emergency department for the prediction of development of respiratory failure in community-acquired pneumonia: A pilot study. Front Med. 9, 960847 (2022).
- Suttapanit, K., Wongkrasunt, S., Savatmongkorngul, S., Supatanakij, P. Ultrasonographic evaluation of the diaphragm in critically ill patients to predict invasive mechanical ventilation. J Intensive Care. 11 (1), 40 (2023).
- Vivier, E., et al. Diaphragm ultrasonography to estimate the work of breathing during non-invasive ventilation. Intensive Care Med. 38 (5), 796-803 (2012).
- Eduardo Garrido-Aguirre, S. a. N. S. Diaphragmatic ultrasonography, a novel approach in critical care. Ultrasound Q. 36, 54-58 (2020).
- Sferrazza Papa, G. F., et al. A review of the ultrasound assessment of diaphragmatic function in clinical practice. Respiration. 91 (5), 403-411 (2016).
- Santana, P. V., Cardenas, L. Z., Albuquerque, A. L. P., Carvalho, C. R. R., Caruso, P. Diaphragmatic ultrasound: A review of its methodological aspects and clinical uses. J Bras Pneumol. 46 (6), e20200064 (2020).
- Qian, Z., Yang, M., Li, L., Chen, Y. Ultrasound assessment of diaphragmatic dysfunction as a predictor of weaning outcome from mechanical ventilation: A systematic review and meta-analysis. BMJ Open. 8 (9), e021189 (2018).
- Kharma, N. Dysfunction of the diaphragm: Imaging as a diagnostic tool. Curr Opin Pulm Med. 19 (4), 394-398 (2013).
- Tsui, J. J., Tsui, B. C. A novel systematic abc approach to diaphragmatic evaluation (abcde). Can J Anaesth. 63 (5), 636-637 (2016).
- Da Conceicao, D., et al. Validation of a novel point-of-care ultrasound method to assess diaphragmatic excursion. Reg Anesth Pain Med. 49 (11), 800-804 (2023).
- Haaksma, M. E., et al. EXpert consensus on diaphragm ultrasonography in the critically ill (EXODUS): A delphi consensus statement on the measurement of diaphragm ultrasound-derived parameters in a critical care setting. Crit Care. 26 (1), 99 (2022).
- El-Boghdadly, K., Goffi, A., Chan, V. Point of care diaphragmatic ultrasound made easy. Can J Anaesth. 64 (3), 327-328 (2017).
- Kim, D. H., et al. Superior trunk block: A phrenic-sparing alternative to the interscalene block: A randomized controlled trial. Anesthesiology. 131 (3), 521-533 (2019).
- Bellissimo, C. A., Morris, I. S., Wong, J., Goligher, E. C. Measuring diaphragm thickness and function using point-of-care ultrasound. J Vis Exp. 201, e65431 (2023).
- Goligher, E. C., et al. Measuring diaphragm thickness with ultrasound in mechanically ventilated patients: feasibility, reproducibility and validity. Intensive Care Med. 41 (4), 642-649 (2015).
- Pereira, R. O. L., et al. Point-of-care lung ultrasound in adults: Image acquisition. J Vis Exp. (193), e64722 (2023).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved