Method Article
Determination of Fatty Acid Oxidation and Lipogenesis in Mouse Primary Hepatocytes
In This Article
Summary
De novo lipogenesis and β-fatty acid oxidation constitute key metabolic pathways in hepatocyte, pathways that are perturbed in several metabolic disorders, including fatty liver disease. Here we demonstrate isolation of mouse primary hepatocytes and describe quantification of β-fatty acid oxidation and lipogenesis.
Abstract
Lipid metabolism in liver is complex. In addition to importing and exporting lipid via lipoproteins, hepatocytes can oxidize lipid via fatty acid oxidation, or alternatively, synthesize new lipid via de novo lipogenesis. The net sum of these pathways is dictated by a number of factors, which in certain disease states leads to fatty liver disease. Excess hepatic lipid accumulation is associated with whole body insulin resistance and coronary heart disease. Tools to study lipid metabolism in hepatocytes are useful to understand the role of hepatic lipid metabolism in certain metabolic disorders.
In the liver, hepatocytes regulate the breakdown and synthesis of fatty acids via β-fatty oxidation and de novo lipogenesis, respectively. Quantifying metabolism in these pathways provides insight into hepatic lipid handling. Unlike in vitro quantification, using primary hepatocytes, making measurements in vivo is technically challenging and resource intensive. Hence, quantifying β-fatty acid oxidation and de novo lipogenesis in cultured mouse hepatocytes provides a straight forward method to assess hepatocyte lipid handling.
Here we describe a method for the isolation of primary mouse hepatocytes, and we demonstrate quantification of β-fatty acid oxidation and de novo lipogenesis, using radiolabeled substrates.
Introduction
Non-alcoholic fatty liver disease is one of the leading causes of liver disease in Westernized cultures1,2. Lipid accumulation within the liver is associated with cell death, fibrosis, and liver failure via yet unknown mechanisms3-6. In fatty liver disease, hepatocyte-mediated β-fatty acid oxidation and de novo lipogenesis are important determinants of net lipid accumulation7,8. This article will, therefore, focus on hepatocyte isolation, followed by quantification of β-fatty acid oxidation and de novo lipogenesis.
Numerous methodologies have been developed to interrogate hepatocyte lipid metabolism. Though it is possible to measure metabolism of fat in vivo using stable isotopes9,10, these methods are costly, and require large numbers of animals. Additionally, the ability to investigate the effect of exogenous chemicals is limited due to the nature of in vivo experimentation. In contrast, the isolation of primary hepatocytes from mouse liver provides an affordable avenue to pursue11. Furthermore, studying hepatocytes in culture allows investigators to study the effects of varying chemicals on lipid processing while circumventing the difficulties of in vivo experimentation. Finally, isolated hepatocytes avoid any confounding from varying genetics since they are derived from the liver of a single animal.
Here we isolate and culture of hepatocytes, and we measure β-fatty acid oxidation and de novo lipogenesis, using radiolabeled palmitate. The protocol detailed below is straight forward, effective, and reproducible.
Protocol
All animal experimentation should be carried out in accordance with local and federal regulations and with the approval of an institutional IACUC and radiation safety administration.
1. Preparation
- Several days prior to the assay, thaw the 500 ml bottle of Liver Digest Medium (LDM) and refreeze ~35 ml aliquots in 50 ml conical tubes. Store at -20 °C until needed.
- One day prior to the assay, pre-sterilize clean dissection tools by autoclave.
- On the day of the assay, treat the necessary amount of 24-well culture plates with collagen.
- Mix 1 part of 3 mg/ml rat tail collagen with 50 parts of PBS. Add approximately 500 µl to each well of a 24-well plate and incubate for 3-5 min at room temperature (RT). Remove the collagen and allow plate to air dry in hood. Repeat at least one additional time.
Note: Collagen coating can be performed up to one week in advance and plates stored at 4 °C sealed in plastic wrap.
- Mix 1 part of 3 mg/ml rat tail collagen with 50 parts of PBS. Add approximately 500 µl to each well of a 24-well plate and incubate for 3-5 min at room temperature (RT). Remove the collagen and allow plate to air dry in hood. Repeat at least one additional time.
- Prepare LDM for assay: Thaw LDM and warm to 37 °C and adjust pH to 7.4 using 1 N KOH. Filter using 0.2 µm syringe filter and place in a recirculating 42 °C water bath.
- Warm 25 ml of Liver Perfusion Medium to 42 °C in recirculating water bath.
- Prepare 90% colloidal silica coated with polyvinylpyrrolidone solution: Add 1 ml of 10x DPBS to 9 ml of colloidal silica coated with polyvinylpyrrolidone. Adjust pH to 7.4 using 0.1 N HCl. Filter with a sterile 0.2 µm syringe filter. Store at RT.
- Warm Plating Medium to 37 °C.
2. Isolation of Primary Mouse Hepatocytes
- Set up peristaltic pump, tubing, and dissection table: Sterilize tubing by flushing with 5 ml 70% ethanol in distilled water, followed by 10 ml of sterile water. Place tubing in Liver Perfusion Medium and run pump to fill entire length of tubing.
- Sacrifice mouse by the institutionally approved method.
- Dissect open the abdominal cavity: Spray the mouse abdomen liberally with 70% ethanol. Using blunt-end scissors, make a midline incision through the dermis the length of the abdomen and reflect laterally. Make a similar incision in the peritoneum to expose the viscera.
- Using a blunt instrument, gently displace the intestines to expose the abdominal vasculature Locate the abdominal inferior vena cava (IVC) and place a suture underneath the blood vessel, distal to the renal vein
- Distal to the suture, place a needle and catheter into the IVC, advancing it beyond the level of the suture. With the needle still in place, tie the suture around the catheter to hold it in place. Carefully remove the needle. If done correctly, blood with flow through the catheter.
- Using a pipet, fill the remaining area in the catheter with perfusion medium, ensuring that no air is present. With great care, attach the tubing to the catheter.
- Dissect open the lung cavity: gently reflect the superior liver lobes to expose the diaphragm. Carefully puncture the diaphragm with sharp tip scissors, then make a lateral incision to expose the pleural cavity, taking care to avoid the gall bladder and pleural vasculature. Place a bulldog clamp around the thoracic inferior vena cava just proximal to the hepatic vein.
- Cut the portal vein and turn on the pump at 3 - 4 ml/min. Perfuse the liver with Liver Perfusion Medium for 5 min using approximately 20 ml of perfusion medium.
Note: The liver should immediately change from red to grey/tan. If portions of the liver remain red, this likely indicates poor perfusion, and the likelihood of successful isolation is greatly diminished. During the perfusion, be careful to ensure the medium does not run out and that no bubbles enter the tubing. - Following the 5 min incubation, stop the pump and transfer the tubing to Liver Digest Medium. Restart the pump and perfuse the liver for another 10 - 15 min (until the medium is exhausted).
- At the end of the perfusion, stop the pump. The liver should have a pinkish hue and appear somewhat enlarged. Excise the liver by careful dissection. Remove the gall bladder and transfer the liver to 10 cm tissue culture dish.
- In a biosafety cabinet, add 10 ml of Plating Medium ( Table 1) to the liver. Gently scrape the liver using either forceps or a scalpel to remove the hepatocytes. Filter the suspension using a 100 µm cell strainer and transfer to a 50 ml conical tube. Wash the plate with an additional 10 ml of Plating Medium and pool in the 50 ml conical tube.
- Pellet the cells by centrifuging for 5 min at 350 x g, 4 °C.
- Aspirate medium and resuspend pellet in 10 ml of Plating Medium and add 10 ml of 90% colloidal silica coated with polyvinylpyrrolidone. Mix gently and centrifuge as in step 2.11. After centrifugation, a layer of dead cells will be floating on the top of the mixture, while the live cells will pellet to the bottom.
- Aspirate dead cells and medium. Wash 2 times with 20 ml of Plating Medium, centrifuge as in 2.11.
- Resuspend cells in 10 ml of Plating Medium. Count cells with a hemocytometer and place 9 x 104 cells/well in collagen-treated 24-well culture dishes. Incubate in a 37 °C tissue culture incubator for 2 hr. Each plate can be used for either the Fatty Acid Oxidation assay OR the Lipogenesis assay.
- Optionally, change wells to Maintenance Medium (Table 1) and culture at 37 °C. Cells can be cultured for 2 - 3 days without affecting assay results.
3. Fatty Acid Oxidation Assay
Warning: Use of radioactivity can be hazardous. All purchasing, storage, handling, and disposal of radioactive material should be carried out in accordance with institutional, state, and federal regulations and guidelines.
- 16 - 20 hr prior to assay, wash cells 2 times with warm PBS. Change the cells to serum-free Serum Starvation Medium (Table 1) with 20 nM glucagon and incubate overnight at 37 °C.
Note: Because fetal bovine serum contains an unknown concentration of metabolic hormones (e.g. insulin, glucagon), serum starve the cells to remove any confounding effects this may have on the assay. Cells are viable in Serum Starvation Medium for >24 hr. Glucagon treatment is used to stimulate fatty acid oxidation within the hepatocytes. - On the morning of the assay, prepare Pre-Incubation Medium: Resuspend an appropriate amount of Sodium Palmitate in ultrapure water to make a 100 mM solution and heat to 70 °C for 10 min. In the meantime, prepare the requisite amount (0.5 ml per well of a 24-well plate) of DMEM with 25 mM HEPES, 1% BSA Fraction V, and 20 nM glucagon. Heat to 37 °C.
- Once solubilized, add Palmitate to a final concentration of 250 µM to the medium. Change hepatocytes to Pre-Incubation Medium and incubate at 37 °C for 2 hr. Reserve some Pre-Incubation Medium at 37 °C for later use.
- During the incubation, dry an appropriate amount of 14C-Palmitate (0.5 µCi/well) by evaporation under nitrogen gas.
- For example, to measure 24 samples in a 24-well plate, transfer 120 µl of 0.1 µCi/ml14 C-Palmitate to a 1.5 ml tube. Slowly evaporate the ethanol solvent by blowing nitrogen gas over the solution from a distance of 3-5 cm in a fume hood. The solvent should evaporate in approximately 30 - 40 min, leaving dry 14C-Palmitate in the bottom of the tube.
- Approximately 15 min before the end of the incubation, resuspend the 14C-Palmitate in 0.1 N NaOH (12.5 µl/µCi). Incubate at 70 °C for 10 min. Add three volumes of warm Pre-Incubation Medium and mix by pipetting up and down.
- Spike each well with 25 µl of diluted 14C-Palmitate. Mix by gently rocking the plate and incubate at 37 °C for 90 min. This is the Assay Plate.
- During the incubation, prepare the Filter Paper Plate (Figure 1).
- Remove the cover from a sterile 24-well plate. Place one 2 cm x 2 cm piece of filter paper in the bottom of each of the wells. Overlay the plate with a piece of 4” x 7” parafilm.
- Using a large rectangular object, such as a micropipettor tip box, rub the parafilm on the wells to perforate the parafilm at the well openings and create a seal over the remainder of the plate. Remove the perforated circles of parafilm now covering the wells. The plate should now be tightly covered with parafilm in all areas except the well openings.
- 10 min before the end of the incubation, add 200 µl of 3 N NaOH to each well of the Filter Paper Plate, making sure the filter paper absorbs all of the liquid.
- Optionally prior to freezing, transfer the medium to a fresh 24-well plate. After washing once with PBS, lyse the remaining cells in 0.1 N HCl and calculate protein content by BCA assay.
- At the end of the incubation, snap-freeze the Assay Plate in liquid nitrogen. Be careful to ensure each well is completely frozen before proceeding.
- Add 100 µl of 70% Perchloric Acid to each well of the Assay Plate. Immediately cover with the Filter Paper Plate. Place the plates on an orbital shaker and rock at orbital speed of 80 rpm at RT for 2 hr.
- Following the incubation, process the samples:
- To measure the CO2 fraction, transfer the filter paper squares to 4 ml liquid scintillation fluid in a scintillation vial and measure 14C signal.
- To measure the acid soluble material, transfer 400 µl of medium to a 1.5 ml microfuge tube. Centrifuge at maximum speed for 10 min. Add 100 µl of the resulting supernatant to 500 µl of 2:1 Chloroform-Methanol (v/v), and vortex briefly.
- Add 250 µl of water to the mixture, and vortex again. Centrifuge samples for 10 min at 3,000 x g. Transfer 200 µl of the upper phase to 4 ml liquid scintillation fluid in a scintillation vial and measure 14C signal.
4. Lipogenesis Assay
- The evening prior to beginning the assay, wash the cells 2 times with warm PBS. Change the hepatocytes to Serum Starvation Medium with 100 nM insulin. Incubate overnight at 37 °C.
Note: Because fetal bovine serum contains an unknown concentration of metabolic hormones (e.g. insulin, glucagon), serum starve the cells to remove any confounding effects this may have on the assay. Cells are viable in Serum Starvation Medium for >24 hr. Insulin is used in this assay to stimulate lipogenesis. - Make Lipogenesis Medium: Serum Starvation Medium with 100 nM insulin, 10 µM cold acetate and 0.5 µCi 3H-Acetate per well. Change cells to Lipogenesis Medium and incubate at 37 °C for 2 hr. Include any compounds of interest to be tested.
- After the incubation period, wash cells 2 times with PBS. Lyse cells by scraping in 120 µl of 0.1 N HCl. Reserve 10 µl for protein assay (assess by BCA assay), and transfer 100 µl to 1.5 ml microfuge tube.
- Extract lipids by addition of 500 µl of 2:1 chloroform-methanol (v/v). Vortex briefly and incubate at RT for 5 min. Add 250 µl of water, vortex and incubate at RT for an additional 5 min. Centrifuge samples 10 min at 3,000 x g, RT. Carefully transfer lower phase to 4 ml liquid scintillation fluid in a scintillation vial and measure 3H activity.
Results
Hepatocyte isolations typically result in 1 - 3 x 107 total cells. After overnight incubation, the cells will appear hexagonal, many of which will be binucleated (Figure 2). Healthy cells should be devoid of granulations or blebs, which are indicative of cell death.
In general, Fatty Acid Oxidation assay is run in three to four replicates per test compound. Counts for the CO2 samples are approximately one-fifth of those derived from the acid soluble material. We typically calculate the ratio of CO2 to acid soluble material as a measure of complete oxidation (that is, the amount oxidized via the citric acid cycle). Substances that promote cellular respiration, such as Carbonyl cyanide-4-(trifluoromethoxy)phenylhydrazone (FCCP) will shift this ratio toward CO2, indicating more oxidation via the TCA cycle. Inhibitors of the respiratory chain will diminish oxidation on the whole (Figure 3). When piloting this assay, it is best to include a no-cell control to verify the procedure is producing cell-specific activity.
The Lipogenesis Assay is most often compared to a zero timepoint control (cells treated with substrate immediately before harvesting). The assay should be linear versus time through at least four hours of incubation. Compounds which enhance fatty acid oxidation, such as FCCP, or diminish ATP synthesis, such as oligomycin, will reduce lipogenic activity (Figure 4).
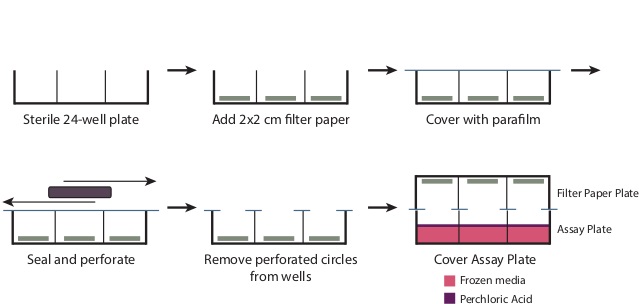
Figure 1: Preparation of the Filter Plate To prepare the filter plate as described in Step 3.6, remove the cover from a sterile 24-well plate. Place a 2 cm x 2 cm piece of filter paper at the base of each well. Overlay the entire plate with a 7” x 4”piece of parafilm, and rub the plate to tightly seal the top of the wells. This will generate perforated circles of parafilm over the well openings, which should be removed. After addition of the perchloric acid in Step 3.9, place the Filter Paper Plate tightly over the Assay Plate to produce a seal.
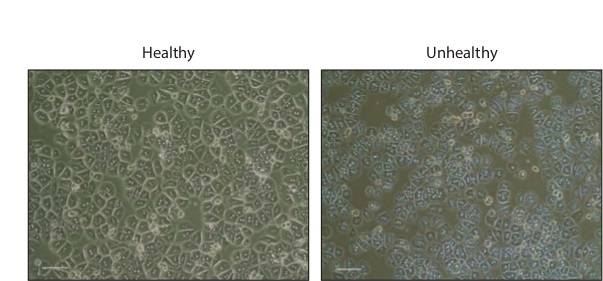
Figure 2: Primary Hepatocytes in Culture Phase contrast images of healthy and unhealthy primary mouse hepatocytes 16 hr after plating in collagen coated 24-well plates. Scale bar, 200 µm. Please click here to view a larger version of this figure.
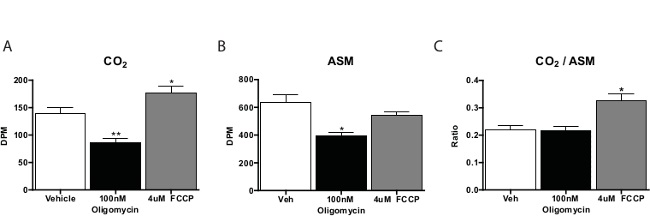
Figure 3: Fatty Acid Oxidation by Primary Mouse Hepatocytes in Culture14C-Palmitate oxidation to (A) CO2, (B) acid soluble material, and (C) the ratio of CO2 to acid soluble material from primary hepatocytes incubated with vehicle, FCCP, or oligomycin. Data are mean ± SEM. *p<0.05, **p<0.01 vs. vehicle by one-tailed Student’s t-test. Please click here to view a larger version of this figure.

Figure 4: Lipogenesis in Cultured Primary Mouse Hepatocytes (A) Lipogenic activity vs. time in primary hepatocytes treated with 3H-acetate and (B) lipogenic activity in the presence of vehicle, oligomycin, or FCCP. Data are mean ± SEM. ***p<0.001 vs. vehicle by one-tailed Student’s t-test.
Table 1: Culture Media Composition of culture media for the isolation of primary hepatocytes.
| Table 1 | |
| Culture Media | |
| Plating Medium | |
| DMEM | 500 ml |
| FBS | 10% |
| Sodium pyruvate | 2 mM |
| Pen/Strep | 2% |
| Dexamethasone | 1 μM |
| Insulin | 0.1 μM |
| Maintenance Medium | |
| DMEM | 500 ml |
| BSA Fraction V | 0.2% |
| Sodium pyruvate | 2 mM |
| Pen/Strep | 2% |
| Dexamethasone | 0.1 μM |
| Insulin | 1 nM |
| Starvation Medium | |
| DMEM | 500 ml |
| BSA Fraction V | 0.2% |
| Sodium pyruvate | 2 mM |
| Pen/Strep | 2% |
Discussion
The time from sacrifice to perfusion should be less than 3 min for ideal perfusion and collagenase digestion of the liver. Once perfusion with Perfusion Medium is initiated, the liver should immediately change appearance to from red to pale. After approximately 10 min of incubation with LDM, the liver will appear swollen and pink. In the event that perfusion is insufficient, the liver may not exhibit these changes, and this will typically result in a lower hepatocyte yield.
Following the washing steps, isolated hepatocytes can be stored for several hours in suspension on ice prior to plating. Once plated, cultured hepatocytes require several hours to adhere and spread out. Following 2 hr of incubation in Plating Medium, the hepatocytes will remain small and round. After an overnight incubation, hepatocytes will take on a more characteristic hexagonal appearance, many of which will be binucleated. If the preparation is unhealthy, the cells will exhibit numerous granulations and occasional blebbing, indicative of cell death. In order to best maintain culture viability, media should be changed every 24 hr and care taken to minimize exposure to open air.
Sodium palmitate is insoluble in water at RT, however, after incubation at 70 °C it should completely dissolve. Exposure to RT will cause the lipid to solidify rapidly, thus it is imperative to work quickly. Once palmitate has been dissolved in Pre-Incubation Medium, it is critical to maintain the medium at 37 °C in order to maintain solubility of the lipids.
As mentioned above, to ensure accurate results from the Fatty Acid Oxidation assay, the medium must be completely frozen in liquid nitrogen. This prevents any escape of CO2 during the addition of perchloric acid to the wells. Immediately following the addition of the perchloric acid to the frozen samples, the assay plate must be tightly covered by the Filter Paper Plate, making sure to align the plates for gas transfer between the wells. Since the filter paper is soaked with an excess of NaOH, the stoichiometry of the reaction is capable of capturing the released CO2 as NaHCO3. Experiments following this protocol have generated reproducible results, with typical replicates having %CV ≤ 10. If needed, acid soluble material from the Fatty Acid Oxidation assay or the cell lysate from the Lipogenesis assay are capable of being stored at -80 °C and processed later without any appreciable effect on results. The assays described above allow for a relatively simple and time efficient mechanism to assess lipid metabolism in primary hepatocytes isolated from mouse liver. Through use of ex vivo culture, these methods allow testing the effects of several conditions on fatty acid oxidation and lipogenesis. This protocol may be adapted to assess the role of genetic alterations on these processes, however, the isolation of hepatocytes is time-limiting and thus in vivo analyses may be more suitable for investigating certain transgenic animal models. If analysis of multiple hepatocyte preparations is necessary, normalization of assay values to protein levels can be performed as described in the optional step 3.7.1 and used for normalization. We recommend assays performed in multiple preparations be compared as relative changes versus a suitable control.
Finally, we have not explored the option of longer culture periods, however, hepatocytes may exhibit equivalent metabolic characteristics after several days in culture. With slight modification, this protocol may be adapted to allow for several day treatments prior to assessing the effects of compounds on lipid metabolism.
Disclosures
The authors indicate they have no conflicts of interest.
Acknowledgements
We would like to acknowledge Susan Gray and Umadevi Chalasani for their help with technical aspects of the hepatocyte isolation protocol. This work was supported by NIDDK grant 5R01DK089185 (to M.P. Cooper) and the DERC Pilot and Feasibility Program at UMMS (to M.P. Cooper).
Materials
| Name | Company | Catalog Number | Comments |
| Liver Perfusion Medium | Life Technologies | 17701038 | |
| Liver Digest Medium | Life Technologies | 17703034 | Aliquot and store at -20 °C |
| PBS | Corning | 21-040-CV | |
| 10X DPBS | Corning | 46-013-CM | |
| DMEM | Corning | 10-017-CV | |
| FBS | Life Technologies | 26140079 | |
| Collagen | Life Technologies | A1048301 | |
| Colloidal silica coated with polyvinylpyrrolidone | GE Life Sciences | 17-0891-01 | |
| Sodium Pyruvate | Cellgro | 25-000-CI | |
| Penicillin / Streptomycin | Cellgro | 30-001-CI | |
| Insulin | Sigma | I0516-5ML | |
| Dexamethasone | Sigma | D2915-100MG | |
| Albumin (BSA), Fraction V | MP Biomedicals | 103703 | |
| 24-Well Culture Dish | Corning Falcon | 353047 | |
| Tygon S3 Tubing | Cole Parmer | 06460-34 | |
| Male Leur Lock to 200 Barb Connectors | Cole Parmer | 45518-00 | |
| 24G x 3/4" Catheter | SurFlo | SROX2419CA | |
| Perma-Hand Silk Suture | Ethicon | 683G | |
| Cell Strainer | Corning Falcon | 08-771-2 | |
| IsoTemp 3013HD Recirculating Water Bath | Fisher | 13-874-3 | |
| MasterFlex C/L Peristaltic Pump | MasterFlex | HV-77122-24 | |
| Microclamp | Roboz | RS-7438 | Pre-sterilize in autoclave |
| 5” Straight, Blunt-Blunt Operating Scissors | Roboz | RS-6810 | Pre-sterilize in autoclave |
| 24mm Blade Straight, Sharp-point Microdissecting Scissors | Roboz | RS-5912 | Pre-sterilize in autoclave |
| 4” 0.8mm Tip Microdissecting Forceps | Roboz | RS-5130 | Pre-sterilize in autoclave |
| 4” 0.8mm Tip Full Curve Microdissecting Forceps | Roboz | RS-5137 | Pre-sterilize in autoclave |
| 60 mL Syringe | Becton Dickinson | 309653 | |
| 50 mL conical tubes | Corning Falcon | 352070 | |
| BCA Protein Assay | Thermo Scientific | 23225 | |
| Biosafety Cabinet | |||
| CO2 Incubator | |||
| Serological pipets | |||
| 1000, 200, 20 μL pipet and tips |
References
- Clark, J. M., Brancati, F. L., Diehl, A. M. The prevalence and etiology of elevated aminotransferase levels in the United States. The American journal of gastroenterology. 98, 960-967 (2003).
- Lazo, M., et al. Prevalence of nonalcoholic Fatty liver disease in the United States: the third national health and nutrition examination survey, 1988-1994. American journal of epidemiology. 178, 38-45 (2013).
- Angulo, P. Nonalcoholic fatty liver disease. The New England journal of medicine. 346, 1221-1231 (2002).
- Adams, L. A., et al. The natural history of nonalcoholic fatty liver disease: a population-based cohort study. Gastroenterology. 129, 113-121 (2005).
- Feldstein, A. E., et al. Hepatocyte apoptosis and fas expression are prominent features of human nonalcoholic steatohepatitis. Gastroenterology. 125, 437-443 (2003).
- Day, C. P., James, O. F. Steatohepatitis: a tale of two 'hits'. Gastroenterology. 114, 842-845 (1998).
- Donnelly, K. L., et al. Sources of fatty acids stored in liver and secreted via lipoproteins in patients with nonalcoholic fatty liver disease. The Journal of clinical investigation. 115, 1343-1351 (2005).
- Lambert, J. E., Ramos-Roman, M. A., Browning, J. D., Parks, E. J. Increased de novo lipogenesis is a distinct characteristic of individuals with nonalcoholic fatty liver disease. Gastroenterology. 146, 726-735 (2014).
- Parks, E. J., Hellerstein, M. K. Thematic review series: patient-oriented research. Recent advances in liver triacylglycerol and fatty acid metabolism using stable isotope labeling techniques. Journal of lipid research. 47, 1651-1660 (2006).
- Befroy, D. E., et al. Direct assessment of hepatic mitochondrial oxidative and anaplerotic fluxes in humans using dynamic 13C magnetic resonance spectroscopy. Nature medicine. 20, 98-102 (2014).
- Goncalves, L. A., Vigario, A. M., Penha-Goncalves, C. Improved isolation of murine hepatocytes for in vitro malaria liver stage studies. Malaria journal. 6, 169 (2007).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved