Method Article
Visualizing Methane-Cycling Microbial Dynamics in Coastal Wetlands
In This Article
Summary
The protocol detects key methane-cycling genes in South Texas coastal wetlands and visualizes their spatial distribution to enhance understanding of methane regulation and its environmental impacts in these dynamic ecosystems.
Abstract
Coastal wetlands are the largest biotic source of methane, where methanogens convert organic matter into methane and methanotrophs oxidize methane, thus playing a critical role in regulating the methane cycle. The wetlands in South Texas, which are subject to frequent weather events, fluctuating salinity levels, and anthropogenic activities due to climate change, influence methane cycling. Despite the ecological importance of these processes, methane cycling in South Texas coastal wetlands remains insufficiently explored. To address this gap, we developed and optimized a method for detecting genes related to methanogens and methanotrophs, including mcrA as a biomarker for methanogens and pmoA1, pmoA2, and mmoX as biomarkers for methanotrophs. Additionally, this study aimed to visualize the spatial and temporal distribution patterns of methanogen and methanotroph abundance utilizing the geographic information system (GIS) software ArcGIS Pro. The integration of these molecular techniques with advanced geospatial visualization provided critical insights into the spatial and temporal distribution of methanogen and methanotroph communities across South Texas wetlands. Thus, the methodology established in this study offers a robust framework for mapping microbial dynamics in wetlands, enhancing our understanding of methane cycling under varying environmental conditions, and supporting broader ecological and environmental change studies.
Introduction
Coastal wetlands are vital ecosystems that contribute to climate regulation, biodiversity conservation, and water management through processes such as carbon sequestration, evapotranspiration, and methane (CH4) emissions1. These ecosystems, including both freshwater and saltwater wetlands2, are highly productive and act as critical zones for uptake of carbon dioxide (CO2) and capture organic matter from terrestrial and marine environments3,4. The dynamic interactions within these wetlands stimulate microbial CH4 production and consumption5, positioning them as one of the largest natural sources of CH46. As the second most important greenhouse gas, CH4 has a global warming potential approximately 27-30x greater than that of CO24,7,8,9, making the study of CH4 emissions from coastal wetlands essential in the era of climate change. The emission of CH4 is influenced by various environmental factors, particularly salinity, playing a crucial role in microbial processes10. Freshwater wetlands contribute significantly to atmospheric methane due to their lower sulfate levels, which facilitates greater microbial CH4 production, whereas saltwater wetlands generally tend to emit less CH4 due to higher sulfate concentrations11,12,13.
CH4 emissions from coastal wetlands are generally controlled by two groups of microorganisms, known as methanogens and methanotrophs14. Methanogens produce CH4 in anoxic sediments by breaking down substrates like formate, acetate, hydrogen, or methylated compounds through a process known as methanogenesis15. The important enzyme in this pathway is methyl-coenzyme M reductase (MCR), as it catalyzes the final and rate-limiting step of methanogenesis15,16,17. The mcrA gene, which encodes the alpha subunit of MCR, is a functional marker that can be found in all methanogenic archaea18. Moreover, in coastal wetlands, the sulfate-methane transition zone (SMTZ) forms above the methanogenic zone, where methane diffusing upward and sulfate moving downward converge and are depleted19. Within this zone, anaerobic methanotrophic archaea (ANME) oxidize methane to carbon dioxide using the MCR enzyme, while sulfate-reducing bacteria (SRB) reduce sulfate to sulfide. SRB outcompete methanogens for hydrogen and acetate, limiting methane production until sulfate is depleted16,17.
In contrast, aerobic methanotrophic bacteria oxidize CH4 in aerobic environments20, utilizing different forms of methane monooxygenase (MMO). These include particulate methane monooxygenase (pMMO), a copper-containing enzyme embedded in the intracytoplasmic membrane, and soluble methane monooxygenase (sMMO), an iron-containing enzyme found in the cytoplasm. However, for pMMO, there are three gene operons pmoCAB21; among them, pmoA gene is the most conservative for all the methanotrophs. There are two different biomarker genes for pmoA: pmoA1 and pmoA222. Moreover, for a comprehensive understanding of methanotrophs, mmoX gene is used as a tool in molecular biology to identify sMMO-containing methanotrophs23. This distinction in metabolic pathways and environmental requirements of methanogens and aerobic methanotrophs highlights the complex microbial interactions regulating methane cycling in coastal wetland ecosystems.
The Boca Chica (BC) wetland, a productive saltwater environment in South Texas, experiences tidal influences from the Gulf of Mexico (GOM), leading to variable surface salinity levels, especially due to its proximity to the hypersaline Laguna Madre24. This tidal action, alternating between high and low tides, causes oxygen levels to fluctuate25 that might alter methanogen and methanotroph activity in sediments26. In contrast, coastal freshwater wetlands are considered to be a significant hotspot for CH4 fluxes27. The coastal freshwater wetlands in South Texas, including Resaca Del Rancho Viejo (RV) and Lozano Banco (LB), distant from the GOM's tidal effects, have distinct hydrological management. RV experiences pulse flows supplemented by river water during low water levels, whereas LB operates as an offline flow system without such supplementation. Moreover, RV and LB maintain lower salinity levels due to a high discharge of artificially pumped freshwater and being an oxbow lake, respectively. The different environmental factors can significantly influence methane cycling across South Texas coastal wetlands. However, methane cycling in South Texas coastal wetlands remains an area that has yet to be thoroughly investigated.
Polymerase chain reaction (PCR) and real-time PCR (also called quantitative PCR [qPCR]) represent fundamental and widely utilized techniques for detecting and quantifying the relative abundance of specific genes in environmental samples. These techniques specifically amplify targeted regions of DNA to indicate the presence and relative quantity of CH4 cycling-related genes, providing indicators of potential methane cycling. Nevertheless, the availability and efficacy of PCR primer sets might be limited by various inhibitory factors in the extracted environmental DNA, being impacted by the types of environments28,29. Thus, this study mainly established an optimal PCR method for detecting the presence of CH4 cycling-related genes in South Texas coastal wetlands (Figure 1) and then visualized their quantified relative abundance in these ecosystems. The results from this study can be applied to other coastal regions to enhance the understanding of CH4 cycling and microbial dynamics in diverse coastal ecosystems.
Protocol
1. Sample collection
- Collect sediment samples using a sediment grab sampler or shovel.
NOTE: Samples were collected from two stations of three distinct coastal wetlands during cool (October-February, the average temperature is 20 °C) and warm (April-June, average temperature is 27 °C) seasons of 2023 and 2024. A sediment grab sampler was used when samples were collected from coastal freshwater wetlands (Figure 2) and a shovel was used for tidal-influenced coastal saltwater wetlands. - Lower the sampler into the shallow waterbody first and allow it to sink to the sediment surface (within the top 50 cm) under its own weight, minimizing disturbance to the sediment structure as shown in Figure 2.
- Pull it out of the water column where the depth was typically 60 cm to 215 cm30, transfer the sediment samples to zip-lock bags, and store them in an ice box immediately.
NOTE: Clean and wash the grab sampler with deionized (DI) water before proceeding to the next stations. - Store all samples at -20 °C immediately in the laboratory.
- In each sampling site, measure surface water quality parameters such as salinity and temperature in situ using a multiparameter water quality meter.
NOTE: The probes were rinsed with deionized water (DI water) after use in each station.
2. Genomic DNA extraction
- Thaw the samples at room temperature before starting the procedure for genomic DNA extraction.
- Transfer approximately 500 mg of sediment samples to a 15 mL tube and centrifuge at 4,250 × g for 3 min to remove all water.
- Extract genomic DNA using a DNA extraction kit for soil following the manufacturer's protocol31 with a little modification and store at -20 °C immediately.
NOTE: The changes were made to reduce redundancy and efficient workflow.- Add up to 500 mg of soil sample to a glass bead/ceramic sphere-containing tube.
- Add 978 µL of Sodium Phosphate Buffer to the sample in the bead/sphere-containing tube.
- Add 122 µL of buffer lysis solution to the sample in the bead/sphere-containing tube to solubilize external contaminants.
- Homogenize using a bead mill homogenizer at 5x the speed level for 20 s and repeat 2x.
NOTE: A bead mill homogenizer was used in this study, which is why the speed was adjusted. - Centrifuge the mixture for 10 min at 14,000 × g.
- Transfer the supernatant to a clean 2.0 mL microcentrifuge tube.
- Add 250 µL of protein precipitation solution (PPS) to separate the solubilized nucleic acids from the cellular debris and lysing matrix. Mix by inverting the tube 10x.
- Centrifuge at 14,000 × g for 5 min to precipitate the pellet, removing the cellular debris and lysing matrix.
- Transfer the supernatant to a clean 15 mL microcentrifuge tube.
- Add 1.0 mL of the Binding Matrix suspension to the supernatant in the 15 mL tube.
NOTE: Shake the Binding Matrix suspension to resuspend before adding it. - Allow the binding of DNA to the Binding Matrix by placing the tubes on a rotator for 2 min.
- Place all the tubes on a rack and incubate for 3 min to allow the settling of the Binding Matrix.
- After 3 min, discard 750 µL and gently mix the remaining supernatant with the pellet using a pipette.
- Transfer 750 µL of the mixture to a SPIN filter and centrifuge at 14,000 × g for 1 min. Empty the catch tube and reuse it. Repeat with the remaining mixture.
- Add 500 µL of the prepared wash solution (with the appropriate amount of ethanol added) to further solubilize impurities. Gently resuspend the pellet using the force of the liquid from the pipet tip.
- Centrifuge at 14,000 × g for 1 min to remove impurities. Empty the catch tube and reuse it.
- Centrifuge again at 14,000 × g for 2 min without adding anything.
- Replace the catch tube with a new, clean catch tube and air dry the SPIN Filter for 5 min at room temperature.
- Add 50 µL DNAse free water (DES) and centrifuge at 14,000 × g for 1 min.
NOTE: In this protocol, 50 µL of DES was used for DNA elution to ensure optimal recovery and stability of the extracted environmental DNA (eDNA).
3. DNA quantification
- Add 1 µL of extracted DNA with 200 µL of fluorescent dye in a 0.5 mL tube and mix thoroughly by pipetting.
- Wrap the tube immediately with aluminum foil so light cannot penetrate and incubate at room temperature for 5 min.
- Measure DNA concentration by using a fluorometer at ONE DNA concentration mode following the manufacturer's protocol.
4. Detection of 16S rRNA, pmoA1 , pmoA2 , mmoX , and mcrA by conventional PCR
- Before running conventional PCR (cPCR), thaw all samples and reagents in an ice bucket.
- Dilute all extracted eDNA samples to 10 ng/µL.
NOTE: A list of primers is given in Table 1. - Prepare a 25 µL cPCR reaction mixture for each sample, including 12.5 µL of 2x PCR Master mix , 0.5 µL of forward and reverse primers (10 µM) (see Table 1 for primer list), 1 µL of 10 ng/µL eDNA, and 10.5 µL of nuclease-free water.
NOTE: Prepare the master mix with enough volume for one extra sample to minimize pipetting errors. - Perform cPCR reaction following the protocol consisting of an initial denaturation at 95 °C for 2 min, followed by 40 cycles of denaturation at 95 °C for 45 s, extension at 72 °C for 30 s, and final extension at 72 °C for 5 m, with varying annealing temperature for different primers (see Table 1 for annealing temperatures of different primers used for different genes).
NOTE: For mcrA gene, ML primers showed the desired band using a slow ramp rate of 0.1 °C/s between the annealing and extension steps for the first five cycles32. - Visualize cPCR products in an agarose gel prestained with ethidium bromide (EtBr).
NOTE: Use 2.5% agarose gel for a 50 bp ladder and 0.9% gel for a 1 kb ladder. Use 1x TAE buffer for a 2.5% agarose gel and 0.5x TAE buffer for a 0.9% agarose gel.
5. Detection of pmoA1 , pmoA2 , mmoX, and mcrA by quantitative real-time PCR
NOTE: Methanogen- and methanotroph-targeted genes such as pmoA1, pmoA2, mmoX, and mcrA abundance were observed by qPCR using a real-Time PCR system.
- Prepare the standards for each gene separately to obtain the standard curve for each gene.
- Amplify the targeted gene with cPCR, using the samples that produced the brightest band during the gel electrophoresis of each gene. Follow the method and primers described in section 4.
- Purify the amplified products using a gel extraction kit and measure the DNA concentration following the method described in section 3 and store at -20 °C.
NOTE: Aliquot the purified standards into separate tubes for further use. - Calculate gene copies from the measured DNA concentration using the copy number calculating website for qPCR.
- Dilute the calculated copy number of the standard with nuclease-free water to prepare each standard, ranging from 108 to 102 copies/µL, before running the qPCR.
- Prepare the standard curve using three replicates of each copy number, including a negative control (NTC, no template DNA).
NOTE: The R2 value of each standard curve was greater than 0.99.
- Prepare a 20 µL qPCR reaction mixture for all samples, standards, and NTC. Perform the qPCR analysis in triplicates for all samples.
- Place all reaction components, including qPCR master mix, primers, nuclease-free water, standards, and samples, on an ice rack before beginning.
- Prepare a 20 µL qPCR reaction mixture for each sample, standard, and NTC, containing 10 µL of SYBR Green master mix, 0.5 µL of each 10 µM forward and reverse primers, 8 µL of nuclease-free water, and 1 µL of either 10 ng/µL template DNA, or standard, or DI water respectively.
NOTE: Use the primer sets shown to yield optimal results for pmoA1 and mcrA genes in conventional PCR for qPCR (see Table 1). To improve accuracy, run each sample in triplicate. The following steps provide an efficient method for preparing triplicate samples with a combined volume of 60 µL per sample.- Prepare the reaction mixture to combine the master mix, forward and reverse primers for the specific gene, and nuclease-free water in a 2 mL tube, except the template DNA.
NOTE: Prepare the reaction mixture volume considering pipetting error. For example, if there are 24 samples and 8 standards, calculate the total volume for 33 reactions instead of 32 reactions to minimize pipetting errors. In this case, the total volume required for triplicate reactions would be as follows: 990 µL of master mix (33 samples x 3 replicates x 10 µL), 49.5 µL of forward primers (33 samples x 3 replicates x 0.5 µL), 49.5 µL of reverse primers (33 samples x 3 replicates x 0.5 µL), and 792 µL of nuclease-free water (33 samples x 3 replicates x 8 µL). - Prepare PCR tubes according to the number of samples and standards.
- Dispense 57 µL of the prepared reaction mixture into each PCR tube.
NOTE: To perform qPCR for each sample in triplicate, prepare a total reaction mixture volume of 57 µL per sample (excluding template DNA, standard, or water). This volume will be divided equally into three wells for one sample, with 19 µL allocated to each well. - Add 3 µL of template DNA, standard, or nuclease-free water to each tube and mix by gently tapping the tube bottom.
NOTE: The total volume of reaction mixture prepared for one sample would be 60 µL now in each tube.
- Prepare the reaction mixture to combine the master mix, forward and reverse primers for the specific gene, and nuclease-free water in a 2 mL tube, except the template DNA.
- Aliquot 20 µL of the prepared reaction mixture from each tube into the designated wells of a 96-well qPCR plate. Seal the PCR plate with adhesive PCR Sealing Film using an applicator.
- Centrifuge the sealed plate at 1,000 × g for 1 min to ensure proper mixing to eliminate any bubbles within the wells.
- Place the PCR plate in the thermal cycler. Turn on the qPCR machine and then open the related software to set up the protocol.
- Set up the protocol according to the qPCR master mix guidelines. Use the following protocol: 95 °C for 10 min, followed by 95 °C for 15 s, and an extension step at 72 °C for 30 s. Perform the annealing step at the annealing temperature specified for the relevant primers in Table 1 for 45 s. Conduct all qPCR runs for 35 cycles.
- Set up the 96-well qPCR plate with standards and NTCs in the same configuration as the sample-containing plate.
- Use the absolute quantification standard curve method to quantify the amplified product and the gene copy number in each sample33.
6. Visualizing methane-cycling genes in the map of South Texas Coastal wetlands
- Open geographic information system (GIS) software ArcGIS Pro and save the project file with the name Study Area in the specified folder on the computer.
- Click on Map in the top left | Basemap and select Terrain with Labels as the basemap.
- Click on Locate | Search and when the search bar opens up, locate the study area by typing the area's name; the area will show up.
- Draw the specific area using georeferencing.
- Click on View | Catalog Pane from the top layer.
- Double-click on Folder from the catalog | File Name.
- Right-click on geodatabase (.gdb) file and then click on New | Feature Class and Create Feature Class will show up.
- Type Name and Alias box and click Finish at the bottom.
- Click on View | Contents. The Alias name will show up in the Contents Pane.
- Click on Edit from the top layer | Create. The Create Features pane will open. Double-click on the Alias in Create Features pane, and the Configure Tool Feedback Options will appear.
- Select Lines, then sketch lines on the map to create an outside boundary of the study area. Double-click on the map when finished.
- Minimize GIS software, then open a spreadsheet. Type the sample name in the first column, enter the latitude in the second column, and the longitude in the third column. Use the next four columns for the qPCR data of pmoA1, pmoA2, mmoX, and mcrA.
- Save the file in CSV format in any specific folder of the computer.
- Open the GIS software again and click Add Data | XY Point Data.
- Select the CSV file from the folder on the computer in the Input Table box. Rename the file name in the Output Feature Class, then click Run to display the sampling points on the map.
- Click on thevsearch bar at the top and search Kriging.
- Select the sampling station file and then select pmoA1.
- Click the Environment | select the sampling station in layer and mask | click Run.
- Follow the protocols mentioned in steps 6.9, 6.10, and 6.11 to create Kriging for pmoA2, mmoX, and mcrA for all the study area.
- Create a layout of the map.
- Click on Insert from the top layer | New Layout, and select ANSI - Landscape.
- Click on Map Frame, select the map with the Kriging, and place all the maps in the layout by drawing a rectangle. This will make the map visible in the layout.
- Select the North Arrow and place it in the layout to indicate the North direction.
- Select Scale Bar to display the scale of the area on the map.
- Click on Legend to display the legends, then place it in the layout.
- Click Grid and select any of the black graticule options. This will create the grid with latitude and longitude and display it in the Contents Pane with the label Black Horizontal Label Graticule.
- Double-click on Black Horizontal Label Graticule, then select Components. Click on Ticks 1 and Grid, and remove these components by clicking on the cross sign to their right.
- Click on Share from the top layer, then click Export Layout. Select the file type as PDF, save the file on the computer using the Name Box, set the vertical resolution to 500 DPI, and click Export to create the PDF file of the map.
Results
To understand the distribution and abundance of CH4 cycling-related genes (mcrA, pmoA1, pmoA2, and mmoX) in the coastal wetlands of South Texas, the extracted eDNA from each sample was analyzed by cPCR and qPCR. Universal primers for each biomarker were selected to run cPCR from previous studies (Table 1)22,34,35,36,37, and modifications were made to optimize annealing temperatures and concentrations based on sample characteristics and environmental conditions. For example, 16S rRNA, pmoA1, and pmoA2 did not amplify using the standard annealing temperature because most of them amplified well below the standard annealing temperature (data not shown). Further, during the cPCR process, a concentration of 10 ng/µL DNA showed a distinct band for pmoA1, pmoA2, and mmoX genes, whereas using 5 ng/µL of template DNA showed brighter band for mcrA gene (Supplemental File 1-Supplemental Figure S1).
The cPCR analysis revealed spatial variability in gene detection across three types of coastal wetlands of South Texas. As shown in Table 2, mcrA genes were detected in all samples from LB and RV, which are coastal freshwater wetlands. However, cPCR could not detect any mcrA gene in the sampled sediment of the saltwater wetland, suggesting a potential link between salinity and methanogen distribution (Supplemental File 1-Supplemental Figure S2). For mcrA detection, four primer pairs were used in this study, including MLF-MLR, MCRf-MCRr, ME1F-ME2R, and McrA 159F-McrA 345R (Table 1); among them, only ML primer pairs were able to detect methanogen community in South Texas wetlands (Supplemental File 1-Supplemental Figure S2, Supplemental Figure S3, and Supplemental Figure S4). In contrast, for aerobic methanotrophs, pmoA1 gene was detected in all RV samples but was absent in LB and BC samples (Supplemental File 1-Supplemental Figure S5). For identifying pmoA1, A189 was used as the forward primer and A682 and mb661 as reverse primers, where A189-A682 primer pairs can also detect amoA35,38. However, this primer pair was able to detect the presence of genes in cPCR in this study where the gel images showed a very faint band, suggesting the low abundance of the pmoA1 gene. Another reverse primer mb661 was designed to identify the pmoA1 gene specifically with A189 as the forward primer38. When mb661 was used as the reverse primer, the cPCR showed a brighter band (Supplemental File 1-Supplemental Figure S6),which was later used in qPCR. Interestingly, the pmoA2 gene was detected in two BC samples and one LB sample but was absent in one RV sample (Supplemental File 1-Supplemental Figure S7). A previous study found the annealing temperature as 66 °C and 60 °C for pmoA2, where at 60 °C two different sized amplicons, 245 and 438 bp, were observed, whereas at 66 °C, 206f-703b primers can amplify only pmoA2 with 438 bp product size22. However, in this study, amplification was successful at an annealing temperature of 60 °C, producing a band of the expected size (438 bp) in LB and BC sites (Supplemental File 1-Supplemental Figure S7). Additionally, the mmoX gene was detected in an LB sample but was absent in one RV sample (Supplemental File 1-Supplemental Figure S8) and was entirely absent in BC samples. The primer pairs used for mmoX were also suitable for studying the South Texas wetlands in this study. An annealing temperature of 55 °C36 produced a bright band for mmoX in cPCR (Supplemental File 1-Supplemental Figure S8). To verify sample integrity, 16S rRNA was used as a positive control with successful amplification of 16S rRNA in all samples during cPCR, suggesting that the samples were not contaminated (Supplemental File 1-Supplemental Figure S9).
To assess temporal and spatial changes in gene abundance, qPCR analysis was conducted on all targeted genes using optimized primer sets identified in cPCR. The qPCR of pmoA2 and mmoX genes was conducted with the same primers used in cPCR. However, for the pmoA1 gene, the A189-mb661 primer pair was used, and for the mcrA gene, the MLF-MLR primer pair was selected, as both produced good results in cPCR. Seasonal differences and salinity variation across wetlands of South Texas were also considered, as they could influence microbial community dynamics. As shown in Figure 3 and Figure 4, there was a difference in methanogens' and methanotrophs' gene abundance between two different sampling periods. While there was no seasonal variation in salinity in the coastal freshwater wetlands, a seasonal difference in salinity was observed in the coastal saltwater wetlands39, which could account for the variation in methanogen and methanotroph abundance. Figure 3 shows that during the cool season, the mmoX was highly abundant in all of these wetlands with the highest abundance of 1.46 × 104 copies/µL. There was a small percentage of pmoA2 in one station of LB, RV, and BC. However, qPCR could not detect pmoA1 and mcrA due to the low abundance during the cool season. In contrast, pmoA1 was mostly abundant in the warm season with the highest abundance of 2.44 × 103 copies/µL (Figure 4). The mmoX and mcrA were also detected in the surface sediment samples of LB (within the top 50 cm), showing the maximum abundance of 1.92 × 102 copies/µL and 2.04 × 102 copies/µL, respectively, despite their relatively low abundance during the warm season. Interestingly, during the warm season, pmoA2 was not detected at all, and none of the target genes were detected in BC samples, likely influenced by environmental factors unique to this saltwater wetland39 during the warm season in South Texas.
This study demonstrates distinct spatial and seasonal variations in the distribution of CH4 cycling-related genes across coastal wetlands. Specifically, mcrA genes were detected only in the freshwater LB sample, suggesting a habitat preference. The mmoX and pmoA2 genes were present across all samples in cool seasons, while pmoA1 exhibited in freshwater environments during warm seasons. These results highlight the influence of both environmental conditions and seasonal shifts on CH4 cycling-related microbial activities. Enhanced visualization using kriging interpolation, as shown in Figure 3 and Figure 4, advanced our understanding of CH4 cycling-related microbial activities in coastal wetland ecosystems.
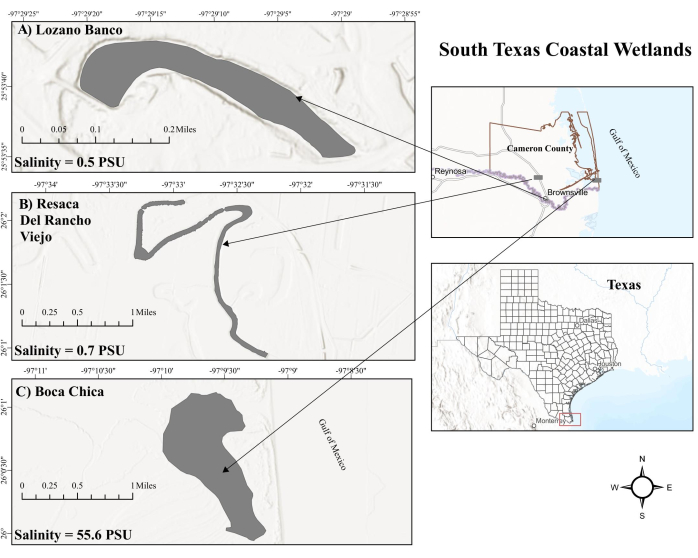
Figure 1: Map of South Texas coastal wetlands. (A) Lozano Banco. LB is a coastal freshwater wetland. (B) Resaca Del Rancho Viejo. RV is also a coastal freshwater wetland. (C) Boca Chica wetland. BC is a tidal-influenced hypersaline coastal saltwater wetland. Abbreviations: LB = Lozano Banco; RV = Resaca Del Rancho Viejo; BC = Boca Chica; PSU = practical salinity units. Please click here to view a larger version of this figure.
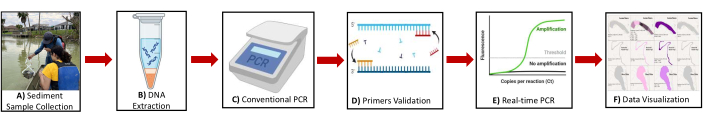
Figure 2: Schematic diagram showing the overview of the protocol. Illustration of the key steps includes (A) Sediment sample collection (B) DNA extraction (C) Conventional PCR (D) Primer's validation (E) Real-time PCR (F) Data visualization for identifying methanogen and methanotrophs' biomarker genes. Please click here to view a larger version of this figure.
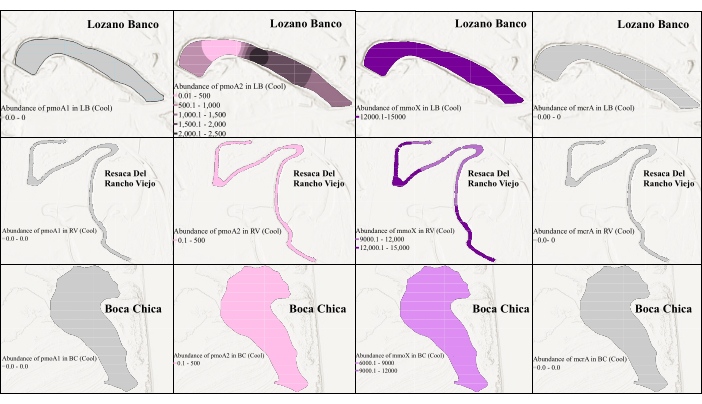
Figure 3: Spatial distribution of methanogen and methanotrophs' biomarker genes in cool season. The figure shows the abundance of mmoX during the cool season. Here, the gray color indicates that the qPCR could not detect the gene because of low abundance. Please click here to view a larger version of this figure.

Figure 4: Spatial distribution of methanogen and methanotrophs' biomarker genes in warm season. Here, the gray color represents that the qPCR could not detect the gene due to its low abundance. The figure shows the abundance of pmoA1 during the warm season in Lozano Banco and Resaca Del Rancho Viejo, while there was no abundance detected in Boca Chica. Please click here to view a larger version of this figure.
| Primer Name | Gene | Annealing Temp. (°C) in this study | Product length (bp) | Sequence (5’-3’) | Reference | |
| 1055F | 16S rRNA | 55 | 337 | ATGGCTGTCGTCAGCT | (Harms et al., 2003) | |
| 1392R | ACGGGCGGTGTGTAC | |||||
| A189F | pmoA1 | 55 | 525 | GGNGACTGGGACTTCTGG | (Holmes et al., 1995) | |
| A682R | GAASGCNGAGAAGAASGC | |||||
| A189F | 56 | 470 | GGNGACTGGGACTTCTGG | (Costello & Lidstrom, 1999) | ||
| mb661R | CCG GMG CAA CGT CYT TACC | |||||
| 206f | pmoA2 | 60 | 438 | GGNGACTGGGACTTCTG GATCGACTTCAAGGATCG | (Tchawa Yimga et al., 2003) | |
| 703b | GAASGCNGAGAAGAASGC GGCGACCGGAACGACGT | |||||
| 536F | mmoX | 55 | 362 | CGCTGTGGAAGGGCATGAAGCG | (Fuse et al., 1998) | |
| 898R | GCTCGACCTTGAACTTGGAGCC | |||||
| MCRf | mcrA | 46 | ~490 | TAYGAYCARATHTGGYT | (Springer et al., 1995) | |
| MCRr | ACRTTCATNGCRTARTT | |||||
| ME1F | 50 | 763 | GCMATGCARATHGGWATGTC | (Hales et al., 1996) | ||
| ME2R | TCATKGCRTAGTTDGGRTAGT | |||||
| MLF | 55 | ~470 | GGTGGTGTMGGATTCA CACARTAYGCWACAGC | (Luton et al., 2002) | ||
| MLR | TTCATTGCRTAGTTWGGRTAGTT | |||||
| McrA 159F | 62 | 186 | AAAGTGCGGAGCAGCAATCACC | (Vaksmaa et al., 2017) | ||
| McrA 345R | TCGTCCCATTCCTGCTGCATTGC | |||||
Table 1: Primer sets used in this study to detect functional genes related to methane cycling. All of these primer pairs were used for conventional PCR, and finally, the bold ones were set up for detecting methanogen and methanotrophs-related genes in South Texas coastal wetlands.
| Gene | Samples | ||||||||
| Lozano Banco | Resaca Del Rancho Viejo | Boca Chica | |||||||
| LB-1 | RV-1 | RV-2 | M1 | M2 | B1 | B2 | MG1 | MG2 | |
| pmoA1 | - | + | + | - | - | - | - | - | - |
| pmoA2 | + | + | + | - | + | - | - | + | - |
| mmoX | + | - | + | - | - | - | - | - | - |
| mcrA | + | + | + | - | - | - | - | - | - |
Table 2: Detection of genes in three different types of water bodies in the cool season using conventional PCR. '+', present; '-', absent. Abbreviations: LB = Lozano Banco, RV = Resaca Del Rancho Viejo, M = Mud, B = Batis, MG = Mangrove.
Supplemental File 1: Gel electrophoresis images for mcrA, pmoA1, pmoA2, mmoX, and 16S rRNA genes. Please click here to download this File.
Discussion
Coastal wetlands are recognized as significant contributors to atmospheric methane, an important greenhouse gas40. Although there have been studies on methane flux and methanogens in wetlands41,42,43, little is known about how methanotrophs operate across different environments or under various management practices, especially in wetlands with fluctuating water levels44. Moreover, the widespread presence of inhibitors such as humic acids in eDNA complicates extraction and amplification processes45, potentially leading to false negative results46. This challenge is further complicated by the variable efficiency of primers working on eDNA, suggesting the need for systematic validation in such studies47. Thus, this research establishes a foundational methodology for understanding methane cycling in the understudied South Texas coastal wetlands. By focusing on the development and validation of PCR techniques, this study advances the detection of key methane-cycling genes in these dynamic ecosystems.
One of the critical aspects of this research is the visualization of microbial methane-cycling-related genes, which plays a crucial role in observing the spatial distribution patterns of these genes across different seasons and salinity gradients. Such visualization is not only a supplementary tool; it is essential for identifying areas of significant methanogenic and methanotrophic microbes' presence indicating there could be potential methane cycling, thereby enhancing our understanding of the environmental conditions that influence these processes.
In this study, the methodology was specified for identifying methanogen and methanotroph marker genes such as pmoA1, pmoA2, mmoX, and mcrA by using cPCR and qPCR. Four primer pairs targeting the mcrA gene and two pairs targeting the pmoA1 gene were used, and only one pair from each set effectively detected these genes in both conventional and real-time PCR. A previous study used three primer pairs for detecting pmoA1; among them, A189-A682 primers could not detect the methanotroph diversity. Instead, they amplified mostly the amoA gene and yielded nonspecific PCR products48. That study found that the primer pair used was effective for detecting the pmoA1 gene only in environments with a high population of methanotrophs. Moreover, the highest abundance of methanotrophs was obtained using the A189-mb661 primer pairs in that study, indicating that these primers are suited for freshwater ecosystems48. Thus, these primer pairs were used for qPCR as further analysis.
Among the four primer pairs used for mcrA, ML primers were able to amplify methanogen species in all environments49. ME primers have a narrow amplification range; they cannot detect all kinds of methanogen species in different types of natural environments. MCR primers have a high level of degeneracy, which makes them sensitive to PCR annealing temperature. This degeneracy allows them to bind to a broad range of target sequences, often accommodating multiple mismatches. However, this also indicates that a small change in annealing temperature can significantly influence their binding efficiency and specificity leading to insufficient amplification and, consequently, false negative results for the methanogen species49,50.
On the other hand, in the low-diversity methanogen community, during the last stages of PCR, high accumulation of PCR products leads to template reannealing and prevents primers from binding, which also fails to detect the desired bands49. Interestingly, McrA 159F-McrA 345R primer pairs were specifically designed to identify nitrate-dependent anaerobic methanotrophic Candidatus M. nitroreducens archaea51, and this study could not detect anaerobic methanotrophs using these primer pairs. However, the anaerobic oxidation of methane (AOM) can be coupled to the reduction of alternative electron acceptors such as iron (Fe), manganese (Mn), and sulfate (SO42-)52, which might explain the inability to detect ANME using this method. Moreover, it might be due to the specific environmental conditions of the study area. Thus, for qPCR, ML primers were used to detect methanogen abundance, as it provided reliable amplification even under conditions of low community diversity49.
In addition, PCR inhibitors such as humic acids or nontarget species DNA are widely present in environmental samples, which can reduce PCR efficiency53. As the high concentration of DNA could not detect any gene by cPCR, all DNA samples were diluted before running cPCR to reduce the effect of PCR inhibitors54. The 16S rRNA gene, as a universal marker for bacteria and archaea, provided a robust baseline for validating our PCR conditions and confirming DNA integrity across all samples55,56. However, saltwater areas are recognized for having higher concentrations of PCR inhibitors46. Moreover, salinity indirectly influences CH4 emissions in coastal wetlands, as it is associated with sulfate concentrations. Higher salinity in saltwater wetlands correlates with elevated sulfate levels that suppress methane production due to competition between sulfate-reducing bacteria and methanogens, whereas lower sulfate concentrations in freshwater wetlands result in higher methane emissions11,41. Additionally, CH4 emission differences between saltwater and freshwater wetlands are also influenced by their tidal connections to the ocean57, which also might explain the unsuccessful detection of methane cycling-related genes by cPCR in BC. In this study, cPCR successfully detected all targeted genes in LB and RV samples but failed to detect the presence of these genes in BC samples, with the pmoA2 gene being the only exception. Moreover, qPCR was able to detect mmoX in all samples, while cPCR could not detect it in BC. This indicates that qPCR shows greater resilience to PCR inhibitors compared to cPCR, attributable to its enhanced sensitivity in detection mechanisms58. Another reason is that qPCR identifies PCR products during the exponential phase of the PCR cycle, whereas cPCR detects them in the plateau phase. This distinction enables qPCR to exhibit reduced susceptibility to product degradation during advanced reaction cycles, where reagents are depleted47.
This study established a PCR technique to identify methane cycling-related genes in South Texas Coastal wetlands to overcome the challenges associated with PCR inhibitors and variable primer efficiencies. Four primer pairs targeting the mcrA gene and two pairs targeting the pmoA1 gene were used, and only one pair from each set effectively detected these genes in both conventional and real-time PCR. This study concludes the set of primer pairs for methane cycling-related genes, including a189-mb6661 for pmoA1, 206f-703b for pmoA2, 536F-898R for mmoX, and ML for mcrA. This suggests the significance of appropriate primer selection for detecting methane cycling-related genes in Coastal wetlands, with those in South Texas as examples. Moreover, the study showed that mmoX was most abundant during the cool season whereas pmoA1 was abundant during the warm season.
Thus, the results of this study provide the groundwork for scientific understanding of microbial processes involved in methane cycling, highlighting the influence of environmental factors across seasonal and spatial variations in South Texas coastal wetlands. Based on these findings, future studies aim to further validate this approach in other ecosystems, such as rice paddies and freshwater wetlands to increase robustness and generalizability. Moreover, these studies will also include geochemical analysis such as sediment methane concentrations and porewater sulfide, and molecular techniques such as metagenomics and transcriptomics, which will be based on this groundwork to provide a more comprehensive understanding of methane cycling dynamics.
Disclosures
The authors have no conflicts of interest to declare.
Acknowledgements
We are thankful to C-REAL members for their assistance in field observation and laboratory analyses.
Materials
| Name | Company | Catalog Number | Comments |
| 0.2 mL PCR tubes | ThermoFisher Scientific | AB0620 | https://www.thermofisher.com/order/catalog/product/AB0620?SID=srch-srp-AB0620 |
| 0.5 mL PCR Tubes | Promega | E4941 | https://www.promega.com/products/biochemicals-and-labware/tips-and-accessories/0_5ml-pcr-tubes/?catNum=E4941 |
| 10 μL tips | ThermoFisher Scientific | 05-408-187 | Fisherbrand SureGrip Pipet Tip Racked or Reload System Tips Natural; 10μL; | Fisher Scientific |
| 15 mL centrifuge tube | ThermoFisher Scientific | 14-959-53A | https://www.fishersci.com/shop/products/falcon-15ml-conical-centrifuge-tubes-5/p-193301 |
| 200 μL tips | ThermoFisher Scientific | 05-408-190 | Fisherbrand SureGrip Pipet Tip Racked or Reload System Tips Natural; 200μL; | Fisher Scientific |
| 1000 μL tips | ThermoFisher Scientific | 02-707-402 | https://www.fishersci.com/shop/products/sureone-micropoint-pipette-tips-specific-standard-fit/02707402?gclid=Cj0KCQiAp NW6BhD5ARIsACmEb kUsQ9Lu0YIq5i4vWege 17qPdtxIYZyvmJH1cDo ARuwereO1V4GLz9UaA lDREALw_wcB&ef_id=C j0KCQiApNW6BhD5ARI sACmEbkUsQ9Lu0YIq5i 4vWege17qPdtxIYZyvmJ H1cDoARuwereO1V4GLz 9UaAlDREALw_wcB:G:s &ppc_id=PLA_goog_2175 7693617_171052169911_02 707402__715434303113_1555 377385658230343&ev_chn=sh op&s_kwcid=AL!4428!3!71543430 3113!!!g!2366517300713!&gad_source=1 |
| Applied Biosystem Power SYBR Green Master Mix | ThermoFisher Scientific | 4368577 | https://www.thermofisher.com/order/catalog/product/4368577 |
| ArcGIS Pro | esri | https://www.esri.com/en-us/arcgis/products/arcgis-pro/overview?srsltid=AfmBOopatJ4 JvHJfscHRcAaDx0Jz5_Jrl8l5 vYkkBvfOqE-uNSsMghN1 | |
| CFX Duet Real-Time PCR system | Bio-Rad | 12016265 | https://www.bio-rad.com/en-us/product/cfx-duet-real-time-pcr-system?ID=97722926-9ed9-16a4-1d83-c92f587e427a |
| Corning Lambda plus single channel pipettor volume 0.5-10 μL | Sigma-Aldrich | CLS4071-1EA | https://www.sigmaaldrich.com/US/en/product/sigma/cls4071 |
| Corning Lambda plus single channel pipettor volume 100-1000 μL | Sigma-Aldrich | CLS4075-1EA | https://www.sigmaaldrich.com/US/en/product/sigma/cls4075 |
| Corning Lambda plus single channel pipettor volume 20-200 μL | Sigma-Aldrich | CLS4074-1EA | https://www.sigmaaldrich.com/US/en/product/sigma/cls4074 |
| FastDNA spin kit for soil | MP Biomedical | 116560200-CF | https://www.mpbio.com/us/116560000-fastdna-spin-kit-for-soil-samp-cf?srsltid=AfmBOoqOxxGilzY3IHNIZR ajegGTr9MoX1oMZUh 3dcbJqe0UvvukY128 |
| Gene copy calculator | Science Primer | https://scienceprimer.com/copy-number-calculator-for-realtime-pcr . | |
| High speed benchtop centrifuge | ThermoFisher Scientific | 75004241 | https://newlifescientific.com/products/thermo-scientific-sorvall-st16-high-speed-benchtop-centrifuge-75004241?gad_source=1&gclid=Cj0KCQiApN W6BhD5ARIsACmEbkVC_-cCIN9j 20TvYq8iDsBlUR5cPK_1_wN OBEcjMdv-CYVoGCfeOLYaAv enEALw_wcB |
| High speed microcentrifuge | VWR | 75838-336 | https://us.vwr.com/store/product/20546590/null |
| Lysing Matrix E tube | glass bead/ceramic sphere-containing tube | ||
| Microcentrifuge tube | ThermoFisher Scientific | 02-681-320 | https://www.fishersci.com/shop/products/fisherbrand-low-retention-microcentrifuge-tubes-8/02681320?gclid=Cj0KCQiAp NW6BhD5ARIsACm EbkWbG4_o3oUiGk HJPU-_31-CuexDwQ fmWPnfyhBOf2BHXsy K3fFW1toaAgJbEALw_ wcB&ef_id=Cj0KCQiAp NW6BhD5ARIsACmEb kWbG4_o3oUiGkHJPU- _31-CuexDwQfmWPnfy hBOf2BHXsyK3fFW1toa AgJbEALw_wcB:G:s&ppc _id=PLA_goog_21757693 617_171052169911_0268 1320__715434303113_10 349826094968484711&ev _chn=shop&s_kwcid=AL!4 428!3!715434303113!!!g!23 66517300713!&gad_source=1 |
| PCR Master mix | Promega | M7502 | https://www.promega.com/products/pcr/taq-polymerase/master-mix-pcr/?catNum=M7502 |
| Quantiflour ONE dsDNA system | Promega | E4871 | https://www.promega.com/products/rna-analysis/dna-and-rna-quantitation/quantifluor-one-dsdna-system/?gad_source=1&gbraid=0AAAAAD _rg189yJTY3cxeVqMdu8RPx10 Ma&gclid=CjwKCAjwxNW2BhAk EiwA24Cm9FUgViPNyWq7UfZL VeeoroLAZ5JIP6w07RGK_4D0w oZgAqf-G1XTmxoCxm8QAvD_B wE&catNum=E4871 |
| Quantus Fluorometer | Promega | E6150 | https://www.promega.com/products/microplate-readers-fluorometers-luminometers/fluorometers/quantus-fluorometer/?catNum=E6150 |
| YSI Pro 2030 | YSI a xylem brand | 603174 | https://www.ysi.com/product/id-p2030/pro2030-kits |
References
- Xu, T., et al. Wetlands of international importance: Status, threats, and future protection. Int J Environ Res Public Health. 16 (10), 1818 (2019).
- Corn, M. L. . Deepwater Horizon oil spill: coastal wetland and wildlife impacts and response. , (2010).
- Hendriks, I. E., Sintes, T., Bouma, T. J., Duarte, C. M. Experimental assessment and modeling evaluation of the effects of the seagrass Posidonia oceanica on flow and particle trapping. Marine Ecology Progress Series. 356, 163-173 (2008).
- Krause, S. J. E., Treude, T. Deciphering cryptic methane cycling: Coupling of methylotrophic methanogenesis and anaerobic oxidation of methane in hypersaline coastal wetland sediment. Geochimica et Cosmochimica Acta. 302, 160-174 (2021).
- Reddy, K. R., DeLaune, R. D., Inglett, P. W. . Biogeochemistry of Wetlands: Science and Applications. , (2022).
- La, W., et al. Sulfate concentrations affect sulfate reduction pathways and methane consumption in coastal wetlands. Water Research. 217, 118441 (2022).
- Derwent, R. G. Global warming potential (GWP) for methane: Monte Carlo analysis of the uncertainties in Global Tropospheric Model predictions. Atmosphere. 11 (5), 486 (2020).
- Potter, C., et al. Methane emissions from natural wetlands in the United States: Satellite-derived estimation based on ecosystem carbon cycling. Earth Interactions. 10 (22), 1-12 (2006).
- . Understanding global warming potentials Available from: https://www.epa.gov/ghgemissions/understanding-global-warming-potentials (2024)
- Wallenius, A. J., Dalcin Martins, P., Slomp, C. P., Jetten, M. S. M. Anthropogenic and environmental constraints on the microbial methane cycle in coastal sediments. Front Microbiol. 12, 631621 (2021).
- Qu, Y., et al. Salinity causes differences in stratigraphic methane sources and sinks. Environmental Science and Ecotechnology. 19, 100334 (2024).
- Vizza, C., West, W. E., Jones, S. E., Hart, J. A., Lamberti, G. A. Regulators of coastal wetland methane production and responses to simulated global change. Biogeosciences. 14 (2), 431-446 (2017).
- van Dijk, G., et al. Salinization lowers nutrient availability in formerly brackish freshwater wetlands; unexpected results from a long-term field experiment. Biogeochemistry. 143 (1), 67-83 (2019).
- Aronson, E., Allison, S., Helliker, B. R. Environmental impacts on the diversity of methane-cycling microbes and their resultant function. Front Microbiol. 4, 225 (2013).
- Reeburgh, W. S. Oceanic methane biogeochemistry. Chem Rev. 107 (2), 486-513 (2007).
- Thauer, R. K. Biochemistry of methanogenesis: a tribute to Marjory Stephenson. Marjory Stephenson Prize Lecture. Microbiology (Reading). 144 (Pt 9), 2377-2406 (1998).
- Thauer, R. K. Anaerobic oxidation of methane with sulfate: on the reversibility of the reactions that are catalyzed by enzymes also involved in methanogenesis from CO2. Curr Opin Microbiol. 14 (3), 292-299 (2011).
- Friedrich, M. W. Methyl-coenzyme M reductase genes: Unique functional markers for methanogenic and anaerobic methane-oxidizing Archaea. Methods in Enzymology. 397, 428-442 (2005).
- Reeburgh, W. S. Oceanic methane biogeochemistry. Chem Rev. 107 (2), 486-513 (2007).
- Rasigraf, O., Schmitt, J., Jetten, M. S. M., Lüke, C. Metagenomic potential for and diversity of N-cycle driving microorganisms in the Bothnian Sea sediment. Microbiologyopen. 6 (4), e00475 (2017).
- McDonald, I. R., Bodrossy, L., Chen, Y., Murrell, J. C. Molecular ecology techniques for the study of aerobic methanotrophs. Appl Environ Microbiol. 74 (5), 1305-1315 (2008).
- Tchawa Yimga, M., Dunfield, P. F., Ricke, P., Heyer, J., Liesack, W. Wide distribution of a novel pmoA-like gene copy among type II methanotrophs, and its expression in Methylocystis strain SC2. Appl Environ Microbiol. 69 (9), 5593-5602 (2003).
- Knief, C. Diversity and habitat preferences of cultivated and uncultivated aerobic methanotrophic bacteria evaluated based on pmoA as molecular marker. Front Microbiol. 6, 1346 (2015).
- Huang, I. -. S., et al. Preliminary assessment of microbial community structure of Wind-Tidal Flats in the Laguna Madre, Texas, USA. Biology. 9 (8), 183 (2020).
- Wilding, T. K., Brown, E., Collier, K. J. Identifying dissolved oxygen variability and stress in tidal freshwater streams of northern New Zealand. Environ Monit Assess. 184 (10), 6045-6060 (2012).
- Roy Chowdhury, T., Mitsch, W. J., Dick, R. P. Seasonal methanotrophy across a hydrological gradient in a freshwater wetland. Ecological Engineering. 72, 116-124 (2014).
- Sun, Q. -. Q., et al. Carbon dioxide and methane fluxes: Seasonal dynamics from inland riparian ecosystems, northeast China. Sci Total Environ. 465, 48-55 (2013).
- Lee, S., et al. Comparison and selection of conventional PCR primer sets for studies associated with nitrogen cycle microorganisms in surface soil. Appl Sci. 12 (20), 10314 (2022).
- Bae, K. -. S., et al. Development of diagnostic systems for wide range and highly sensitive detection of two waterborne hepatitis viruses from groundwater using the conventional reverse transcription nested PCR assay. J Virol Methods. 299, 114344 (2022).
- Lecusay, D. . Assessment and Monitoring of Deltaic Wetlands and Fluvial Systems: Refining and Validating a Multimetric Index of Resaca Ecosystem Health. , (2021).
- . FastDNA SPIN Kit for Soil (Cat No. 116560200) Available from: https://www.mpbio.com/media/productattachment/LS082019-EN-FastDNA-SPIN-Kit-for-Soil-116560200-Manual.pdf (2025)
- Luton, P. E., Wayne, J. M., Sharp, R. J., Riley, P. W. The mcrA gene as an alternative to 16S rRNA in the phylogenetic analysis of methanogen populations in landfill. Microbiology (Reading, England). 148 (Pt 11), 3521-3530 (2002).
- Changsoo, L., Jaai, K., Seung Gu, S., Seokhwan, H. Absolute and relative QPCR quantification of plasmid copy number in Escherichia coli. J Biotechnol. 123 (3), 273-280 (2006).
- Harms, G., et al. Real-time PCR quantification of nitrifying bacteria in a municipal wastewater treatment plant. Environ Sci Technol. 37 (2), 343-351 (2003).
- Holmes, A. J., Costello, A., Lidstrom, M. E., Murrell, J. C. Evidence that particulate methane monooxygenase and ammonia monooxygenase may be evolutionarily related. FEMS Microbiol Lett. 132 (3), 203-208 (1995).
- Fuse, H., et al. Oxidation of trichloroethylene and dimethyl sulfide by a marine Methylomicrobium strain containing soluble methane monooxygenase. Biosci Biotechnol Biochem. 62 (10), 1925-1931 (1998).
- Springer, E., Sachs, M. S., Woese, C. R., Boone, D. R. Partial gene sequences for the A subunit of methyl-coenzyme M reductase (mcrI) as a phylogenetic tool for the family Methanosarcinaceae. Int J Syst Bacteriol. 45 (3), 554-559 (1995).
- Costello, A. M., Lidstrom, M. E. Molecular characterization of functional and phylogenetic genes from natural populations of methanotrophs in lake sediments. Appl Environ Microbiol. 65 (11), 5066-5074 (1999).
- Flores, E. A. . Effects of Nutrient Enrichment on Mangrove and Saltmarsh Habitats. , (2022).
- Minjie, H., Jordi, S., Xianyu, Y., Josep, P., Chuan, T. Patterns and environmental drivers of greenhouse gas fluxes in the coastal wetlands of China: A systematic review and synthesis. Environ Res. 186, 109576 (2020).
- Bridgham, S. D., Cadillo-Quiroz, H., Keller, J. K., Zhuang, Q. Methane emissions from wetlands: biogeochemical, microbial, and modeling perspectives from local to global scales. Glob Chang Biol. 19 (5), 1325-1346 (2013).
- Liu, D. Y., Ding, W. X., Jia, Z. J., Cai, Z. C. Relation between methanogenic archaea and methane production potential in selected natural wetland ecosystems across China. Biogeosciences. 8 (2), 329-338 (2011).
- Ke, Z., et al. Methane emissions and methanogenic community investigation from constructed wetlands in Chengdu City. Urban Climate. 39, 100956 (2021).
- Chowdhury, T. R., Dick, R. P. Ecology of aerobic methanotrophs in controlling methane fluxes from wetlands. Applied Soil Ecology. 65, 8-22 (2013).
- Maja, S., et al. Humic substances cause fluorescence inhibition in real-time polymerase chain reaction. Anal Biochem. 487, 30-37 (2015).
- Sanches, T. M., Schreier, A. D. Optimizing an eDNA protocol for estuarine environments: Balancing sensitivity, cost and time. PLOS ONE. 15 (5), e0233522 (2020).
- Xia, Z., et al. Conventional versus real-time quantitative PCR for rare species detection. Ecol Evol. 8 (23), 11799-11807 (2018).
- Bourne, D. G., McDonald, I. R., Murrell, J. C. Comparison of pmoA PCR primer sets as tools for investigating methanotroph diversity in three Danish soils. Appl Environ Microbiol. 67 (9), 3802-3809 (2001).
- Juottonen, H., Galand, P. E., Yrjala, K. Detection of methanogenic Archaea in peat: comparison of PCR primers targeting the mcrA gene. Res Microbiol. 157 (10), 914-921 (2006).
- Lueders, T., Friedrich, M. W. Evaluation of PCR amplification bias by terminal restriction fragment length polymorphism analysis of small-subunit rRNA and mcrA genes by using defined template mixtures of methanogenic pure cultures and soil DNA extracts. Appl Environ Microbiol. 69 (1), 320-326 (2003).
- Vaksmaa, A., Jetten, M. S., Ettwig, K. F., Luke, C. McrA primers for the detection and quantification of the anaerobic archaeal methanotroph 'Candidatus Methanoperedens nitroreducens'. Appl Microbiol Biotechnol. 101 (4), 1631-1641 (2017).
- Ren, G., et al. Electron acceptors for anaerobic oxidation of methane drive microbial community structure and diversity in mud volcanoes. Environ Microbiol. 20 (7), 2370-2385 (2018).
- Goldberg, C. S., et al. Critical considerations for the application of environmental DNA methods to detect aquatic species. Methods in Ecology and Evolution. 7 (11), 1299-1307 (2016).
- McKee, A. M., Spear, S. F., Pierson, T. W. The effect of dilution and the use of a post-extraction nucleic acid purification column on the accuracy, precision, and inhibition of environmental DNA samples. Biological Conservation. 183, 70-76 (2015).
- Gohl, D. M., et al. Systematic improvement of amplicon marker gene methods for increased accuracy in microbiome studies. Nat Biotechnol. 34 (9), 942-949 (2016).
- Ballarini, A., Segata, N., Huttenhower, C., Jousson, O. Simultaneous quantification of multiple bacteria by the BactoChip microarray designed to target species-specific marker genes. PLOS ONE. 8 (2), e55764 (2013).
- Le Mer, J., Roger, P. Production, oxidation, emission and consumption of methane by soils: A review. Eur J Soil Biol. 37 (1), 25-50 (2001).
- Smith, C. J., Osborn, A. M. Advantages and limitations of quantitative PCR (Q-PCR)-based approaches in microbial ecology. FEMS Microbiol Ecol. 67 (1), 6-20 (2009).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved