Method Article
Microfluidics in Assessing Platelet Function
W tym Artykule
Podsumowanie
Platelet function under flow can be assessed and simulated hemostatic resuscitation can be modeled using a microfluidic device, which has applications in trauma and transfusion medicine.
Streszczenie
Microfluidics incorporate physiologically relevant substrates and flows that mimic the vasculature and are, therefore, a valuable tool for studying aspects of thrombosis and hemostasis. At high-shear environments simulating arterial flow, a microfluidic assay facilitates the study of platelet function, as platelet-rich thrombi form in a localized stenotic region of a flow channel. Utilizing devices that allow for small sample volume can additionally aid in evaluating platelet function under flow from volume-limited patient samples or animal models. Studying trauma patient samples or samples following platelet product transfusion may aid in directing therapeutic strategies for patient populations in which platelet function is critical. Effects of platelet inhibition via pharmacological agents can also be studied in this model. The objective of this protocol is to establish a microfluidic platform that incorporates physiologic flow, biological surfaces, and relevant hemostatic mechanisms to assess platelet function with implications for the study of trauma induced coagulopathy and transfusion medicine.
Wprowadzenie
Trauma is a leading global cause of death and disability. Severe injury is frequently complicated by a unique, endogenous disturbance of hemostasis and thrombosis, termed trauma-induced coagulopathy (TIC)1. Platelets play a critical role in TIC, and they have been described as having both adaptive and maladaptive functions2. The mechanisms of platelet dysfunction after injury remain unclear, and there is a critical need to better understand the cellular response to guide the development of improved resuscitation and therapy. An additional vexing problem regarding platelet function after injury is the uncertainty of the reliability of present readouts of platelet function in the trauma patient.
Multiple studies have shown that even mildly injured patients, with no known clinical bleeding phenotype, have abnormal platelet function using conventional platelet function testing such as aggregometry3,4. However, limitations in aggregometry to assess platelet function in an injury setting include a lack of physiologically relevant injury surface, a reductionist approach to agonist stimulation, sample dilution with whole blood impedance aggregometry, plasma separation with optical light transmission aggregometry, and stagnant sample assessment. Additionally, whether this sensitivity of platelet function represents true cellular dysfunction or a measurement artifact, such as increased baseline electrical impedance, in the setting of injury remains unclear2. Thus, studying relevant platelet functions in the context of trauma is crucial to understanding TIC, and there is substantial room for innovation and improvement in this area.
Platforms traditionally used to study platelet function do not include fluid dynamics and flow, which may be critical in understanding platelet dysfunction pertaining to trauma and trauma-induced coagulopathy5. Mechanisms of hemostasis that are dependent on flow include von Willebrand factor (VWF) elongation at high shear, above a critical shear rate, and platelet capture via glycoprotein 1b6,7,8, which are not captured using stagnant platelet function assays. Additionally, platelets preferentially bind VWF or fibrinogen depending on the flow regime and elicit differential roles in arterial versus venous thrombosis9,10. Arterial thrombi are mainly comprised of platelets while venous thrombi are mainly comprised of red blood cells, based, in part, on flow regimes11. Assays that incorporate flow regimes can aid in elucidating dysfunctions pertaining to the spectrum of TIC phenotypes, from hypocoagulability and bleeding phenotypes to hypercoagulability and thrombotic phenotypes. Finally, blood volume sampling constraints with trauma patient populations may make traditional platelet function testing challenging. While assays such as flow cytometry can and should be utilized in these circumstances, results often depict a physical characterization of a sample and not a hemostatic functional assessment.
While mechanisms of platelet dysfunction may not be completely understood in trauma, modeling platelet dysfunction in vitro, with P2Y12 antagonists for example, can also help guide the study of therapeutic interventions. Hemostatic resuscitation is critically important in trauma patients where blood products are transfused in a balanced approach to address shock, coagulopathy, and endothelial injury with either whole blood or blood components (red blood cells, plasma, and platelet concentrates) in a 1:1:1 unit ratio12,13,14. In trauma patients, early use of blood products is associated with improved survival15,16. To extend shelf life, cold-stored platelet products have been increasingly studied. Examination of cold-stored platelets shows increased hemostatic activity, as well as safety when transfused following injury17,18.
The evolution of cold-stored platelet resuscitation emphasizes the need for additional testing to understand the most efficacious platelet product available for trauma. However, traditional platelet function assays are often over- or under-potentiated to detect dysfunction, occurring both in the trauma patient receiving therapeutic platelet transfusion as well as in the transfused product itself seen in platelet storage lesions. Determining the origin of dysfunction can be challenging, given the limitations in current platelet function assays, including the static nature of most of these tests. Therefore, when studying hemostatic resuscitation in vitro, the platform and detection methods for both recipient and product platelet populations are of critical importance in determining optimal therapeutic interventions.
Microfluidic testing offers flow profiles and biofidelic surfaces to create a physiologically relevant assay on which to study platelets. Microfluidic devices can be customized to study particular pathophysiology or injury types, such as vessel puncture19 or endothelial damage20. These devices are generally comprised of polydimethylsiloxane (PDMS) bonded to a glass microscope slide with surface modifications, such as collagen, to mimic sub-endothelium and tissue injury. Utilizing these types of flow-based devices can aid in guiding trauma-related platelet dysfunction research and aid in examining optimal transfusion medicine approaches to ameliorate platelet dysfunction. These strategies may help to clarify the existing confusion about the relevance of static platelet assays such as aggregometry in the injured patient.
Protokół
All research was performed in compliance with institutional guidelines. Approval from the University of Pittsburgh Human Research Protection Office was obtained and informed consent from healthy human volunteers was obtained.
1. Microfluidic device preparation
- To fabricate the PDMS portion of the device, prepare a master mold using brass via computer numerical control (CNC) micromachining.
NOTE: Depending on channel dimensions, photolithography techniques may be used to create a master mold. The device used in this protocol includes eight parallel micromachined channels approximately 480 µm wide, 140 µm tall at the device inlet and outlet, and 40 µm tall at the device stenosis, with a ramp length to/from the stenotic region of approximately 0.3 mm. Channel lengths are approximately 6 mm. - With a master mold obtained, pour the Silicone Elastomer Base (obtained from the elastomer kit) into a weighing dish. Add the Silicone Curing Agent (obtained from the elastomer kit), which facilitates the cross-linking of the silicone polymer chains to transform the liquid PDMS into a durable and flexible solid, at a ratio of 10:1 (base agent) and stir the mixture well.
- Place the mold into a Petri dish and pour the uncured PDMS onto the mold. Place the Petri dish inside a vacuum desiccator for 30 min to remove bubbles.
- Finish curing the PDMS in the master mold by placing it in an oven set to 70 °C for 90 min.
- After complete PDMS curing, cut out the microfluidic cast using a razor blade or scalpel. Punch holes at the edges of the channels (1.5 mm in diameter on both sides).
- Using lab tape, clean the surface of a glass slide and the etched side of the microfluidic cast. Use compressed air as needed to remove remaining debris.
- Place the glass slide and the microfluidic cast with etched side upward into a Plasma Cleaner. Start the vacuum pump, seal the chamber, and turn on the plasma cleaner to the high setting. Leave the slide and PDMS in the plasma cleaner for 30 s, then turn the plasma cleaner off and remove vacuum.
- Bind the plasma-cleaned cast and glass slide together by gently pressing together the sides that were face up in the plasma cleaner. Then, place the microfluidic device in an oven/hotplate at 70 °C for 10 min.
NOTE: Do not apply too much pressure when bonding the cast and glass slide together as that could lead to a loss in channel morphology. - Rinse each chamber with 10-30 µL of 70% ethanol to sterilize and allow the microfluidic device to dry on a 70 °C hot plate.
NOTE: Devices should be made at least 24 h in advance but may be made weeks to months in advance. Devices should be stored in an airtight container or covered Petri dish at room temperature. - The day before experimentation with the microfluidic device, re-rinse each chamber with 10-30 µL of 70% ethanol to sterilize and allow the microfluidic device to dry on a 70 °C hotplate.
- Coat the chamber with Type 1 equine fibrillar collagen reagent (1 mg/mL), diluted in 0.9% NaCl in a 1:5 volumetric ratio through a designated outlet to designated inlet. Make sure directionality is conserved within an experiment. Store the device in a warm, humid closed container to prevent evaporation of the coated collagen within the channel.
- After 1 h, rinse with phosphate-buffered saline (PBS) to flush out the collagen solution. Flush in the opposite direction of the coating. When not in use, store the device again in a warm, humid, closed container.
2. Blood sample preparation
- Obtain a citrated whole blood sample via venipuncture. Incubate citrated whole blood with FC receptor blocking solution (1:600).
NOTE: Blood samples are stored at room temperature prior to and during experimentation. - Incubate citrated whole blood with fluorescence-conjugated (using a fluorophore of choice) CD41 antibody (1:600). Stain for 30 min on a nutating rocker.
- As a positive control for platelet inhibition, add Ticagrelor, a P2Y12 receptor antagonist reconstituted in a solution of 30% w/v 2-hydroxypropyl-β-cyclodextrin (HP-β-CD) in PBS.
NOTE: Ticagrelor stocks up to 6.4 mM should be prepared and further dilution of Ticagrelor stocks in HP-β-CD solution (30% HP-β-CD dissolved in PBS) can be done prior to experimentation (1,000x stock concentrations are recommended). - Incubate the citrated sample with Ticagrelor (final concentration up to 6.4 µM) for 30 min to observe platelet inhibition.
- If mixing the blood product with the citrated sample, stain the blood product with FC receptor blocking solution (1:600) and fluorescence-conjugated (using a separate and distinct fluorophore to the citrated sample) CD41 antibody (1:600).
- Mix a volumetric equivalent of the transfused product units with the citrated sample. For example, to simulate 2 units of platelet products transfused into a hemorrhaging person (approximately 250 mL per product into a total blood volume of 5,000 mL), mix 100 µL of blood product into 1,000 µL of citrated blood sample.
- Immediately before experimentation, filter the blood sample through a 40 µm filter into a sterile 1.5 mL microcentrifuge tube.
3. Platelet function testing under flow (Method 1)
- Turn on the microscope and associated software.
- Set a withdrawal syringe pump level with the stage of the microscope. Adjust the settings on the syringe pump.
- Calculate a volumetric flow rate (Q) for a desired average wall shear rate (γ) of 3,500 s-1 at the stenotic region of the channel using equations (1) and (2)21.
 (1)
(1)
 (2)
(2)
Where A is the cross-sectional area of the channel, P is the wetted perimeter, λ is the shape factor, b is the short side of the rectangle (height), and a is the long side of the rectangle (width).
NOTE: The value of 3,500 s-1 is chosen due to VWF critical shear and is in the arterial regime7,22,23.
- Calculate a volumetric flow rate (Q) for a desired average wall shear rate (γ) of 3,500 s-1 at the stenotic region of the channel using equations (1) and (2)21.
- Place the microfluidic device on the microscope stage. Tape the edges of the microfluidic device to the stage to avoid movement. Ensure that the outlet is facing the back of the microscope.
- Connect one end of 1/16” ID tubing (approximately 30 cm long) to an elbow connector and the other end to a 10 mL syringe with 1/16” ID connector.
- Fill the syringe with sterile PBS and connect it to the syringe pump.
- Place the elbow connector into the device outlet.
- Prepare inlet lines approximately 10 cm long with the elbow connector on one end and the angled cut on the other end.
- Connect the inlet elbow connector into the device inlet.
- Position the inlet line into a waste microcentrifuge tube on an angled holder.
- Use the 10x objective for channel dimensions approximately 500 µm in width and focus on the microfluidic device channel edges.
- Prime the lines with PBS and clear the channel of any PDMS/debris by advancing the syringe pump manually. Be sure to check near the channel inlet and outlet.
- Open the saved image capture settings or create an image capture procedure for time series images captured every 1–2 s with a brightfield channel and fluorescent channels corresponding to the fluorescent CD41 antibodies used in the blood sample.
- Obtain the filtered citrated blood sample and mix by pipetting up and down just prior to the experiment. Place the sample on the angled microcentrifuge holder.
- Position the inlet line into the sample.
- Start recording the image capture.
- Slowly, withdraw the syringe to fill the dead volume in the tubing. Once blood reaches the channel, immediately hit play on the syringe pump to resume flow at the desired shear rate.
- Adjust the focus if needed.
- Run the experiment until platelets have completely occluded the stenotic region of the microfluidic device or until an experimental endpoint (i.e., 10 min).
- Ensure the inlet tubing is submerged in the blood sample for the duration of the experiment.
- Once the experiment is complete, stop the image capture and stop the syringe pump. Save the image capture.
- Remove the inlet elbow connector and empty the contents of the tubing into a waste conical. Empty the contents of syringe and outlet lines into waste conical as well.
- Replace the inlet and outlet connectors and tubing as necessary for subsequent samples.
4. Platelet function testing under flow with low-volume samples (below 1 mL) (Method 2)
- Repeat steps 1.1 through 1.4 as above.
- Punch a 1.5 mm in diameter outlet and 3 mm in diameter inlet at the edges of the channels.
- Repeat steps 1.6 through 3.6 as above.
- Use the 10x objective for channel dimensions approximately 500 µm in width and focus on the microfluidic device channel edges.
- Clear the channel of any PDMS/debris by advancing the syringe pump manually and wicking away access fluid with a laboratory wipe. Remove any debris with lab tape on the top of the microfluidic device.
- Manually advance the pump to fill the 3 mm inlet reservoir with PBS.
- Open the saved image capture settings or create an image capture procedure for time series images captured every 1–2 s with a brightfield channel and fluorescent channels corresponding to the fluorescent CD41 antibodies used in the blood sample.
- Start withdrawal on the syringe pump (manually or with set speed) and once PBS is advancing into the channel and the fill line in the reservoir is going down, pause the withdrawal on the pump.
- Remove excess PBS from the reservoir with a pipette.
- Start the image capture.
- Pipette the blood sample into the reservoir (approximately 40 μL) and immediately start the withdrawal syringe. Ensure flow starts.
NOTE: If the syringe pump motor for withdrawal was not primed in step 4.8, flow will not start immediately, and the experiment should be repeated. - Refill the blood reservoir for the duration of the experiment, ensuring no air pockets enter the channel.
- Run the experiment until the platelets have completely occluded the stenotic region of the microfluidic device or until an experimental endpoint (i.e., 10 min).
- Once the experiment is complete, stop image capture and stop the syringe pump. Save the image capture.
- Remove excess blood in the reservoir by pipetting. Empty the contents of the withdrawal tubing into a waste conical tube.
- Replace the connectors and tubing as necessary for subsequent samples.
5. Decontamination
- Clear the inlet and outlet lines of blood by flushing into a 10% bleach solution.
- If all the channels on the microfluidic device have been used, then discard the device in the Sharps biohazardous waste container.
NOTE: All biological waste should be disposed of in biohazard waste appropriately.
6. Image analysis
- Export the images from kinetic experiments using software by clicking File | Export/Import | Export.
- Select the following parameters: filetype: Tagged Image File Format (TIFF); compression: LZW; check Original Data and Shift Pixel; uncheck Apply display curve and channel color; check Define Subset (Region, Rectangle region, and select the channel area with a conserve width and height measurement between conditions). Export to the desired folder and check Create folder.
- Note the below experimental values on the software: start frame when blood enters the channel; frame rate (Info, Time Series).
- Measure the normalized mean fluorescence intensity of each frame in an experiment utilizing Matlab code provided as Supplemental File 1, changing the following inputs for each experiment: start frame/end frame based on experiment length (ensure the experiment length is conserved between conditions); experiment name/folder name; cropping X, Y, H, and W parameters. Run the code once for the recipient platelet fluorescent channel and once for the product platelet fluorescent channel. Report normalized MFI (column 2) and normalized AUC values (normalized to start frame). Subtract the experiment length value from the normalized AUC value.
Wyniki
Microfluidic experiments following the use of this method should show platelet-rich thrombi formation in the region of stenosis of the flow channel (Figure 1). Figure 1A illustrates representative results where functional platelets formed a thrombus in the stenotic region of the channel to block blood flow through the channel. Mean fluorescence intensity (MFI) curves of kinetic images taken for the duration of the experiment illustrate a lag, growth, and plateau phase of platelet incorporation in the growing thrombus (Figure 2A,C). Increasing concentrations of a P2Y12 antagonist, Ticagrelor, decreases platelet MFI as well as the calculated area under the curve (AUC) of MFI curves (Figure 2B,D). Longer incubation times are needed (30 min compared to 5 min) to observe pronounced platelet dysfunction, in accordance with a more pronounced dose-response relationship utilizing whole blood impedance aggregometry after a 30 min Ticagrelor incubation in comparison to 5 min incubation (Supplemental Figure S1). Under differing vehicle conditions, similar time-dependent effects of P2Y12 inhibition were observed in the microfluidic model (Supplemental Figure S2).
Visualization of two platelet populations in the simulated hemostatic resuscitation method illustrates the incorporation of both platelet populations within the thrombus (Figure 3A). The incorporation of both platelet populations in their corresponding fluorescent signal can be quantified kinetically over the duration of the experiment via MFI measurements (Figure 3B). Steeper slopes in the growth phase as well as increased endpoint MFI measurements demonstrate increased platelet functionality and hemostatic potential, which can be seen in the quantification of recipient platelets when the blood sample was induced with platelet dysfunction via P2Y12 inhibition, and then, resuscitation simulated by mixing fresh autologous whole blood in a 1:10 volumetric ratio stained with a distinct platelet fluorophore. With autologous whole blood mixing, more of the platelets were incorporated from the simulated transfused product compared to an apheresis platelet product at room temperature storage day 5, which showed minimal product incorporation in the forming thrombus. Day 5 room temperature platelet product mixing also showed lower recipient platelet incorporation compared to mixing with fresh autologous whole blood (Figure 3B).
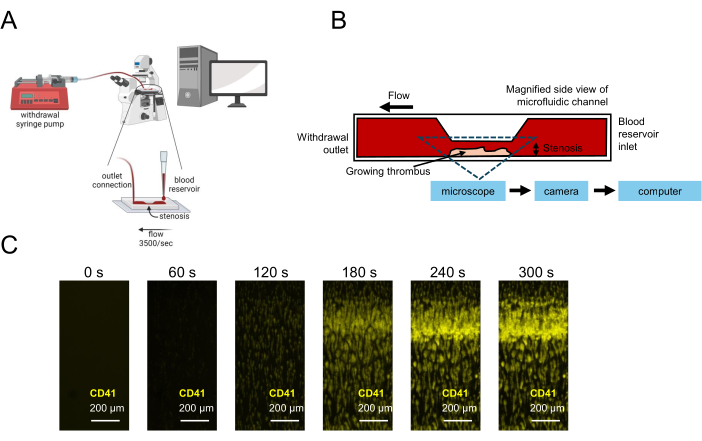
Figure 1: Representation of platelet function testing using microfluidics. (A) Schematic microfluidic experimental setup for platelet function testing using low-volume blood samples is shown, including a withdrawal syringe pump pulling blood through a stenotic flow chamber, allowing for image capture in real time. (B) A side view of a stenotic flow chamber is shown, including a collagen-coated surface on which platelets will adhere, activate, and aggregate, resulting in a growing thrombus at the stenotic region. (C) Real-time image capture of a citrated blood sample under flow in a stenotic microfluidic channel (3,500 s-1) and a growing thrombus formed over 300 s. Scale bars = 200 µm (C). Please click here to view a larger version of this figure.
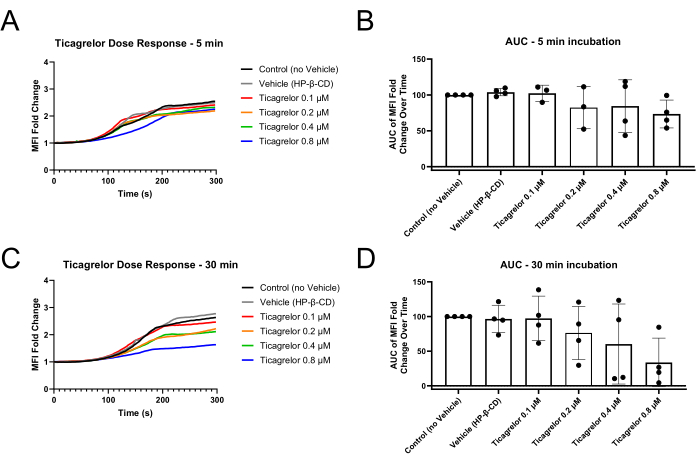
Figure 2: Reduced platelet MFI fold changes and area under the curve after 30 min of incubation with P2Y12 antagonist Ticagrelor. (A) Incubation with P2Y12 antagonist for only 5 min demonstrates slightly extended lag phases of the MFI curves and (B) slight decreases in MFI AUC. (C) Incubation for 30 min with P2Y12 antagonist results in extended lag phases and reduced endpoint MFI values as well as (D) more robust dysfunction demonstrated via MFI AUC. Individual data points represent biological replicates and mean ± standard deviation is shown. Abbreviations: MFI = mean fluorescence intensity; AUC = area under the curve; HP-β-CD = 2-hydroxypropyl-β-cyclodextrin. Please click here to view a larger version of this figure.

Figure 3: Blood product mixing with coagulopathic P2Y12 inhibition in the recipient sample, simulating patient platelet dysfunction, in the microfluidic chamber. (A) Apheresis platelet unit (plasma, storage day 7) (CD41 in cyan) mixed in a 1:10 ratio with citrated blood pre-incubated with 0.8 µM Ticagrelor for 30 min (CD41 in red). (B) Representative normalized mean fluorescence intensity curves of recipient blood sample receiving either apheresis platelet unit (storage day 5) or autologous fresh whole blood (1:10 ratio) along with the recipient platelet kinetics over time. Recipient citrated blood was pre-incubated with 0.8 µM Ticagrelor for 30 min before mixing with blood products. (C) Representative z-stack image from a modified device bonded to a coverslip for confocal microscopy. Apheresis platelet product was mixed with a thrombocytopenic blood sample in a 1:5 volumetric ratio (product:blood) in addition to a von Willebrand factor antibody (1:600). Please click here to view a larger version of this figure.
Supplemental File 1: Matlab code for image analysis. Please click here to download this File.
Supplemental Figure S1: Whole blood impedance aggregometry time-dependence of P2Y12 inhibition with Ticagrelor. Incubation for 30 min results in a more pronounced dose-response relationship compared to 5 min. Vehicle and drug solutions were utilized at 0.1% v/v. Individual data points represent biological replicates and mean ± standard deviation is shown. Abbreviations: AUC = area under the curve; HP-β-CD = 2-hydroxypropyl-β-cyclodextrin. Please click here to download this File.
Supplemental Figure S2:Time-dependent P2Y12 inhibition with Ticagrelor in a microfluidic model of platelet function. Representative MFI curves and area under the curve values for technical replicates from an individual healthy donor are shown with ethanol vehicle (1% v/v) utilized for drug solubility. Abbreviations: MFI = mean fluorescence intensity; AUC = area under the curve. Please click here to download this File.
Dyskusje
The above protocol has some critical steps to ensure the reliability and reproducibility of experiments. First, fluorescent antibodies should be carefully considered. The antibodies used to detect platelets in the sample should not block the function of the glycoprotein Ib (GPIb) platelet receptor. Lot matching, whenever possible between experiments, is also critical to ensure the reproducibility of the fluorescent signal. Another critical step in this protocol is using sterile consumables and solutions and filtered samples whenever possible. Filtering blood samples immediately prior to experimentation will prevent debris or platelet clumps larger than the channel dimensions from blocking flow, which is another critical parameter to keep consistent between experiments. Additionally, blood samples should be tested within 4 h of collection as described in the circular of information for the use of human blood and blood components prepared by AABB, the American Red Cross, America's Blood Centers, and the Armed Services Blood Program, except when testing storage lesion of blood products.
Modifications can be considered in this protocol, such as bonding the PDMS to a coverslip for confocal microscopy, illustrated in Figure 3C. If necessary, prior to imaging, a fixative solution may be perfused through the channels and washed with PBS for storage prior to imaging. Additionally, while reproducible channel dimensions are key to ensuring conserved shear rates between conditions, the height of the stenotic region can be modified but will directly affect the timing of thrombus formation, even if matching shear rates. Since larger channel dimensions will require more time to reach substantial thrombus formation, more sample volume will be necessary. When troubleshooting this protocol, an important consideration should be the coating strategy. Specific collagen type, timing, dilution, and storage conditions are all factors that may affect the surface coat. While this protocol has been validated for this particular collagen reagent (Table of Materials), collagen alternatives that can reliably adhere to the device surface and adhesion validated via immunofluorescence staining or other measures can be considered and have been previously used by other groups successfully24.
A potential limitation of this protocol is a lack of direct endothelial response. However, coating strategies and modifications can be made to include platelet function testing in response to endothelial damage. For example, inflammatory factors that endothelial cells secrete in response to injury could be modified in the coating strategy of this protocol or added exogenously to the blood sample tested. The addition of these factors without the complexities of cellularization would allow for a targeted approach to examine the effects of endotheliopathy on platelet function. In a similar vein, patient plasma from disease or healthy control states can be spiked into the system to test the impact of plasma soluble mediators on platelet function.
The study of platelet function under flow following this protocol can facilitate the study of trauma-induced coagulopathy and transfusion medicine approaches in trauma. Current platelet function testing is often over or under potentiated to see a dysfunctional response in a trauma patient. This method allows for flexibility and design modifications to observe platelet function in trauma patient samples, even with volume constraints, in addition to simulation of platelet dysfunction to assess therapeutic interventions. Beyond the trauma patient, this method could be considered for postpartum hemorrhage patients, cardiac surgery patients, or cancer patients to assess platelet functionality and potential therapeutic interventions. Importantly, this method incorporates flow dynamics that are of critical importance to the mechanisms of platelet plug formation and hemostatic function.
Ujawnienia
The authors have no conflicts of interest to declare.
Podziękowania
The authors acknowledge and thank all blood donors who participated, as well as the Trauma and Transfusion Medicine Research Lab phlebotomists and the UPMC Montefiore Clinical and Translational Research Center for assistance in collections. SMS is supported by K25HL161401. MDN is supported by 1R01HL166944-01A1.
Materiały
| Name | Company | Catalog Number | Comments |
| Equipments | |||
| Axio Observer | Zeiss | 491917-0001-000 | |
| Bel-Art Space Saver Vacuum Desiccators | Fisher Scientific | 08-594-15A | |
| Fisherbrand Isotemp Digital Hotplate Stirrer | Fisher Scientific | FB30786161 | |
| Nutating Mixer | Fischer Scientific | 88-861-043 | |
| OHAUS Scout Balance Scale | Uline | H-5852 | |
| Oven | Fisher Scientific | 15-103-0520 | |
| Plasma cleaner | Harrick | PDC-32G (115V) | |
| Syringe Pump (PHD ULTRA CP, I/W PROGRAMMABLE) | Harvard Apparatus | 883015 | |
| Zen 3.4 | Zeiss | Blue edition | Software |
| Material | |||
| 1/16 inch ID - Barbed Elbow Connectors | Qosina | 11691 | |
| 10 mL syringe | Fischer Scientific | 14-955-459 | |
| 2-Hydroxypropyl-β-cyclodextrin | Cayman Chemicals | 16169 | 30% Dissolved in Phosphate buffered saline |
| 40-micron filters | Fischer Scientific | NC1469671 | |
| CD41 antibody | Novus Biologicals | NB100-2614 | 1:600 Ratio in Whole Blood |
| Chrono-Par Collagen Reagent | Chrono Log Corporation | 385 | 1:5 Ratio in 0.9% Saline |
| Electron Microscopy Sciences Miltex Biopsy Punch with Plunger, 3.0 mm | Fisher Scientific | NC0856599 | |
| Eppendorf Snap-Cap Microcentrifuge SafeLock Tubes, 1.5 mL | Fisher Scientific | 05-402-25 | |
| Essendant 121oz. Clorox Germicidal Bleach | Fischer Scientific | 50371500 | |
| Ethanol | Fisher Scientific | 07-678-005 | 70% |
| Falcon Safety Dust Off DPSXLRCP Compressed Gas | Supra | 1381978 | |
| Human TruStain | Biolegend | 422302 | 1:600 Ratio in Whole Blood |
| LevGo smartSpatula Disposable Polypropylene Spatula | Fisher Scientific | 18-001-017 | |
| Microscope Slides | Fisher Scientific | 12-550-A3 | |
| Phosphate buffered saline | Gibco | 10010-023 | |
| Safety Scalpel | Fisher Scientific | 22-079-718 | |
| Saline | Millipore | 567442 | 0.90% |
| Sartorius Polystyrene Weighing Boats | Fisher Scientific | 13-735-744 | |
| Superslip Cover Slips - Superslip No. 1.5 | Fisher Scientific | 12-541-055 | |
| SYLGARD 184 Silicone Elastomer Kit | Fisher Scientific | NC9285739 | Polydimethylsiloxane (PDMS) |
| Ticagrelor | Cayman Chemicals | 15425 | |
| Tygon PVC Clear Tubing 1/16" ID, 1/8" OD, 50 ft length | McMaster-Carr | 6516T11 | |
| Ultra-Machinable 360 Brass Bar | McMaster-Carr | 8954K721 | For master mold fabrication |
| Vacutainers | BD | 363083 | |
| World Precision Instrument Reusable Biopsy Punch, 1.5mm | Fisher Scientific | NC1215626 |
Odniesienia
- Moore, E. E., et al. Trauma-induced coagulopathy. Nat Rev Dis Primers. 7 (1), 1-23 (2021).
- Vulliamy, P., et al. Alterations in platelet behavior after major trauma: adaptive or maladaptive. Platelets. 32 (3), 295-304 (2021).
- Starr, N. E., et al. Identification of injury and shock driven effects on ex vivo platelet aggregometry: A cautionary tale of phenotyping. J Trauma Acute Care Surg. 89 (1), 20-28 (2020).
- Kutcher, M. E., et al. Characterization of platelet dysfunction after trauma. J Trauma Acute Care Surg. 73 (1), 13-19 (2012).
- Yakusheva, A. A., et al. Traumatic vessel injuries initiating hemostasis generate high shear conditions. Blood Adv. 6 (16), 4834-4846 (2022).
- Colace, T. V., Diamond, S. L. Direct observation of von Willebrand factor elongation and fiber formation on collagen during acute whole blood exposure to pathological flow. Arterioscler Thromb Vasc Biol. 33 (1), 105-113 (2013).
- Schneider, S. W., et al. Shear-induced unfolding triggers adhesion of von Willebrand factor fibers. Proc Natl Acad Sci USA. 104 (19), 7899-7903 (2007).
- Savage, B., Almus-Jacobs, F., Ruggeri, Z. M. Specific synergy of multiple substrate-receptor interactions in platelet thrombus formation under flow. Cell. 94 (5), 657-666 (1998).
- Savage, B., Saldívar, E., Ruggeri, Z. M. Initiation of platelet adhesion by arrest onto fibrinogen or translocation on von Willebrand factor. Cell. 84 (2), 289-297 (1996).
- Ruggeri, Z. M., Mendolicchio, G. L. Adhesion mechanisms in platelet function. Circ Res. 100 (12), 1673-1685 (2007).
- Chernysh, I. N., et al. The distinctive structure and composition of arterial and venous thrombi and pulmonary emboli. Sci Rep. 10 (1), 5112(2020).
- Holcomb, J. B., et al. Transfusion of plasma, platelets, and red blood cells in a 1:1:1 vs a 1:1:2 ratio and mortality in patients with severe trauma: The PROPPR randomized clinical trial. JAMA. 313 (5), 471-482 (2015).
- Shea, S. M., et al. Doing more with less: low-titer group O whole blood resulted in less total transfusions and an independent association with survival in adults with severe traumatic hemorrhage. J Thromb Haemost. 22 (1), 140-151 (2024).
- Cardenas, J. C., et al. Platelet transfusions improve hemostasis and survival in a substudy of the prospective, randomized PROPPR trial. Blood Adv. 2 (14), 1696-1704 (2018).
- Sperry, J. L., et al. Prehospital plasma during air medical transport in trauma patients at risk for hemorrhagic shock. N Engl J Med. 379 (4), 315-326 (2018).
- Meyer, D. E., et al. Every minute counts: Time to delivery of initial massive transfusion cooler and its impact on mortality. J Trauma Acute Care Surg. 83 (1), 19-24 (2017).
- Shea, S. M., et al. Cold-stored platelet hemostatic capacity is maintained for three weeks of storage and associated with taurine metabolism. J Thromb Haemost. 22 (4), 1154-1166 (2024).
- Sperry, J. L., et al. Early cold stored platelet transfusion following severe injury: a randomized clinical trial. Ann Surg. 280 (2), 212-221 (2024).
- Schoeman, R. M., et al. A microfluidic model of hemostasis sensitive to platelet function and coagulation. Cell Mol Bioeng. 10 (1), 3-15 (2017).
- Sakurai, Y., et al. A microengineered vascularized bleeding model that integrates the principal components of hemostasis. Nat Commun. 9 (1), 509(2018).
- Miller, C. Predicting non-Newtonian flow behavior in ducts of unusual cross section. Ind Eng Chem Fundamentals. 11 (4), 524-528 (1972).
- Kim, D., Bresette, C., Liu, Z., Ku, D. N. Occlusive thrombosis in arteries. APL Bioeng. 3 (4), 041502(2019).
- Kroll, M. H., Hellums, J. D., McIntire, L. V., Schafer, A. I., Moake, J. L. Platelets and shear stress. Blood. 88 (5), 1525-1541 (1996).
- Sorrells, M. G., Neeves, K. B. Adsorption and absorption of collagen peptides to polydimethlysiloxane and its influence on platelet adhesion flow assays. Micromachines. 11 (1), 62(2020).
Przedruki i uprawnienia
Zapytaj o uprawnienia na użycie tekstu lub obrazów z tego artykułu JoVE
Zapytaj o uprawnieniaThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. Wszelkie prawa zastrzeżone