Method Article
Establishing a Swine Model of Post-myocardial Infarction Heart Failure for Stem Cell Treatment
* Wspomniani autorzy wnieśli do projektu równy wkład.
W tym Artykule
Podsumowanie
We sought to establish a swine model of heart failure induced by left circumflex artery blockage and rapid pacing to test the effect and safety of intramyocardial administration of stem cells for cell-based therapies.
Streszczenie
Although advances have been achieved in the treatment of heart failure (HF) following myocardial infarction (MI), HF following MI remains one of the major causes of mortality and morbidity around the world. Cell-based therapies for cardiac repair and improvement of left ventricular function after MI have attracted considerable attention. Accordingly, the safety and efficacy of these cell transplantations should be tested in a preclinical large animal model of HF prior to clinical use. Pigs are widely used for cardiovascular disease research due to their similarity to humans in terms of heart size and coronary anatomy. Therefore, we sought to present an effective protocol for the establishment of a porcine chronic HF model using closed-chest coronary balloon occlusion of the left circumflex artery (LCX), followed by rapid ventricular pacing induced with pacemaker implantation. Eight weeks later, the stem cells were administered by intramyocardial injection in the peri-infarct area. Then the infarct size, cell survival, and left ventricular function (including echocardiography, hemodynamic parameters, and electrophysiology) were evaluated. This study helps establish a stable preclinical large animal HF model for stem cell treatment.
Wprowadzenie
Cardiovascular diseases, coronary artery disease (CAD) in particular, remain the major cause of morbidity and mortality in Hong Kong and worldwide1. In Hong Kong, a 26% increase from 2012 to 2017 of the number of CAD patients treated under the Hospital Authority was projected2. Among all CADs, acute myocardial infarction (MI) is a leading cause of death and subsequent complications, such as heart failure (HF). These contribute to significant medical, social, and financial burdens. In patients with MI, thrombolytic therapy or primary percutaneous coronary intervention (PCI) is an effective therapy in preserving life, but these therapies can only reduce cardiomyocyte (CM) loss during MI. The treatments available are unable to replenish the permanent loss of CMs, which leads to cardiac fibrosis, myocardial remodeling, cardiac arrhythmia, and eventually heart failure. The mortality rate at 1-year post-MI is around 7% with more than 20% patients developing HF3. In end-stage HF patients, heart transplantation is the only available effective therapy, but it is limited by a shortage of available organs. Novel therapies are necessary to reverse the development of post-MI HF. As a result, cell-based therapy is considered an attractive approach to repair the impaired CMs and ameliorate left ventricular (LV) function in HF following MI. Our previous studies found stem cell transplantation to be beneficial for heart function improvement after direct intramyocardial transplantation in small animal models of MI4,5. Standardized preclinical large animal HF protocols are thus needed to further test the efficacy and safety of stem cell transplantation before clinical use.
Recent decades have witnessed the widespread use of pigs in cardiovascular research for stem cell therapy. HF pigs are a promising model of translational research due to their similarity to humans in terms of cardiac size, weight, rhythm, function, and coronary artery anatomy. Moreover, porcine HF models can mimic post-MI HF patients in terms of CM metabolism, electrophysiological properties, and neuroendocrine changes under ischemic conditions6. The protocol presented here uses such a standardized pig HF model, employing a closed-chest coronary balloon occlusion of the left circumflex artery (LCX) followed by rapid pacing induced by pacemaker implantation. The study also optimizes the route of intramyocardial administration of stem cells for the treatment of post-MI HF. The purpose is to produce a porcine animal model of chronic myocardial infarction that can be used to develop treatments that are clinically relevant for patients with severe CAD.
Protokół
All animal experiments were performed in accordance with the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health and regulations of the University of Hong Kong, and the protocol was approved by the Committee on the Use of Live Animals in Teaching and Research (CULTAR) at the University of Hong Kong.
NOTE: Female farm pigs weighing 35-40 kg (9-12 months old) were used for this study. The flowchart of this experiment is shown in Figure 1.
1. Surgical procedures
- Anesthesia and preparation of the animal
- Fast the animals for 12 h and subject to water deprivation for 4 h before the experiment.
- Anesthetize the pigs through an intramuscular injection of tiletamine+zolezepam (2-7 mg/kg) and xylazine (0.5-1 mg/kg) prepared in 20 mL of normal saline. Monitor the animal's palpebral reflexes until they are absent.
- Remove the pig's hair and sterilize the skin at the neck and the groin for sections 1.3-1.5. Disinfect the operation area 3x with 70% ethanol and betadine.
- Place a 7 mm endotracheal tube into the porcine trachea and place a 22 G venous indwelling needle into the ear vena.
- Move the pig onto the operating table and place in a supine position. Connect the endotracheal tube to the respirator and mechanically ventilate (inspiratory/expiratory time ratio 1:2) the animal with isoflurane (1.5%-2.0% inhalation) and oxygen (0.5-1.5 L/min inhalation).
- Monitor the surface electrocardiogram and blood pressure, and continuously monitor the heart rate, heart rhythm, and arterial blood pressure via electrophysiology recording systems.
- Echocardiography
- Move the pig to the left lateral decubitus position and fix on the table.
- Put the probe on the pericardial region and perform serial echocardiography, including 2D and M-mode imaging, using a high-resolution echocardiographic system and a 3-9 MHz transducer at the baseline, before cell transplantation and 8 weeks after cell transplantation (Supplemental Figure 1).
- Analyze all the obtained images using commercial software. Calculate the LV end-diastolic dimension (LVEDD), LV end-systolic dimension (LVESD), LV end-diastolic volume (LVEDV), LV end-systolic volume (LVESV), LV ejection fraction (LVEF), and wall thickness after standard echocardiographic images are obtained from the parasternal long-axis view.
NOTE: All the off-line analyses were conducted by another independent operator using a computer workstation. The variability of the measurements between different observers was 4% based on 20 repeated random images. All the echocardiographic measurements were performed in accordance with the American Society of Echocardiography recommendations.
- Pacemaker implantation
- Move the pig to the supine position and fix the limbs of the pig on the table with straps.
- Locate the right carotid artery and jugular vein in the carotid triangle (behind the sternocleidomastoid and surrounded by the stylohyoid, the digastric muscle, and the omohyoid) and isolate the right carotid artery and jugular vein with hemostatic forceps under sterile conditions (Supplemental Figure 2). Ligate the distal end of the right carotid artery and jugular vein. Sew the two muscles with 2-0 Vicryl.
- Cannulate the right jugular vein with an angiocath and insert a pacemaker lead to the right ventricle under X-ray guidance (Figure 2).
- Isolate the sternocleidomastoid and the anterior scalene muscle using forceps. Implant a pacemaker between the two muscles and sew the two muscles with 2-0 silk. Connect the pacemaker to the lead.
- Reprogram the pacemaker to backup VVI mode (35 bpm) by a pacemaker generator after the transplantation.
- Apply rapid ventricular pacing (150 beats/min) to induce HF by a pacemaker generator 4 weeks after MI induction. Then set the pacemaker back to backup VVI mode at 8 weeks.
- Invasive pressure volume loop analysis
NOTE: Perform invasive hemodynamic assessment at baseline, before cell transplantation and 8 weeks after cell transplantation to assess changes in LV function.- Isolate the right femoral artery and femoral vein in the femoral triangle (surrounded by the inguinal ligament, sartorius muscle, and adductor longus muscle) (Supplemental Figure 2).
- Cannulate the right femoral artery with an angiocath and place a guidewire into the artery via the angiocath. Remove the angiocath and cannulate a 9F sheath into the artery under the guidance of the guidewire. Remove the guidewire.
- Cannulate the right femoral vein with a 12F sheath as described in step 1.4.2. Insert a balloon catheter from the placed 12F sheath into the inferior vena cava (IVC) under X-ray guidance.
- Calibrate a 7 Fr pressure-volume (PV) catheter in isotonic saline with a PV signal processor.
- Insert the PV catheter into the LV apex from the placed 9F sheath under X-ray guidance. Suspend ventilation and measure the left ventricular maximal positive pressure derivative (+dP/dt), end-systolic pressure (ESP), and end-diastolic pressures (EDP) with the PV signal processor.
- Measure the end systolic pressure-volume relationship (ESPVR) by the PV signal processor during the occlusion of the IVC.
- Restart ventilation when the procedure is finished.
- Induction of MI
- Intravenously administer amiodarone (5 mg/kg intravenously over 1 h) and lidocaine (1.5 mg/kg intravenous bolus) to the animal before induction of MI to prevent ventricular arrhythmias.
- Cannulate the right carotid artery with an 8F sheath as mentioned in step 1.4.3.
- Perform the coronary angiography through a 6F JR4 over-the-wire guiding catheter via the placed sheath guided by standard C arm fluoroscopy equipment.
- Occlude the left circumflex coronary artery (LCX) distal to the first obtuse marginal branch with percutaneous transluminal coronary angioplasty (PTCA) dilatation balloon catheter inflation under X-ray guidance (Figure 2).
- Inject 1 mL of 700 µm sponge microspheres mixed with 3 mL of saline prepared in a 10 mL syringe through the balloon catheter to block the LCX, then deflate the balloon and perform an angiogram to confirm the occlusion.
- Repeat the injection procedure to achieve successful complete blockage.
- Monitor the animal heart rate and rhythm to detect cardiac arrhythmias. If ventricular fibrillation happened, use an external, biphasic defibrillator to reestablish a sinus rhythm using 150-300 J shocks.
- Stem cell injection
- Randomly assign all the animals with notable impairment of heart function (LVEF < 40% at 8 weeks after induction of MI) to two different groups: one that will receive intramyocardial administration of 2 x 108 human induced pluripotent stem cell-derived mesenchymal stem cells (hiPSC-MSCs), and the other that will not receive hiPSC-MSCs.
- Prepare the hiPSC-MSCs in 2 mL of normal saline for intramyocardial transplantation. Before intramyocardial hiPSC-MSCs transplantation, repeat the anesthesia and animal preparation steps mentioned in section 1.1, this time sterilizing 10 cm around the apex beat area. Perform left thoracotomy at the 4-5 intercostal space with a retractor. Perform pericardiotomy to expose the infarcted lateral wall.
NOTE: The length of the incision was 10-12 cm. - Use 5-8 intramyocardial injections (~0.3 mL per injection) around the infarcted area to administer culture medium (Table of Materials) to one group of animals or 2 x 108 hiPSC-MSCs to the other group (Figure 3). Carefully avoid any damage to coronary arteries to reduce the risk of hemorrhage.
- Close the intercostal space with iron wire and close the muscle layer with 2-0 silk. Sew the subcutaneous tissue and skin with 2-0 vicryl.
- Intracardiac programmed electrical stimulation
- Perform programmed electrical stimulation using a programmable stimulator to assess the inducibility of ventricular tachyarrhythmia (VT) after the cell transplantation therapy.
- Insert a 6F electrophysiological catheter into the right ventricular apex via the femoral vein before sacrificing all the animals.
- Display the intracardiac recordings with the surface electrocardiogram leads I, II, and III on the electrophysiological recording system at a speed of 200 mm/s. Deliver a 2 ms pulse width at 2x the diastolic threshold using a stimulator.
- Deliver a pacing train of eight stimuli (S1) at two drive cycle lengths (200 ms and 300 ms), followed by one (S2) or two (S2 and S3) premature extra stimuli.
- Sequentially shorten the coupling intervals until a ventricular effective refractory period or arrhythmia is induced. Note the presence of inducible sustained VT (>10 s).
2. Postoperative protocol
- Postoperative medicine
- Perform conventional pharmacological therapies for HF. In brief, orally administer metoprolol succinate (25 mg) and ramipril (2.5 mg) to all animals daily.
- Intramuscularly administer enrofloxacin (5 mg/kg) and buprenorphine (0.01 mg/kg) to all animals daily for 1 week after surgery to prevent infection and relieve pain.
- To minimize immunological rejection, orally administer a steroid (40 mg/day orally) and cyclosporine (200 mg/day orally) to all animals from 3 days prior to cell transplantation to 8 weeks after.
- Infarct size assessment
- Euthanize the animals by an overdose of dorminal (pentobarbital sodium, 100 mg/kg, IV) at the end of the experiment.
- Open the chest and collect the heart. Rinse the heart in 0.9% saline.
- Serially section LV tissue samples with a scalpel at 1 cm thicknesses in the LV transverse direction.
- Select portions of the slices that contain the infarcted myocardium to measure the wall thickness and the infarct area.
- Capture the image of these slices and quantitatively analyze the wall thickness and the infarct area using commercial image analysis software.
- Fix the tissue in 10% formalin at 4 °C for a month. Embed the tissue within, adjacent and remote to the infarct sites (~1 cm2 pieces) in paraffin. Section into 5 µm slices using a microtome for histological examination.
- Cell survival
- Detect the engraftment of the transplanted cells by immunohistochemical staining with anti-human nuclear antigen (HNA) according to the protocol provided by the manufacturer.
- Capture the image in three different sections at five random fields in each animal and quantitatively analyze the positive cells in the peri-infarct zone.
NOTE: The image capturing system and image analysis software were used to capture and analyze the images of the heart sections.
Wyniki
Mortality
A total of 24 pigs were used in this study. Three of them died during MI induction because of sustained VT. One animal died in the open-heart surgery for cell injection because of wound bleeding. Two animals died because of severe infection. Two animals were excluded because of slight EF reduction (LVEF reduction > 40% of baseline). As a result, 16 animals completed the whole study protocol.
Cardiac function and remodeling
Serial echocardiographic examination showed that LVEF significantly decreased from 68.23 ± 3.52% at baseline to 39.37 ± 3.22%. LVEDD significantly increased from 3.6 ± 0.5 to 4.8 ± 0.4 and LVESD significantly increased from 2.5 ± 0.3 to 3.9 ± 0.4 (Figure 4A) at 8 weeks after induction of MI. LVEF and LVESD significantly improved to 52.9 ± 4.27% and 3.3 ± 0.3 respectively in the hiPSC-MSCs group 8 weeks after the transplantation, compared with the MI status (Figure 4A).
The +dP/dt and ESPVR significantly decreased from 1,325 ± 63 mmHg/s and 3.9 ± 0.4 at baseline to 978 ± 45 mmHg/s and 1.8 ± 0.2 at 8 weeks after induction of MI. Intramyocardial administration of hiPSC-MSCs increased the +dP/dt and ESPVR to 1,127.4 ± 50 mmHg/s and 2.6 ± 0.3 at 8 weeks after iPSC-MSCs transplantation, compared with the MI status (Figure 4B).
Infarct wall thickness
The average LV infarct wall thickness was measured from 5-7 serial 1 cm thickness section samples in each animal (Figure 5). The percentage of LV infarction was 16 ± 2%.
Cell Survival after the transplantation
There was no cell survival around the injection site in the infarct area 8 weeks after the transplantation, but a small number of the survival hiPSC-MSCs were visible in the peri-infarct area (Figure 6).
Inducible ventricular arrhythmia
The incidence of inducible sustained ventricular tachyarrhythmias could be easily increased in animals with HF (10% at baseline vs. 75% 8 weeks after induction of MI). The hiPSC-MSCs transplantation does not significantly modify the underlying myocardial substrate to reduce susceptibility to VT (62.5% in hiPSC-MSCs group 8 weeks after intramyocardial administration of hiPSC-MSCs, Figure 7).
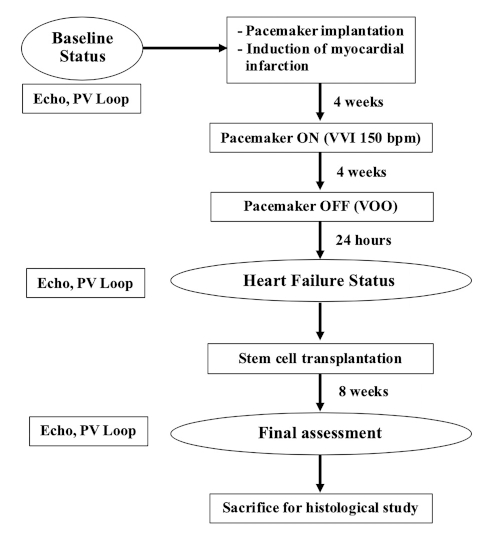
Figure 1: Flow chart of the experiment. Please click here to view a larger version of this figure.
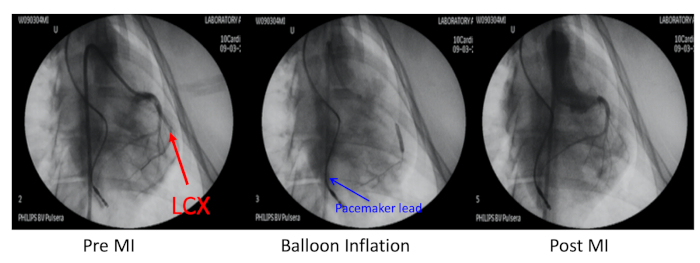
Figure 2: Porcine model of myocardial infarction. The porcine model of myocardial infarction (MI) was induced by embolization of the left circumflex coronary artery (LCX, red arrow) distal to the first obtuse marginal branch. This coronary artery was occluded with balloon inflation and an injection of 700 µm microspheres. Coronary angiography at pre-MI, balloon inflation, and post-MI was performed through a 6F JR4 guiding catheter via the right carotid artery. The pacemaker lead was inserted into the right ventricle wall (blue arrow). Please click here to view a larger version of this figure.
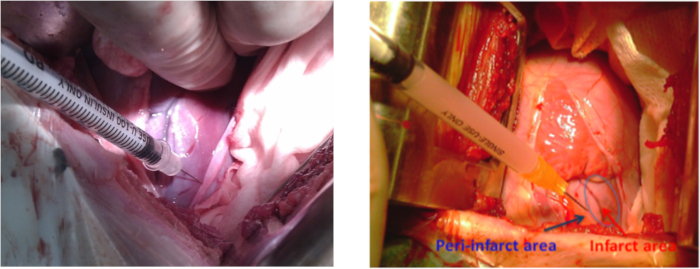
Figure 3: Cell transplantation in a porcine model of MI. Cell injection sites at the lateral wall around the infarct area of the left ventricle during left thoracotomy. The blue arrow shows the peri-infarct area and the red arrow shows the infarct area. Please click here to view a larger version of this figure.
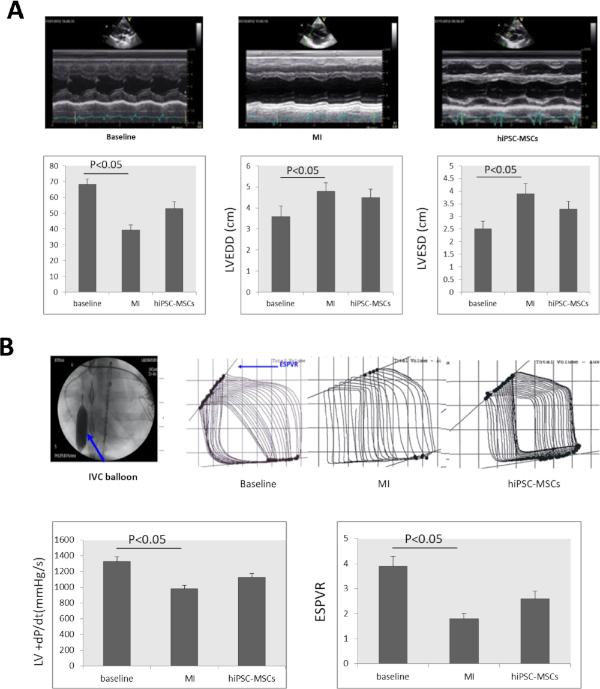
Figure 4: Heart function changes after MI. (A) A LV M-mode echocardiogram image at baseline, MI, and cell transplantation. LVEF, LVEDD, LVESD significantly decreased 8 weeks after MI induction and significantly increased in the hiPSC-MSCs group 8 weeks after cell transplantation. (B) To assess the cardiac function of the pigs with heart failure, the +dP/dt value and the ESPVR were measured with a PV signal processor. The inferior vena cava (IVC) was occluded by balloon inflation (blue arrow) during the ESPVR assessment. Both the +dP/dt and the ESPVR significantly decreased after MI induction, and then significantly increased in the hiPSC-MSC groups 8 weeks after the transplantation. ANOVA followed by Student-Newman-Keuls post hoc testing (SPSS, version 14) was used with α = 0.05 for significance. Please click here to view a larger version of this figure.

Figure 5: Infarct area changes after MI. LV transverse direction samples sectioned at 1 cm thicknesses in each heart containing infarcted myocardium. Please click here to view a larger version of this figure.
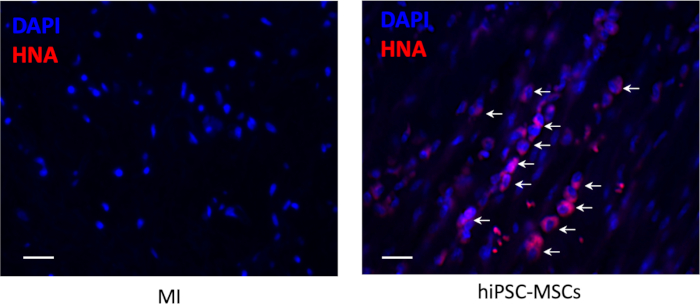
Figure 6: Cell survival after the transplantation. The engraftment of the transplanted hiPSC-MSCs was detected by immunohistochemical staining foranti-human nuclear antigen (red color). Scale bar = 100 µm. Arrows represent positive cells. Please click here to view a larger version of this figure.

Figure 7: The incidences of sustained ventricular tachyarrhythmias. (A) Ventricular tachyarrhythmias (VT, red arrow) induced by in vivo intracardiac programmed electrical stimulation. (B) The incidence of VT significantly increased after MI induction. Cell transplantation did not increase the incidence of VT. Please click here to view a larger version of this figure.

Supplemental Figure 1: Echocardiogram acquisition. The left panel shows the animal's position. The right panel shows the probe position. The middle panel shows the echocardiographic image under this position. Please click here to view a larger version of this figure.
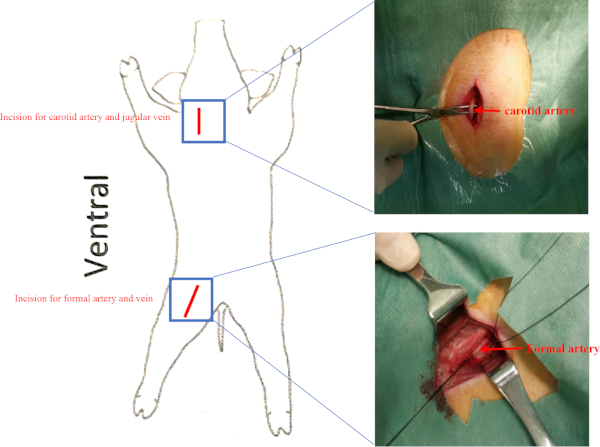
Supplemental Figure 2: Location of vessels. Pigs were placed in the supine position. Incisions for the carotid artery and femoral artery are presented as a red line. The jugular vein and femoral vein were beneath the carotid artery and femoral artery respectively. Please click here to view a larger version of this figure.
Dyskusje
Standard animal models are of paramount importance to understand the pathophysiology and mechanisms of diseases and test novel therapeutics. Our protocol establishes a porcine model of HF induced by left circumflex artery blockage and rapid pacing. Eight weeks after the induction of MI, the animals developed significant impairment of LVEF, LVEDD, LVESD, +dP/dt, and ESPVR. This protocol also tests the administration method of stem cell therapy for heart regeneration by intramyocardial injection. The infarct size, and cardiac systolic and diastolic function are evaluated. This study helps establish a stable and reproducible preclinical large animal HF model for stem cell treatment, which is similar to clinical cases.
LCX blockage and rapid pacing has been used extensively to create animal models of HF in our previous studies7,8. The LCX distal to the first obtuse marginal branch was occluded, followed by 4 weeks of rapid right ventricular pacing. Myocardium ischemia results in loss of cardiomyocytes during MI, which causes cardiac fibrosis, myocardial remodeling, and cardiac arrhythmia. Ventricular pacing results in significant LV dilation, nonischemic impairment of left ventricular contractility, and severe LV dysfunction9,10. Longer durations of ischemia and rapid pacing produce a progressive experimental low-output HF model for translational research. Previous studies established heart failure models by inducing MI10. However, the mortality of severe MI was higher and the LVEF reduction of MI was unstable. Therefore, we apply rapid right ventricular pacing after LCX blockage to induce significant impairment of cardiac function. As can be seen in our prior studies, the model presented here yields stable infarct size, and the LVEF of this model is reduced to at least below 40% normal6,7,8. Had there been fewer infections and bleeding, our model success rate could have been around 80%.
One of the major hurdles to the clinical application of stem cells is their poor survival and engraftment following transplantation. Recent clinical studies and meta-analysis11,12,13,14,15 have failed to demonstrate any consistent improvement in LV function or infarct size following such therapy. One of the potential reasons is the low survival rate of transplanted cells. Discovering an optimal administration method plays a critical role in stem cell therapies. Comparing the three methods of cell transplantation, intramyocardial administration is more efficient than intravenous and intracoronary administration due to higher cell retention16,17. Therefore, we selected an intramyocardial administration route for iPSC-MSCs delivery in this study. Echocardiographic results and invasive hemodynamic results demonstrated that intramyocardial administration of iPSC-MSCs ameliorated LV function of post-MI HF pigs 8 weeks after cell transplantation. Despite the administration of immunosuppressive drugs (a steroid and cyclosporine), only a few transplanted cells were detected in the peri-infarct area. No surviving cell was detected in the infarcted area around the injected site. Previous studies have also found an extremely small portion of stem cells in the infarcted myocardium after the transplantation18,19,20,21. Cell loss during the intramyocardial administration might affect the experimental outcomes. How to improve the administration methods and increase the residence rate should be clarified in future studies.
Safety, especially arrhythmogenesis, is another vital concern regarding clinical practice with cell-based therapies. Our recent study demonstrated that intramyocardial administration of human embryo stem cell (hESC) derived CMs increased the incidence of spontaneous non-sustained ventricular tachyarrhythmias4. In our post-MI HF porcine model, the incidence of spontaneous non-sustained ventricular tachyarrhythmia (rate >180 bpm and >12 beats) recorded by telemetry monitoring from the pacemaker was 25% after MI induction, but sustained VT could be easily induced (80%). In this study, the incidence of sudden death remains unchanged with or without hiPSC-MSCs administration. Moreover, hiPSC-MSCs transplantation did not modify the underlying myocardial substrate to reduce or increase susceptibility to ventricular arrhythmias. This result suggests that the large animal chronic HF model could be used for cell safety assessment.
Avoidance of infection and hemorrhage are of paramount important to successful animal model establishment. To reduce the risk of hemorrhage, attention should be paid to avoid any damage to coronary arteries and cardiac veins. As two animals died of severe infection, an appropriate postoperative medical strategy will be benefit. Here, we provide a postoperative medical strategy as below: Intramuscularly administer enrofloxacin (7.5 mg/kg, SID) and buprenorphine (0.02 mg/kg, BID) combined with orally administer Amoxycillin/Clavulanic Acid (12.5mg/kg, SID) and Carprofen (2 mg/kg, SID) to all animals for 1 week after surgery to prevent infection and relieve pain.
In summary, the current method provides a stable and reproducible clinically relevant large animal model of heart failure for cell-based therapies.
Ujawnienia
The authors have nothing to disclose.
Podziękowania
The authors acknowledge Alfreda and Kung Tak Chung for their excellent technical support during the animal experiments.
Materiały
| Name | Company | Catalog Number | Comments |
| Amiodarone | Mylan | - | - |
| Anaesthetic machines and respirator | Drager | Fabius plus XL | - |
| Angiocath | Becton Dickinson | 381147 | - |
| Anti-human nuclear antigen | abcam | ab19118 | - |
| Axio Plus image capturing system | Zeiss | Axioskop 2 PLUS | Axioskop 2 plus |
| AxioVision Rel. 4.5 software | Zeiss | - | - |
| Baytril | Bayer | - | enrofloxacin |
| Betadine | Mundipharma | - | - |
| CardioLab Electrophysiology Recording Systems | GE Healthcare | G220f | - |
| Culture media | MesenCult | 05420 | - |
| Cyclosporine | Novartis | - | - |
| Defibrillator | GE Healthcare | CardioServ | - |
| Dorminal | TEVA | - | - |
| Echocardiographic system | GE Vingmed | Vivid i | - |
| EchoPac software | GE Vingmed | - | - |
| Electrophysiological catheter | Cordis Corp | - | - |
| Embozene Microsphere | Boston Scientific | 17020-S1 | 700 μm |
| Endotracheal tube | Vet Care | VCPET70PCW | Size 7 |
| Ethanol | VWR chemicals | 20821.33 | - |
| Formalin | Sigma | HT501320 | 10% |
| IVC balloon Dilatation Catheter | Boston Scientific | 3917112041 | Mustang |
| JR4 guiding catheter | Cordis Corp | 67208200 | 6F |
| Lidocaine | Quala | - | - |
| Mersilk | Ethicon | W584 | 2-0 |
| Metoprolol succinate | Wockhardt | - | - |
| Microtome | Leica | RM2125RT | - |
| Mobile C arm fluoroscopy equipment | GE Healthcare | OEC 9900 Elite | - |
| Pacemaker | St Jude Medical | PM1272 | Assurity MRI pacemaker |
| Pacemaker generator | St Jude Medical | Merlln model 3330 | - |
| Pressure-volume catheter | CD Leycom | CA-71103-PL | 7F |
| Pressure–volume signal processor | CD Leycom | SIGMA-M | - |
| Programmable Stimulator | Medtronic Inc | 5328 | - |
| PTCA Dilatation balloon Catheter | Boston Scientific | H7493919120250 | MAVERICK over the wire |
| Ramipril | TEVA | - | - |
| Sheath introducer | Cordis Corp | 504608X | 8F, 9F, 12F |
| Steroid | Versus Arthritis | - | - |
| Temgesic | Nindivior | - | buprenorphine |
| Venous indwelling needle | TERUMO | SR+OX2225C | 22G |
| Vicryl | Ethicon | VCP320H | 2-0 |
| Xylazine | Alfasan International B.V. | - | - |
| Zoletil | Virbac New Zealand Limited | - | tiletamine+zolezepam |
Odniesienia
- Mozaffarian, D., et al. Heart disease and stroke statistics-2015 update: a report from the American Heart Association. Circulation. 131, e29 (2015).
- Hospital Authority. . Hospital Authority Statistical Report 2013. , (2013).
- Cung, T. T., et al. Cyclosporine before PCI in Patients with Acute Myocardial Infarction. The New England Journal of Medicine. 373 (11), 1021-1031 (2015).
- Liao, S. Y., et al. Proarrhythmic risk of embryonic stem cell-derived cardiomyocyte transplantation in infarcted myocardium. Heart Rhythm. 7, 1852-1859 (2010).
- Liao, S. Y., et al. Overexpression of Kir2.1 channel in embryonic stem cell-derived cardiomyocytes attenuates posttransplantation proarrhythmic risk in myocardial infarction. Heart Rhythm. 10, 273-282 (2013).
- Liu, Y., et al. Thoracic spinal cord stimulation improves cardiac contractile function and myocardial oxygen consumption in a porcine model of ischemic heart failure. Journal of Cardiovascular Electrophysiology. 23, 534-540 (2012).
- Liao, S. Y., et al. Improvement of Myocardial Function Following Catheter-Based Renal Denervation in Heart Failure. JACC: Basic to Translational Science. 2 (3), 270-281 (2017).
- Liao, S. Y., et al. Remodelling of cardiac sympathetic re-innervation with thoracic spinal cord stimulation improves left ventricular function in a porcine model of heart failure. Europace. 17 (12), 1875-1883 (2015).
- Daehnert, I., Rotzsch, C., Wiener, M., Schneider, P. Rapid right ventricular pacing is an alternative to adenosine in catheter interventional procedures for congenital heart disease. Heart. 90 (9), 1047-1050 (2004).
- Hála, P., et al. Tachycardia-Induced Cardiomyopathy as a Chronic Heart Failure Model in Swine. Journal of Visualized Experiments. (132), e57030 (2018).
- Santoso, T., et al. Endomyocardial implantation of autologous bone marrow mononuclear cells in advanced ischemic heart failure: a randomized placebo-controlled trial (END-HF). Journal of Cardiovascular Translational Research. 7, 545-552 (2014).
- Traverse, J. H., et al. Cardiovascular Cell Therapy Research Network. Effect of intracoronary delivery of autologous bone marrow mononuclear cells 2 to 3 weeks following acute myocardial infarction on left ventricular function: the LateTIME randomized trial. Journal of the American Medical Association. 306, 2110-2119 (2011).
- Traverse, J. H., et al. Cardiovascular Cell Therapy Research Network (CCTRN). Effect of the use and timing of bone marrow mononuclear cell delivery on left ventricular function after acute myocardial infarction: the TIME randomized trial. Journal of the American Medical Association. 308, 2380-2389 (2012).
- de Jong, R., Houtgraaf, J. H., Samiei, S., Boersma, E., Duckers, H. J. Intracoronary stem cell infusion after myocardial infarction. A meta-analysis and update on clinical trials. Circulation: Cardiovascular Interventions. 7, 156-167 (2014).
- Nowbar, A. N., et al. DAMASCENE writing group. Discrepancies in autologous bone marrow stem cell trials and enhancement of ejection fraction (DAMASCENE): weighted regression and meta-analysis. British Medical Journal. 348, g2688 (2014).
- Kanelidis, A. J., Premer, C., Lopez, J., Balkan, W., Hare, J. M. Route of Delivery Modulates the Efficacy of Mesenchymal Stem Cell Therapy for Myocardial Infarction: A Meta-Analysis of Preclinical Studies and Clinical Trials. Circulation Research. 120 (7), 1139-1150 (2017).
- Hou, D., et al. Radiolabeled cell distribution after intramyocardial, intracoronary, and interstitial retrograde coronary venous delivery: implications for current clinical trials. Circulation. 112 (9 Suppl), I150-I156 (2005).
- Hu, X., et al. A Large-Scale Investigation of Hypoxia-Preconditioned Allogeneic Mesenchymal Stem Cells for Myocardial Repair in Nonhuman Primates: Paracrine Activity Without Remuscularization. Circulation Research. 118, 970-983 (2016).
- Chong, J. J., et al. Human embryonic-stem-cell-derived cardiomyocytes regenerate non-human primate hearts. Nature. 510, 273-277 (2014).
- Martens, A., et al. Substantial early loss of induced pluripotent stem cells following transplantation in myocardial infarction. Artificial Organs. 38, 978-984 (2014).
- Shiba, Y., et al. Allogeneic transplantation of iPS cell-derived cardiomyocytes regenerates primate hearts. Nature. 538, 388-391 (2016).
Przedruki i uprawnienia
Zapytaj o uprawnienia na użycie tekstu lub obrazów z tego artykułu JoVE
Zapytaj o uprawnieniaThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. Wszelkie prawa zastrzeżone