Method Article
A Comparative Analysis of Recombinant Protein Expression in Different Biofactories: Bacteria, Insect Cells and Plant Systems
W tym Artykule
Podsumowanie
In this study the expression of a target human recombinant protein in different production platforms was compared. We focused on traditional fermenter-based cultures and on plants, describing the set-up of each system and highlighting, on the basis of the reported results, the inherent limits and advantages for each platform.
Streszczenie
Plant-based systems are considered a valuable platform for the production of recombinant proteins as a result of their well-documented potential for the flexible, low-cost production of high-quality, bioactive products.
In this study, we compared the expression of a target human recombinant protein in traditional fermenter-based cell cultures (bacterial and insect) with plant-based expression systems, both transient and stable.
For each platform, we described the set-up, optimization and length of the production process, the final product quality and the yields and we evaluated provisional production costs, specific for the selected target recombinant protein.
Overall, our results indicate that bacteria are unsuitable for the production of the target protein due to its accumulation within insoluble inclusion bodies. On the other hand, plant-based systems are versatile platforms that allow the production of the selected protein at lower-costs than Baculovirus/insect cell system. In particular, stable transgenic lines displayed the highest-yield of the final product and transient expressing plants the fastest process development. However, not all recombinant proteins may benefit from plant-based systems but the best production platform should be determined empirically with a case-by-case approach, as described here.
Wprowadzenie
Recombinant proteins are commercially mass-produced in heterologous expression systems with the aid of emerging biotechnology tools. Key factors that have to be considered when choosing the heterologous expression system include: protein quality, functionality, process speed, yield and cost.
In the recombinant protein field, the market for pharmaceuticals is expanding rapidly and, consequently, most biopharmaceuticals produced today are recombinant. Proteins can be expressed in cell cultures of bacteria, yeasts, molds, mammals, plants and insects, as well as in plant systems (either via stable- or transient-transformation) and transgenic animals; each expression system has its inherent advantages and limitations and for each target recombinant protein the optimal production system has to be carefully evaluated.
Plant-based platforms are arising as an important alternative to traditional fermenter-based systems for safe and cost-effective recombinant protein production. Although downstream processing costs are comparable to those of microbial and mammalian cells, the lower up-front investment required for commercial production in plants and the potential economy of scale, provided by cultivation over large areas, are key advantages.
We evaluated plants as bioreactors for the expression of the 65 kDa isoform of human glutamic acid decarboxylase (hGAD65), one of the major autoantigen in Type 1 autoimmune diabetes (T1D). hGAD65 is largely adopted as a marker, both for classifying and monitoring the progression of the disease and its role in T1D prevention is currently under investigation in clinical trials. If these trials are successful, the global demand for recombinant hGAD65 will increase dramatically.
Here, we focus on the expression of the enzymatically inactive counterpart of hGAD65, hGAD65mut, a mutant generated by substituting the lysine residue that binds the cofactor PLP (pyridoxal-5'-phosphate) with an arginine residue (K396R)1.
hGAD65mut retains its immunogenicity and, in plant and insect cells, accumulates up to ten-fold higher than hGAD65, its wild-type counterpart. It was hypothesized that the enzymatic activity of hGAD65 interferes with plant cell metabolism to such an extent that it suppresses its own synthesis, whereas hGAD65mut, the enzymatically-inactive form, can be accumulated in plant cells to higher levels.
For the expression of hGAD65mut, the use of different technologies, widely used in plant biotechnology, was explored here and compared to traditional expression platforms (Escherichia coli and Baculovirus/insect cell-based).
In this work, the recombinant platforms developed for the expression of hGAD65mut comprising traditional and plant-based systems were reviewed and compared on the basis of process speed and yield, and of final product quality and functionality.
Protokół
1. Construction of Expression Vectors
- Commercial recombination cloning system:
- Amplify the full-length sequence of the target gene (hGAD65mut) with appropriate primers allowing the addition of a CACC clamp at the 5’-end of the gene as previously described2.
- Clone the gel-purified amplification product, according to the directional cloning kit specifications, in the entry vector (topoisomerase bound) by assembling the reaction in a total volume of 6 µl, using a 1.5:1 molar ratio insert:vector and 1 µl of salt solution (0.2 M NaCl and 0.01 MgCl2). Incubate for 5 min at room temperature (RT).
- Transform the entire reaction into chemically competent E. coli cells according to directional cloning kit specifications and screen obtained colonies by colony PCR3, using M13 forward and reverse primers included in the directional cloning kit. Isolate the plasmid from a positive colony according to plasmid DNA preparation kit specifications and sequence the insert using M13 forward and reverse primers to ensure the absence of mutations3.
- Use the obtained entry clone to perform the recombination reaction by the Lambda Recombinase Clonase II Enzyme (LR) with specific destination vectors: for recombinant protein expression in bacterial cells with pDEST17 (yielding pDEST17.G65mut), in plants (transient or stable) with pK7WG2 (yielding pK7WG2.G65mut), in Baculovirus/insect cell system with the linearized viral DNA (yielding Baculo.G65mut)4. Assemble the reaction, according to clonase kit specifications, with 100 ng of entry vector, 150 ng of destination vector and 2 µl of enzyme mix in a final volume of 10 µl. Incubate the mix at RT for 1 hr.
- Transform the recombination product into chemically competent E. coli cells according to clonase kit specifications. Screen obtained colonies by PCR3 using for all the transformation reaction derived colonies the same reverse GAD65mut-specific primer (5’-CACACGCCGGCAGCAGGT-3’) and the following specific destination vectors forward primers: for pDEST17: 5’-TAATACGACTCACTATAGGG-3’; for pK7WG2: 5’-AAGATGCCTCTGCCGACAGT-3’; for Baculo linearized vector: 5’-AAATGATAACCATCTCGC-3’.
- Isolate plasmid DNA using a commercial minipreparation DNA kit and confirm the presence of the target sequence by PCR3, using the same specific primer combinations described in the previous step.
- Use the obtained PCR-positive plasmids to transform different target organisms, depending on the platform chosen for recombinant protein expression as described in the following steps.
- MagnICON system5-7:
- Clone the target gene in the 3’-module pICH31070, as previously described in detail7, yielding pICH31070.G65mut. Use the following specific reaction cycle: 30 min incubation at 37 °C, 5 min at 50 °C and then 5 min at 80 °C.
2. Recombinant Protein Expression
- Bacterial cell system:
- Transform pDEST17.G65mut into E. coli BL21 (DE3) electrocompetent cells using standard techniques and screen the colonies grown on ampicillin-containing (100 µg/ml) LB-medium, by colony PCR using the following transgene-specific primers: 5’-CTGGTGCCAAGTGGCTCAGA-3’ and 5’-CACACGCCGGCAGCAGGT-3’, with an annealing temperature of 58 °C and an elongation time of 20 sec. Carry out the PCR reaction in a total volume of 20 µl after dissolving bacterial cells with a plastic tip in the tube.
- Inoculate a single colony overnight at 37 °C in 3 ml of ampicillin-containing (100 µg/ml) LB-medium.
- Dilute the bacterial culture 1:100 in 100 ml of fresh LB medium (including ampicillin) and incubate at 37 °C for 1-6 hr until a final OD600 of 0.8.
- Induce recombinant protein expression by addition of isopropil-β-D-1-tiogalattopiranoside (IPTG) at a final concentration of 1 mM and incubate culture with vigorous shaking for 3 hr at 37 °C.
- Collect cells by centrifugation at 4,000 x g for 20 min and use bacterial pellet for protein extraction (step 3.1.1.1).
- Baculovirus/insect cell system:
- Seed Spodoptera frugiperda (Sf9) cells into 6-well plates (8 x 105 cells per well) and wash twice with 2 ml of non-supplemented Grace’s Insect Medium.
- Remove and replace the medium drop-wise with transfection mixture (5 µl LR recombinant reaction, 6 µl Celfectin solution and 200 µl non-supplemented Grace’s Insect Medium).
- Incubate plates at 27 °C for 5 hr.
- Remove and replace transfection mixture with 2 ml of fresh Sf-900 medium, supplemented with 10% fetal bovine serum, 10 µg/ml gentamycin and 100 µM ganciclovir to select recombinant Baculovirus clones.
- After incubation for 96 hr at 27 °C, collect medium (V1 viral stock), centrifuge at 4,000 x g to remove cells and large debris and store in the dark at 4 °C.
- Seed 1 x 106 Sf9 cells per well in 2.5 ml Sf-900 medium containing 10% fetal bovine serum, 10 µg/ml gentamycin and 100 µM ganciclovir and infect with 100 µl of V1 stock in every well.
- Incubate cells for 3 days at 27 °C.
- Collect and centrifuge medium at 4,000 x g.
- Store the supernatant (V2 high-titer stock) at 4 °C.
- Seed 1 x 106 Sf9 cells per well in 2.5 ml Sf-900 medium containing 10% fetal bovine serum, 10 µg/ml gentamycin and 100 µM ganciclovir and infect with 25 µl of V2 stock in every well.
- Incubate cells for 3 days at 27 °C.
- Collect cells by centrifuge at 4,000 x g and use insect cell pellet for protein extraction (step 3.1.2.1).
- Nicotiana benthamiana transient expression systems:
- Grow N. benthamiana plants in a greenhouse, under natural light within the temperature range 18-23 °C. For agroinfection use 4-5 week old plants.
- Keep agroinfected plants in a climate chamber at 22 °C with a 13 hr day/11 hr night photoperiod.
- Commercial recombination cloning system:
- Introduce pK7WG2.G65mut in Agrobacterium tumefaciens strain EHA105 electrocompetent cells as previously described8 and screen the colonies, grown on LB medium containing rifampicin (50 µg/ml), streptomycin (300 µg/ml) and spectinomycin (100 µg/ml) after 2 days of incubation at 28 °C, by colony PCR8 using the following transgene-specific primers: 5’-CATGGTGGAGCACGACACGCT-3’ and 5’-CACACGCCGGCAGCAGGT-3’, with an annealing temperature of 58 °C and an elongation time of 50 sec. Carry out the PCR reaction in a total volume of 20 µl.
- Inoculate bacteria in 30 ml of LB medium containing rifampicin (50 µg/ml), streptomycin (300 µg/ml) and spectinomycin (100 µg/ml) for two days at 28 °C.
- Collect bacteria by centrifugation at 4,000 x g for 20 min and resuspend pellet in 10 ml of infiltration buffer (10 mM MES, 10 mM MgCl2, 100 µM acetosyringone, pH 5.6). Measure the OD600 value and then adjust it to 0.9 by diluting in the same buffer.
NOTE: the total volume needed for infiltrating three N. benthamiana plants is 30 ml. - Use a 2.5 ml needleless syringe to infiltrate the Agrobacterium suspension in N. benthamiana leaves. Carefully and slowly inject leaf panels with the suspension from the syringe. Infiltrate three expanded leaves per plant and use three plants as triplicates.
NOTE: for health and safety reasons wear eye protection during infiltration process. - Collect agroinfiltrated leaves 2 days post infection (dpi) and freeze in liquid nitrogen. Store plant tissue at -80 °C.
- MagnICON system:
- Introduce the MagnICON vectors - the 5’ module (pICH20111), the 3’ module (pICH31070.G65mut), and the integrase module (pICH14011) - in A. tumefaciens GV3101 strain using standard techniques. Screen the colonies, grown on LB medium containing 50 µg/ml rifampicin and appropriate vector-specific antibiotic (50 µg/ml carbenicillin for pICH20111 and pICH14011, 50 µg/ml kanamycin for pICH31070), by colony PCR using specific primers for each vector.
- Inoculate separately the three A. tumefaciens strains in 5 ml of LB medium containing 50 µg/ml rifampicin and appropriate vector-specific antibiotic and shake overnight at 28 °C.
- Pellet overnight bacterial cultures by centrifugation at 4,000 x g for 20 min and resuspend them in two volumes of the initial bacterial culture of 10 mM MES (pH 5.5) and 10 mM MgSO4.
- Mix equal volumes of bacterial suspensions containing the three vectors and use the suspension mix for syringe infiltration of N. benthamiana leaves. Infiltrate three expanded leaves per plant.
- Collect agroinfiltrated leaves at 4 dpi and freeze in liquid nitrogen.
- Store plant tissue at -80 °C.
- Nicotiana tabacum stable expression system:
- Grow and maintain in vitro plantlets of N. tabacum (var. Sr1) on solid plant culture medium (4.4 g/L Murashige and Skoog - MS - medium including vitamins, 30 g/L sucrose, pH 5.8, 7 g/L plant agar) under controlled conditions in climate chamber at 25 °C with 16 hr/8 hr day/night regime.
- Start a pre-culture of A. tumefaciens EHA105 harboring the expression vector pK7WG2.G65mut in 5 ml of liquid YEB medium with appropriate antibiotics (rifampicin 50 µg/ml, streptomycin 300 µg/ml, spectinomycin 100 µg/ml) and grow overnight at 28 °C in an orbital shaker set at 200 rpm.
- Use 1 ml of the pre-culture to inoculate a 50 ml Agrobacterium culture in YEB medium plus antibiotics (rifampicin 50 µg/ml, streptomycin 300 µg/ml, spectinomycin 100 µg/ml) and grow for 24 hr, until the bacterial culture is saturated (OD600 value of 0.5-1.0).
- Collect bacteria by centrifugation at 4,000 x g for 20 min and resuspend pellet in 50 ml of liquid plant culture medium. Repeat this step twice to completely remove antibiotics.
- Take first healthy fully expanded leaves from in vitro tobacco plants and cut them into approximately 1 cm squares.
- Transfer leaf pieces to deep Petri dishes containing bacterial suspension and leave in the dark for 20 min.
- Remove leaf pieces from suspension and blot dry on sterile filter paper.
- Place leaf pieces with adaxial side (upper leaf surface) on solid plant culture medium containing 1.0 µg/ml 6-benzylaminopurine (BAP) and 0.1 µg/ml naphthalene acetic acid (NAA) and incubate plates for two days in a climate chamber at controlled conditions (25 °C, 16 hr day/8 hr night).
- Transfer leaf pieces onto solid culture medium (including 1.0 µg/ml BAP, 0.1 µg/ml NAA, 500 µg/ml cefotaxime, 100 µg/ml kanamycin) with abaxial surface (lower surface of leaf) in contact with medium.
- Incubate plates for 2-3 weeks in the climate chamber at 25 °C with 16 hr/8 hr day/night regime to induce shoot formation. Subculture every 2 weeks by transferring leaf explants to fresh solid plant culture medium containing 1.0 µg/ml BAP, 0.1 µg/ml NAA, 500 µg/ml cefotaxime and 100 µg/ml kanamycin.
- When shoots appear transfer them to Magenta boxes containing solid culture medium including 500 µg/ml cefotaxime and 100 µg/ml kanamycin to induce root formation. Incubate plantlets with 16 hr day/8 hr night photoperiod at 25 °C for 1-2 weeks.
- When roots form, transfer the plants to soil in the greenhouse.
- Collect a leaf piece for each plant and isolate genomic DNA with a commercial kit.
- Detect the presence of the transgene by specific PCR. Use 30 ng of genomic DNA as template for PCR amplification using the following transgene-specific primers: 5’-CTGGTGCCAAGTGGCTCAGA-3’ and 5’-CACACGCCGGCAGCAGGT-3’, with an annealing temperature of 58 °C and an elongation time of 20 sec. Carry out the PCR reaction in a total volume of 50 µl.
- Analyze the 220 bp product by electrophoresis on 1% agarose gel in Tris-acetate-EDTA (TAE) buffer (40 mM Tris, 20 mM acetic acid, and 1 mM EDTA).
- Select transgenic plants containing the target gene.
- Collect a leaf piece from each of the selected transgenic plant and freeze in liquid nitrogen immediately.
- Prepare total protein extracts from plant leaf tissue (step 3.1.3) and test each sample by western blot analysis as previously described2 to select the best recombinant protein expressing tobacco plant.
- Bag flowers of the selected best performing plant before blooming to prevent cross-pollination.
- After blooming, fruit ripening and seed drying, collect bags.
- Separate seeds from the chaff and store them in a dry room at controlled temperature (20-24 °C).
- Sow the dried seeds to produce the second generation transgenic plants and subsequently select the best performing plant to be self-pollinated.
- Repeat the same procedure described in steps 2.4.19-2.4.21 for subsequent generations.
3. Recombinant Protein Expression Analyses
- Total protein extraction:
- Bacterial cells:
- Resuspend the bacterial cell pellet, collected as described in step 2.1.5, in half the culture volume of Tris-buffered saline (TBS - 2 mM Tris/HCl , 500 mM NaCl) pH 7.4 supplemented with 1 mM phenylmethanesulfonylfluoride (PMSF).
- Sonicate resuspended cell pellet three times for 40 sec at half power, while keeping sample on ice.
- Clarify lysate by centrifugation at 14,000 x g for 20 min at 4 °C.
- Transfer supernatant to a clean tube and store both supernatant and pellet separately at -80 °C.
- Solubilize the inclusion bodies, collected in the pellet, in half cellular lysate volume of TBS pH 8.0 supplemented with 6 M urea by vigorous shaking overnight at room temperature.
- Centrifuge at 10,000 x g for 25 min at room temperature and collect supernatant in a clean tube.
- Store both supernatant and pellet at -80 °C.
- Insect cells:
- Wash infected insect cell pellet, collected as described in step 2.2.12, with 1 ml of phosphate buffered saline (PBS - 137 mM NaCl, 2.7 mM KCl, 10 mM Na2HPO4, 1.8 mM KH2PO4) pH 7.4.
- Resuspend cells in 200 µl of lysis buffer (20 mM Tris/HCl pH 8.0, 0.5 M NaCl, 3 mM β-mercaptoethanol and 1% Tween-20) and incubate on ice for 30 min.
- Centrifuge solubilized cells at 14,000 x g for 20 min at 4 °C.
- Collect soluble fractions and store at -80 °C and discard the pellet.
- Plant leaf tissue:
- Grind N. benthamiana or N. tabacum tissue to fine powder in liquid nitrogen using mortar and pestle.
- Homogenize 100 mg of ground tissue in 300 µl of extraction buffer (40 mM Hepes pH 7.9, 5 mM DTT, 1.5% CHAPS), supplemented with 3 µl of protease inhibitor cocktail and thaw on ice.
NOTE: the selected ratio between plant tissue weight (g) to buffer volume (ml) is 1:3. - Centrifuge extract at 30,000 x g for 30 min at 4 °C.
- Collect supernatant in a clean tube and store at -80 °C.
- Bacterial cells:
- Coomassie gel staining:
- Prepare an appropriate dilution of total protein extracts (e.g., 2 µl of plant extract, 5 µl of bacterial and insect extracts) to a final volume of 10 µl of extraction buffer and add 5 µl of 3x sample buffer (1.5 M Tris/HCl pH 6.8, 3% SDS, 15% glycerol, 4% β–mercaptoethanol) to a final 1x concentration.
- Boil samples for 10 min.
- Separate proteins by 10% SDS-PAGE.
- After electrophoresis, heat gel in the presence of Coomassie solution A (0.05% Brilliant Blue R-250, 25% isopropanol, 10% acetic acid) in a microwave oven for about two minutes until boiling point.
- Cool down gel to room temperature with gentle shaking.
- Discard Coomassie staining solution A.
- Destain gel by heating in the presence of Coomassie solution B (0.05% Brilliant Blue R-250, 25% isopropanol), C (0.002% Brilliant Blue R-250, 10% acetic acid) and D (10% acetic acid) each time following the same protocol for staining.
- After the last heating step with solution D, leave gel destaining till background is clear9.
- Western blot analysis:
- After electrophoresis, transfer separated proteins onto a nitrocellulose membrane using standard techniques and block with 4% milk in PBS pH 7.4 at room temperature for 1 hr.
- Incubate the blot overnight at 4 °C or for 4 hr at room temperature in the blocking solution containing rabbit primary antibody raised against the target protein at 1:10,000 and supplemented with 0.1% Tween-20.
- After primary antibody labeling, wash the membrane 3 times for 5 min each with blocking solution containing 0.1% Tween-20.
- Incubate with horseradish peroxidase (HRP)-conjugate anti-rabbit antibody at 1:10,000 for 1.5 hr.
- Wash membrane 5 times for 5 min each with PBS-T (PBS supplemented with 0.1% Tween-20).
- Process western blot using the chemiluminescent peroxidase substrate.
- Radioimmuno assay (RIA):
- Allow reagents (antiserum, tracer and protein A Sepharose) to reach room temperature and pipet 20 µl of protein extract samples and standards at different concentrations (from 300-2,400 ng/ml of commercial recombinant hGAD65) into 12 x 75 mm polystyrene tubes.
- Add 20 µl of antiserum, prepared diluting 1:30 the serum from plasmapheresis of a T1D patient.
- Add 50 µl of tracer (125-I-GAD65) and incubate for 2 hr at room temperature. Set a tube with tracer only to estimate the total activity.
- Add 50 µl of protein A Sepharose and incubate for 1 hr at room temperature.
- Dispense 1 ml of cold PBS buffer into each tube and centrifuge at 1,500 x g at 4 °C for 30 min.
- Decant the tubes to remove supernatant and absorb residual liquid on blotting paper by gently tapping tube.
- Measure immunoprecipitated radioactivity in all tubes by counting for 1 min in a gamma-counter.
- Plot in log scale the binding rates (B/T %) of calibrators related to total activity, to establish the standard curve, and read GAD concentration of samples off the calibration curve10.
NOTE: Restricted areas should be designed for storage, handling and disposal of radioactive material.
Wyniki
An experimental design for the heterologous expression of a target recombinant protein in different production systems is described here. The first focus was the set-up of the different platforms by establishing the optimal conditions for the expression of the target protein in each system.
The expression of the target protein, hGAD65mut, was induced in triplicate E. coli cultures. Following 3 hr of expression at 37 °C, bacterial cells were collected by centrifugation and lysed by sonication. After a centrifugation step, soluble proteins were separated from insoluble inclusion bodies and initial analyses demonstrated that hGAD65mut accumulated prevalently in insoluble inclusion bodies (data not shown). Recombinant protein solubilisation required the use of urea 6 M, which, for its strong denaturing properties, interferes with RIA analysis, making impossible a proper quantification of hGAD65mut. Several strategies have been reported for limiting the formation of inclusion bodies, comprising growing the microbial cells at low temperature11. The cultures were grown at 15 °C and 20 °C, and recombinant protein expression was induced at the same temperatures. As shown in Figure 1A, the solubilisation of hGAD65mut produced at both temperatures, again requires urea (lanes S2). Thus, low temperatures in this experiment do not prevent hGAD65mut from forming insoluble aggregates.
Baculovirus vectors containing the hGAD65mut sequence (Baculo.G65mut) was expressed in adherent Sf9 cell cultures. V1 and V2 high-titer stocks were prepared and the best-performing infection conditions were set up evaluating different viral stock volumes from 5-50 μl. As shown in Figure 1B, the optimal viral stock volume was identified as 25 μl, yielding 11.8 ± 0.8 μg of recombinant protein per ml of culture medium, as evaluated by RIA analysis (Table 1).
Following infiltration, time course analysis of agroinfected N. benthamiana leaves was carried out in the two transient expression systems. For the pK7WG2-based system, leaf samples were pooled daily in the range 1-5 dpi, total soluble proteins (TSP) were extracted and equal amounts of TSP were analyzed by western blot (Figure 1C). This analysis highlighted that the maximum accumulation of target recombinant protein is reached 2 dpi. Therefore, the leaves were harvested 2 dpi and the protein extracts were analyzed by RIA for measuring recombinant protein accumulation, which shows an average of 67.8 µg/g FLW (fresh leaf weight, Table 1). Recombinant protein expression levels, using this system, may be further improved, by co-infiltrating the leaves with a suppressor of Post-Transcriptional Gene Silencing (PTGS) like P19 or HC Pro12.
The same time-course detection was carried out with N. benthamiana leaves agroinfected with the MagnICON vectors: infected leaves were collected 1-8 dpi and equal amounts of TSP were analyzed by western blot. This analysis demonstrated that maximum recombinant protein accumulation is obtained 4 dpi (Figure 1D), with an average accumulation of the recombinant protein of 78.8 µg/g FLW (Table 1), as evaluated by RIA.
The expression of hGAD65mut in transgenic tobacco plants has been previously reported12, showing that recombinant protein levels varied significantly among independently transformed lines. The best-performing hGAD65mut T1 transgenic plant was self-crossed and the derived plants (T2) were analyzed to select again the best performing one. This procedure was repeated over several generations to develop a homogeneous production platform, checking the performance in each generation by RIA until no further improvement was achieved (data not shown). In Figure 1E, a representative western blot of T5 transgenic plants is reported, where the homogeneity of recombinant protein yields is evident. As shown in Figure 1F, the average hGAD65mut yield increased from T2 to T6, reaching level of 99.1 µg/g FLW (Table 1) and, during the selection process, the standard deviation of the expression level declined.
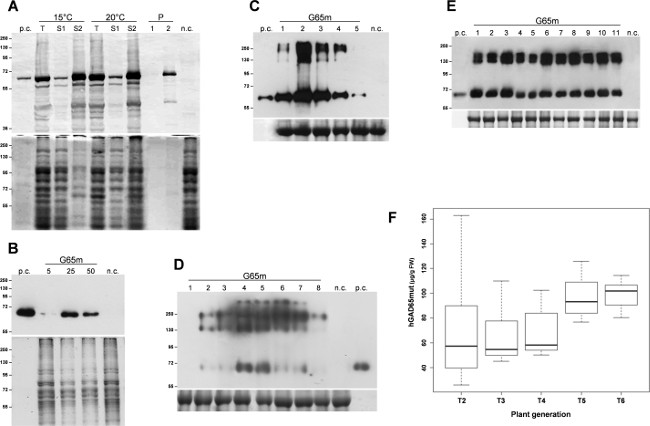
Figure 1: Platform set-up. Set up of best conditions for hGAD65mut expression in each platform. (A) E. coli inducible expression platform. Bacterial cells were grown at 15 °C or 20 °C. Upper panel, western blot of hGAD65mut in cell extracts (2 µg TSP per lane). Lower panel, loading control stained with Coomassie. n.c. = negative control, bacterial cells transformed with the pDEST17 vector containing the chloramphenicol-resistance gene; T: Total samples; S1: Supernatant after sonication and centrifugation; S2 and P: Supernatant (1) and Pellet (2) after centrifugation of the sample solubilized in urea-containing buffer. (B) Baculovirus/insect cell platform. The following viral stock volumes were tested: 5, 25 and 50 µl. Upper panel, western blot of hGAD65mut in cell extracts (5 µg TSP per lane). Lower panel, loading control stained with Coomassie. n.c. = negative control, extract of non-transformed insect cells. (C) Transient expression in N. benthamiana plants using the pK7WG2 vector. Samples were collected daily, 1-5 dpi (lanes 1-5). Upper panel, western blot of hGAD65mut in leaf extracts (2.5 µg TSP per lane). Lower panel, loading control stained with Coomassie, where the large subunit of Rubisco is evident. n.c. = negative control, plants infiltrated with the pK7WG2 vector carrying the gfp marker gene. (D) Transient expression in N. benthamiana plants using MagnICON vectors. Samples were collected daily, 1-8 dpi (lanes 1-8). Upper panel, western blot of hGAD65mut in leaf extracts (5 µg TSP per lane). Lower panel, loading control stained with Coomassie where the large subunit of Rubisco is evident. n.c. = negative control, plants infiltrated only with the pICH20111 5’-module and pICH14011 integrase-module. Numbers indicate molecular mass marker in kDa. p.c. = positive control, 15 ng of commercial hGAD65 produced in the Baculovirus/insect cell system. (E) Stable expression in N. tabacum plants. Leaf samples were collected from different T5 plants (lanes 1-11). Upper panel, western blot of hGAD65mut in leaf extracts (5 µg TSP per lane). Lower panel, loading control stained with Coomassie. n.c. = negative control, wild-type plants. Numbers indicate molecular mass marker in kDa. p.c.= positive control, 15 ng of commercial hGAD65 produced in the Baculovirus/insect cell system. (F) Stable expression in N. tabacum plants. Boxplot representation of hGAD65mut accumulation over several generations derived from best-performing hGAD65mut T1 transgenic tobacco plant reported as µg/g FLW calculated from RIA data. Please click here to view a larger version of this figure.
| System | [hGAD65mut] (µg/ml) | [hGAD65mut] |
| Baculo/insect | 117.5 ± 7.7 | 11.8 ± 0.8 µg/ml culture medium |
| Transient | 22.6 ± 0.9 | 67.8 ± 2.7 µg/g FLW |
| MagnICON | 26.3 ± 5.9 | 78.8 ± 17.8 µg/g FLW |
| Elite T6 | 33.0 ± 3.8 | 99.1 ± 11.3 µg/g FLW |
Table 1: hGAD65mut yields hGAD65mut yields in different platforms - fermenter-based (Baculo/Insect) and plant-based (pK7WG2- and MagnICON in N. benthamiana and elite T6 in N. tabacum). Second column - hGAD65mut concentration in protein extracts (µg/ml). Third column - hGAD65mut content in fresh leaf weight (µg/g FLW) for plant-based platforms and in cell culture medium (µg/ml) for fermenter-based platform.
Dyskusje
In this study three different platforms were compared for the expression of a recombinant human protein: bacterial cells, Baculovirus/insect cells and plants. The plant-based platform was further explored by exploiting three widely used expression technologies (i.e., transient - MagnICON and pK7WG2 based - and stable). The target protein chosen for this experiment, hGAD65mut, has been previously expressed in different systems13, and its production and functionality are easily detectable and measurable14.
Bacterial cells were not an effective production platform because hGAD65mut formed inclusion bodies, even at low-temperature growing conditions, thus requiring laborious solubilization and refolding to achieve its native conformation. Indeed, the main failure of this platform for the expression of complex recombinant proteins is the right conformation of the final product.
The Baculovirus/insect cell platform mediated a high expression of the immunoreactive recombinant protein, but the main limitation of this expression system is the high cost of the culture media, required to grow insect cells. It was estimated that total production costs for 1 g of hGAD65mut could reach 700 euro in this production platform (considering 9 L of insect cell culture media required). A further limitation of this expression platform is the need of sterile cultivation of insect cells, which requires personnel with aseptic manipulation skills. To ensure efficient recombinant protein accumulation two critical parameters must be carefully controlled in this expression system: the viral stock volumes used to infect cells and the timing of viral infection. Furthermore, the detergent, used for total soluble protein extraction from Sf9 insect cells, drastically influences recombinant protein solubilisation.
Plant-based systems were the most beneficial platform to express hGAD65mut: the plant-made recombinant protein was immunoreactive and accumulated at high levels in leaf tissues. Comparing different plant-based expression systems, the highest yields were achieved in stable transformed tobacco plants (Table 1) and, if considering the total biomass of tobacco compared to N. benthamiana, used for transient expression, the overall higher productivity of tobacco is evident. However, the main limitation of stable transformed tobacco plant-based platform is the time required for the set-up of the system, which in our study took 20 months. Indeed, a homozygous line should be selected for recombinant protein expression homogeneity and it may require repeated self-cross cycles, starting from the highest T1 expressing lines. In particular, when the selected T1 bears more than one copy of the transgenic trait, the breeding program may take up to 3 years.
Transient expression systems offered the advantage of rapid upscaling due to a short interval between transformation and expression and the set-up of the expression platform required 4 days. However, a limitation of the plant-based transient systems is that their automation is hardly implementable on a lab-scale unless dedicated high-grade equipment for agro-infiltration is used. Hence, a proper calculation for the large-scale production of hGAD65 using transient-based systems cannot be performed here. On the other hand, we speculate that total production costs for 1 g of recombinant protein using T6 stable tobacco line may be calculated at less than 5 euro (considering soil for growing 60 tobacco plants). To ensure the efficient agroinfiltration and protein production several critical parameters should be carefully controlled (plant developmental stage, grow and infiltration condition of Agrobacterium), as previously reported15. Furthermore, for each expression experiment a specific time-course analysis should be performed to select the dpi that allows the highest accumulation of the recombinant protein.
The example discussed here can be considered a proof-of-principle case study, that highlights some of the specific advantages of plant-based production over traditional systems. In particular, tobacco transgenic lines homogeneously expressing the recombinant protein can be considered a valuable platform for the mass production of recombinant proteins that are required in large-quantities.
Ujawnienia
The authors declare that there is no conflict of interests regarding the publication of this paper.
Podziękowania
This work was supported by the COST action ‘Molecular pharming: Plants as a production platform for high-value proteins’ FA0804. The Authors thank Dr Anatoli Giritch and Prof. Yuri Gleba for providing the MagnICON vectors for research purposes.
Materiały
| Name | Company | Catalog Number | Comments |
| Yeast extract | Sigma | Y1333 | |
| Tryptone | Formedium | TRP03 | |
| Agar Bacteriological Grade | Applichem | A0949 | |
| Sf-900 II SFM medium | Gibco | 10902-088 | |
| Grace’s Insect Medium, unsupplemented | Gibco | 11595-030 | |
| Cellfectin II Reagent | Invitrogen | 10362-100 | |
| MS medium including vitamins | Duchefa Biochemie | M0222 | |
| Sucrose | Duchefa Biochemie | S0809 | |
| Plant agar | Duchefa Biochemie | P1001 | |
| Ampicillin sodium | Duchefa Biochemie | A0104 | Toxic |
| Gentamycin sulphate | Duchefa Biochemie | G0124 | Toxic |
| Ganciclovir | Invitrogen | I2562-023 | |
| Carbenicillin disodium | Duchefa Biochemie | C0109 | Toxic |
| Kanamycin sulfate | Sigma | K4000 | Toxic |
| Rifampicin | Duchefa Biochemie | R0146 | Toxic – 25 mg/ml stock in DMSO |
| Streptomycin sulfate | Duchefa Biochemie | S0148 | Toxic |
| Spectinomycin dihydrochloride | Duchefa Biochemie | S0188 | |
| IPTG (isopropil-β-D-1-tiogalattopiranoside) | Sigma | I5502 | Toxic |
| MES hydrate | Sigma | M8250 | |
| MgCl2 | Biochemical | 436994U | |
| Acetosyringone | Sigma | D134406 | Toxic – 0.1 M stock in DMSO |
| Syringe (1 ml) | Terumo | ||
| MgSO4 | Fluka | 63136 | |
| BAP (6-Benzylaminopurine) | Sigma | B3408 | Toxic |
| NAA (Naphtalene acetic acid) | Duchefa Biochemie | N0903 | Irritant |
| Cefotaxime | Mylan Generics | ||
| Trizma base | Sigma | T1503 | Adjust pH with 1 N HCl to make Tris-HCl buffer |
| HCl | Sigma | H1758 | Corrosive |
| NaCl | Sigma | S3014 | 1 M stock |
| KCl | Sigma | P9541 | |
| Na2HPO4 | Sigma | S7907 | |
| KH2PO4 | Sigma | P9791 | |
| PMSF (Phenylmethanesulfonylfluoride) | Sigma | P7626 | Corrosive, toxic |
| Urea | Sigma | U5378 | |
| β-mercaptoethanol | Sigma | M3148 | Toxic |
| Tween-20 | Sigma | P5927 | |
| Hepes | Sigma | H3375 | |
| DTT (Dithiothreitol) | Sigma | D0632 | Toxic – 1 M stock, store at -20 °C |
| CHAPS | Duchefa Biochemie | C1374 | Toxic |
| Plant protease inhibitor cocktail | Sigma | P9599 | Do not freeze/thaw too many times |
| SDS (Sodium dodecyl sulphate) | Sigma | L3771 | Flammable, toxic, corrosive – 10% stock |
| Glycerol | Sigma | G5516 | |
| Brilliant Blue R-250 | Sigma | B7920 | |
| Isopropanol | Sigma | 24137 | Flammable |
| Acetic acid | Sigma | 27221 | Corrosive |
| Anti-Glutamic acid decarboxylase 65/67 | Sigma | G5163 | Do not freeze/thaw too many times |
| Horseradish peroxidase (HRP)-conjugate anti-rabbit antibody | Sigma | A6154 | Do not freeze/thaw too many times |
| Sf9 Cells | Life Technologies | 11496 | |
| BL21 Competent E. coli | New England Biolabs | C2530H | |
| Protein A Sepharose | Sigma | P2545 | |
| Cell culture plates | Sigma | CLS3516 | |
| Radio Immuno Assay kit | Techno Genetics | 12650805 | Radioactive material |
Odniesienia
- Hampe, C. S., Hammerle, L. P., Falorni, A., Robertson, J., Lernmark, A. Site-directed mutagenesis of K396R of the 65 kDa glutamic acid decarboxylase active site obliterates enzyme activity but not antibody binding. FEBS Lett. 488 (3), 185-189 (2001).
- Avesani, L., et al. Recombinant human GAD65 accumulates to high levels in transgenic tobacco plants when expressed as an enzymatically inactive mutant. Plant Biotechnol. J. 9 (8), 862-872 (2010).
- Sambrook, J., et al. . Molecular Cloning: A laboratory manual. Second Edition. , (1989).
- Avesani, L., et al. Comparative analysis of different biofactories for the production of a major diabetes autoantigen. Transgenic Res. 23, 281-291 (2014).
- Marillonnet, S., Giritch, A., Gils, M., Kandzia, R., Klimyuk, V., Gleba, Y. In planta engineering of viral RNA replicons: efficient assembly by recombination of DNA modules delivered by Agrobacterium. Proc. Natl. Acad. Sci. (USA). 101 (18), 6852-6857 (2004).
- Gleba, Y., Klimyuk, V., Marillonnet, S. Viral vectors for the expression of proteins in plants). Curr. Opin. Biotechnol. 18, 134-141 (2007).
- Engler, C., Kandzia, R., Marillonnet, S. A one pot, one step, precision cloning method with high throughput capability. PLoS One. 3 (11), (2008).
- Xu, R., Li, Q. Q. Protocol: streamline cloning of genes into binary vectors in Agrobacterium via the Gateway TOPO vector system. Plant Methods. 4 (4), 1-7 (2008).
- Fairbanks, G., Steck, T. L., Wallach, D. F. Electrophoretic analysis of the major polypeptides of the human erythrocyte membrane. Biochemistry. 10 (13), 2606-2617 (1971).
- Falorni, A., et al. Radioimmunoassay detects the frequent occurrence of autoantibodies to the Mr 65,000 isoform of glutamic acid decarboxylase in Japanese insulin-dependent diabetes. Autoimmunity. 19, 113-125 (1994).
- Hunt, I. From gene to protein: a review of new and enabling technologies for multi-parallel protein expression. Protein Expr. Purif. 40 (1), 1-22 (2005).
- Arzola, L., et al. Transient co-expression of post-transcriptional silencing suppressor for increased in planta expression of a recombinant anthrax receptor fusion protein. Int. J. Mol. Sci. 12 (8), 4975-4990 (2011).
- Merlin, M., Gecchele, E., Capaldi, S., Pezzotti, M., Avesani, L. Comparative evaluation of recombinant protein production in different biofactories: the green perspective. Biomed. Res. Int. 2014, 136419 (2014).
- Avesani, L., et al. Improved in planta expression of the human islet autoantigen glutamic acid decarboxylase (GAD65). Transgenic Res. 12 (2), 203-212 (2003).
- Leuzinger, K., et al. Efficient agroinfiltration of Plants for high-level transient expression of recombinant proteins. J Vis Exp. (77), (2013).
Przedruki i uprawnienia
Zapytaj o uprawnienia na użycie tekstu lub obrazów z tego artykułu JoVE
Zapytaj o uprawnieniaThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. Wszelkie prawa zastrzeżone