Method Article
Monitoring Kinase and Phosphatase Activities Through the Cell Cycle by Ratiometric FRET
W tym Artykule
Podsumowanie
FRET-based reporters are increasingly used to monitor kinase and phosphatase activities in live cells. Here we describe a method on how to use FRET-based reporters to assess cell cycle-dependent changes in target phosphorylation.
Streszczenie
Förster resonance energy transfer (FRET)-based reporters1 allow the assessment of endogenous kinase and phosphatase activities in living cells. Such probes typically consist of variants of CFP and YFP, intervened by a phosphorylatable sequence and a phospho-binding domain. Upon phosphorylation, the probe changes conformation, which results in a change of the distance or orientation between CFP and YFP, leading to a change in FRET efficiency (Fig 1). Several probes have been published during the last decade, monitoring the activity balance of multiple kinases and phosphatases, including reporters of PKA2, PKB3, PKC4, PKD5, ERK6, JNK7, Cdk18, Aurora B9 and Plk19. Given the modular design, additional probes are likely to emerge in the near future10.
Progression through the cell cycle is affected by stress signaling pathways 11. Notably, the cell cycle is regulated differently during unperturbed growth compared to when cells are recovering from stress12.Time-lapse imaging of cells through the cell cycle therefore requires particular caution. This becomes a problem particularly when employing ratiometric imaging, since two images with a high signal to noise ratio are required to correctly interpret the results. Ratiometric FRET imaging of cell cycle dependent changes in kinase and phosphatase activities has predominately been restricted to sub-sections of the cell cycle8,9,13,14.
Here, we discuss a method to monitor FRET-based probes using ratiometric imaging throughout the human cell cycle. The method relies on equipment that is available to many researchers in life sciences and does not require expert knowledge of microscopy or image processing.
Protokół
1. Introducing the probe to cells
- Co-transfect cells with a FRET-based probe and a plasmid conferring resistance. Choose a transfection method that is efficient in your cell-type of interest. For U2OS cells, standard calcium phosphate transfection methods give adequate results15.
- Select cells in the appropriate antibiotic for at least seven days. This enriches the amount of cells expressing the probe and limits the amount of cells with toxic expression levels, or expression levels that severely affect the cell cycle.
- (optional) Select for stable clones.
- Using fixed or live cells, verify that both CFP and YFP are present in all cells at approximately the same ratio.
- In case some cells contain only CFP or YFP, recombination has likely occurred. Recombination can occur either during bacterial growth or after transfection of the probe and may in the latter case depend on plasmid quality. Repeat plasmid preparation and linearize the plasmid before transfection if the problem persists.
2. Monitoring ratiometric FRET
- Seed cells on glass bottom dishes, or cover slips for mounting in a chamber. Make sure that dishes/cover slips are no 1.5 (170 μm thick).
- Mount cells in growth medium without phenol red on a motorized epifluorescence microscope with temperature control set to 37 °C. Ensure pH regulation of media, either by CO2 or by using CO2-independent media such as Liebowitz-15.
- Focus on cells using transmitted light. Do not look on cells using fluorescent light.
- Start by acquiring a 12 or 16 bit image using YFP excitation (YFPex) and YFP emission (YFPem) filters and a suitable dichroic mirror. Use maximal binning available that still permits the required subcellular resolution. Using a high binning to decrease exposure time or intensity is crucial to avoid phototoxicity.
- Measure or estimate the background intensity and the approximate noise by measuring or estimating the rough average, minimum and maximum pixel values in an area devoid of cells. Change exposure time and neutral density filters so that the average signal in the cell is approximately the background intensity plus five to ten times the difference between max and min background intensities.
- Verify that images are not close to being saturated. The maximum intensity in the image should be less than half of the dynamic range (e.g. max 2000 in a 12 bit image) to allow for intensity differences when cells enter mitosis.
- Repeat step 2.4 to 2.6 using CFP excitation and YFP emission filters and a suitable dichroic mirror.
- Select multiple regions with transfected cells, excluding the regions used for point 2.4 to 2.7, and ensure that maximum intensity is less than half of the dynamic range in all cells.
- Start a time-lapse experiment acquiring two images every 45 min using YFPex - YFPem and CFPex - YFPem filter sets.
3. Verifying and optimizing conditions
- Open the acquired images and monitor the cell cycle time from mitosis to mitosis for cells that express the transfected probe.
- Compare the measured cell cycle time to published data for the cell type used. Alternatively, film untransfected cells by acquiring one image of transmitted light only (e.g. DIC or phase-contrast) every hour to get reference cell cycle times.
- In case the time from mitosis to mitosis corresponds to reference cell cycle timings, proceed to step 4. Alternatively, re-acquire images using harder exposure, more time-points or multiple z-levels and repeat step 3.
- In case the time from mitosis to mitosis does not correspond to reference cell cycle timings, film transfected cells by acquiring one image of transmitted light only (e.g. DIC or phase-contrast) every hour followed by one image at the end of the experiment to identify transfected cells.
- If the transfected cells still show longer cell cycle times, repeat step 1 but transfect less probe or select for stable clones that contain very low amounts of probe.
- If the transfected cells show normal cell cycle times in the absence of fluorescent light, repeat step 2 but lower exposure by modifying binning, neutral density filters, exposure time, and time between images.
4. Analysing FRET
- The analysis can be performed by most software dedicated to microscopy. Here we describe how to perform the analysis using the freeware ImageJ (http://rsb.info.nih.gov/ij) utilizing the plugin Ratio Plus (http://imagej.nih.gov/ij/plugins/ratio-plus.html).
- Open the images in ImageJ. In case your images are in the format of a multicolor stack (e.g. Deltavision), separate the individual channels by first converting them to a hyperstack: Image-hyperstack-stack to hyperstack and subsequently split them to single channel stacks using: Image-hyperstack-reduce dimensionality
- Multiply the YFPex - YFPem stack with 3 using: Process-Math-Multiply This step is optional, as it is only intended to increase the visual clarity of the images by ensuring that ratios are not in the range between 0 and 1.
- In the YFPex - YFPem stack, draw a region of interest (ROI) in an area that is devoid of cells, but that is close to a cell to be measured. Different cells in the same image may require different regions, as both background and signal intensity typically is strongest in the center of an image.
- Add the ROI to ROI manager: Analyze-tools-ROI manager.
- Measure the average intensity of the ROI in both the YFPex - YFPem and the CFPex - YFPem stack: Analyze-Measure.
- Repeat at multiple timepoints and verify that background intensities close to the cell of interest are similar throughout the film.
- Set measurements to include minimal intensity: Analyze-Set measurements-Min and Max grey value.
- Draw a region of interest covering most of a cell and measure the minimal intensity in both the YFPex - YFPem and the CFPex - YFPem stack. Take the difference between the measured minimal intensity and the background intensity from 4.6 and divide it by two. This provides a starting estimate for a clipping value.
- Open Ratio Plus. Select the YFPex - YFPem and the CFPex - YFPem stack. For FRET ratio, select CFPex - YFPem as stack 1, for inverted FRET ratio visualizing probes where FRET efficiency decreases upon phosphorylation, select YFPex - YFPem as stack 1.
- Insert the measured background intensities and clipping values.
- Set the scaling of the resulting ratio stack and apply a suitable look up table (LUT) to visualize ratio changes.
5. Representative Results
Plk1 activity is first visible in the nucleus in G2 phase and peaks during mitosis. Figure 3 shows an experiment using minimal phototoxicity settings as described in section 2. Please note that this is a representative initial result and that exposure conditions or time between images can be modified to increase signal to noise ratio or temporal resolution. Figure 3D shows that a majority of the cells expressing the probe proliferate with a cell cycle time of between 20 and 25 hours, indicating that the imaging conditions and expression levels of the probe do not affect cell cycle timings. Although there is considerable noise, the trend of Plk1 activity increasing in G2 and peaking in mitosis14 is clearly visible (fig 3A). Processing the raw data, here by mean filtering presented as a kymograph (fig 3B), or quantification of the average inverted ratio (fig 3C) can enhance clarity.
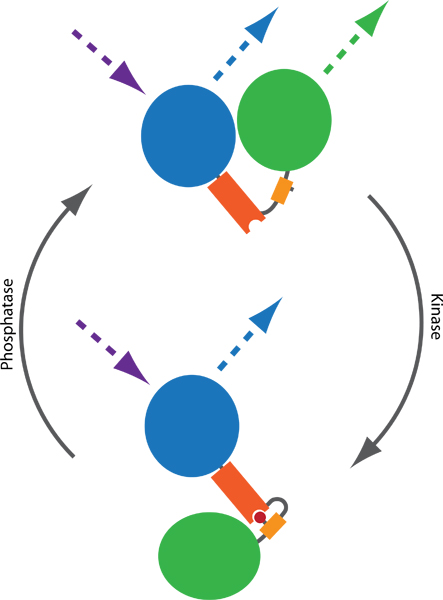
Figure 1. Principle of a FRET-based probe to monitor kinase and phosphatase activities. Two fluorophores, typically CFP (blue) and YFP (green), are connected by a phospho-binding domain (orange) and a phosphorylatable sequence (yellow). Phosphorylation (red) mediates binding to the phospho-binding domain, thereby inducing a conformational change in the probe. The conformational change results in a difference in the distance or orientation between the two fluorophores, which affects the FRET efficiency between CFP and YFP. FRET can be visualized by exciting CFP and monitoring YFP emission (dotted lines).

Figure 2. Schematic outline of the experimental procedure.
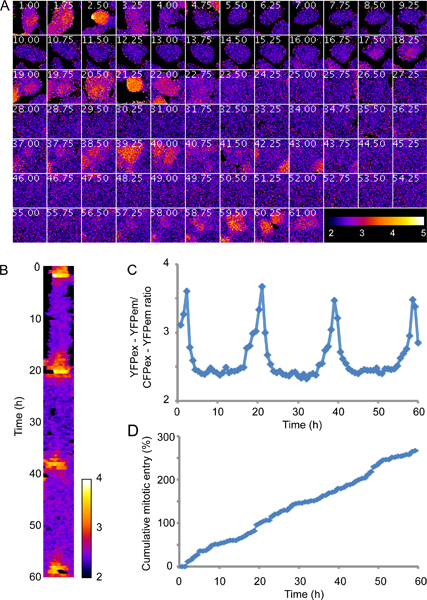
Figure 3. Plk1 activity is first visible in the nucleus in G2 phase and peaks during mitosis. U2OS cells expressing a FRET-based probe monitoring Plk1 activity9,14 were filmed for 60 hr using a Deltavision Spectris Imaging system equipped with a 20x NA 0.7 air objective and a mercury lamp. Imaging conditions were selected to cause minimal phototoxicity, as outlined in section 2, using 4x binning and neutral density filters that block 99% of the incoming light. A, false color representation of inverted FRET-ratio, following one cell through 4 divisions. B, kymograph of the cell shown in A after applying a mean filter. C, quantification of the inverted FRET ratio of the cell shown in A. D, cumulative mitotic entry of 50 FRET-probe expressing cells, including divisions of daughter cells.
Dyskusje
Monitoring FRET throughout the cell cycle requires considerations that are less crucial when assessing short-term responses to external stimuli. First, cell cycle progression is easily perturbed by stress signaling, requiring that phototoxicity is kept to a minimum. Second, all reporters can potentially affect cellular processes by titrating out kinases, phosphatases or interaction domains. The probably most straightforward way to assess if the experimental conditions are adequate is to measure the cell cycle length from mitosis to mitosis and compare it to experiments independent of fluorescence imaging and expression of a probe.
A crucial factor in monitoring FRET ratios throughout the cell cycle is to withstand the temptation to acquire beautiful images with high resolution. Although the exact settings depend on the sensitivity of the microscope system and expression level and nature of the particular probe, it is important to use a level of binning that corresponds to what is absolutely necessary to see. Although the time between CFPex – YFPem and YFPex – YFPem images need to be short to avoid changes in cell morphology, in our hands it is better to keep exposure times at more than 0.1 seconds and reduce the intensity of the fluorescent light by neutral density filters.
How phototoxicity affects the cell cycle depends largely on expression levels and localization of the probe. A probe that is localized to chromatin, for example by fusion with a Histone, renders cells more sensitive to fluorescent light, presumably since phototoxic byproducts are created in the vicinity of DNA. It is therefore crucial to monitor cells that express relatively low levels of the reporters and to re-check cell-cycle timings if localizing the probe to different subcellular structures.
Several control experiments are required to verify that FRET is present in the experimental setup and that ratio changes reflect actual phosphorylation of a probe. Except functional controls, such as inhibition or depletion of a kinase, it is advisable to perform biochemical experiments to verify that the probe is phosphorylated, mutation of the expected phospho-acceptor site within the probe to a non-phosphorylatable residue, and acceptor photobleaching to prove the occurrence of FRET. For an example of an extensive validation, see supplementary figure 3 in ref 14.
Although it is theoretically preferable to assess FRET changes by monitoring both CFP and YFP emission, such setups are very sensitive to focus changes and illumination conditions and can easily lead to artifacts. Sensitivity to focus changes is due to chromatic aberration, where CFP and YFP emission do not focus on the same point. Illumination changes depend largely on different alignment of the light path. This is particularly prominent when using filter cubes containing a dichroic mirror. Focus and illumination change can be reduced using corrected objectives and carefully aligned filter cubes. However, in our experience, keeping the emission filter constant gives superior results, preferably by using a setup with separate control of excitation and emission filters.
The magnitude of the observed FRET-change is reduced by bleedthrough from CFP fluorescence to the YFP emission filter. In case bleedthrough is substantial, an extra CFPex – CFPem image can be acquired to correct bleedthrough into the CFPex – YFPem image. However, such corrections are very sensitive to focus differences and may introduce artifacts. In addition, they require an extra image, which may produce phototoxicity.
Although less of an issue when only monitoring YFP emission, keeping the cells in focus throughout a 24h+ time-lapse experiment can be challenging. To avoid focus drifts, make sure that the microscope and the dish is pre-warmed and that the temperature in the room does not fluctuate. Alternatively, use an autofocus system that is not based on imaging of the transfected probe.
The choice of which objective to use depends on the particular application. An oil-based high NA objective collects more light and gives increased resolution, but is more sensitive to focus changes and is unpractical in high-content setups. We prefer to use an air objective with a relatively high NA to study cytoplasmic or nuclear FRET-ratios, and use high NA oil objectives only when higher subcellular resolution is required.
This paper discusses a straightforward method to film FRET-based probes through the cell cycle that uses equipment that are commonly available to many life science researchers and require only a basal knowledge of microscopy and image processing. More specialized alternatives include the use of beam-splitters, Fluorescence Lifetime Microscopy (FLIM)1 and software to automate object segmentation16 and calculation of FRET ratio17.
Ujawnienia
We have nothing to disclose.
Podziękowania
The authors are supported by the Swedish research council, the Swedish foundation for strategic research, the Swedish cancer society, the Swedish child cancer society, Åke Wibergs foundation and Jeanssons foundation.
Materiały
| Name | Company | Catalog Number | Comments |
| Leibovitz L-15, no phenol red | GIBCO, by Life Technologies | 21083-027 | |
| DMEM+Glutamax-I | GIBCO, by Life Technologies | 31966 | |
| Fetal Bovine Serum (FBS) | Hyclone | SV30160.03 | |
| 0.05% Trypsin EDTA | Hyclone | SH30236.01 | |
| Penicillin-Streptomycin | Hyclone | SV30010 | |
| DPBS | GIBCO, by Life Technologies | 14287 | |
| Puromycin | Sigma-Aldrich | P8833 |
Odniesienia
- Sun, Y., Wallrabe, H., Seo, S. A., Periasamy, A. FRET microscopy in 2010: the legacy of Theodor Forster on the 100th anniversary of his birth. Chemphyschem. 12, 462-474 (2011).
- Allen, M. D., Zhang, J. Subcellular dynamics of protein kinase A activity visualized by FRET-based reporters. Biochem. Biophys. Res. Commun. 348, 716-721 (2006).
- Kunkel, M. T., Ni, Q., Tsien, R. Y., Zhang, J., Newton, A. C. Spatio-temporal dynamics of protein kinase B/Akt signaling revealed by a genetically encoded fluorescent reporter. J. Biol. Chem. 280, (2005).
- Violin, J. D., Zhang, J., Tsien, R. Y., Newton, A. C. A genetically encoded fluorescent reporter reveals oscillatory phosphorylation by protein kinase. C. J. Cell. Biol. 161, 899-909 (2003).
- Kunkel, M. T., Toker, A., Tsien, R. Y., Newton, A. C. Calcium-dependent regulation of protein kinase D revealed by a genetically encoded kinase activity reporter. Journal of Biological Chemistry. 282, 6733-6742 (2007).
- Harvey, C. D. A genetically encoded fluorescent sensor of ERK activity. Proc. Natl. Acad. Sci. U.S.A. 105, 19264-19269 (2008).
- Fosbrink, M., Aye-Han, N. N., Cheong, R., Levchenko, A., Zhang, J. Visualization of JNK activity dynamics with a genetically encoded fluorescent biosensor. Proc. Natl. Acad. Sci. U.S.A. 107, 5459-5464 (2010).
- Gavet, O., Pines, J. Progressive activation of CyclinB1-Cdk1 coordinates entry to mitosis. Dev. Cell. 18, 533-543 (2010).
- Fuller, B. G. Midzone activation of aurora B in anaphase produces an intracellular phosphorylation gradient. Nature. 453, 1132-1136 (2008).
- Ni, Q., Titov, D. V., Zhang, J. Analyzing protein kinase dynamics in living cells with FRET reporters. Methods. 40, 279-286 .
- Morgan, D. O. . The cell cycle : principles of control. , (2007).
- Lindqvist, A., Rodriguez-Bravo, V., Medema, R. H. The decision to enter mitosis: feedback and redundancy in the mitotic entry network. J. Cell. Biol. 185, 193-202 (2009).
- Gavet, O., Pines, J. Activation of cyclin B1-Cdk1 synchronizes events in the nucleus and the cytoplasm at mitosis. J. Cell. Biol. 189, 247-259 (2010).
- Macurek, L. Polo-like kinase-1 is activated by aurora A to promote checkpoint recovery. Nature. 455, 119-123 (2008).
- van der Eb, A. J., Graham, F. L. Assay of transforming activity of tumor virus DNA. Methods. Enzymol. 65, 826-839 (1980).
- Lamprecht, M. R., Sabatini, D. M., Carpenter, A. E. CellProfiler: free, versatile software for automated biological image analysis. Biotechniques. 42, 71-75 (2007).
- Roszik, J., Lisboa, D., Szollosi, J., Vereb, G. Evaluation of intensity-based ratiometric FRET in image cytometry--approaches and a software solution. Cytometry A. 75, 761-767 (2009).
Przedruki i uprawnienia
Zapytaj o uprawnienia na użycie tekstu lub obrazów z tego artykułu JoVE
Zapytaj o uprawnieniaPrzeglądaj więcej artyków
This article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. Wszelkie prawa zastrzeżone