Method Article
The Isolation and Characterization of Low- and Normal- Density Neutrophils from Whole Blood
In This Article
Summary
Here, we provide a reliable approach for isolating low- and normal-density neutrophils from whole blood using magnetic isolation (negative selection) and discontinuous density gradient medium. It ensures untouched isolation of high-purity cells (≥93%), facilitating accurate downstream analysis of neutrophil subpopulations, crucial for understanding their roles in health and disease.
Abstract
Emerging research shows that the circulating neutrophil population in humans consists of diverse subtypes and should not be studied as a single population, as has been done historically. In particular, low-density and normal-density neutrophils (LDNs, NDNs) have been shown to have functionally and metabolically distinct profiles, a factor that must be considered when publishing neutrophil research. Here, we present a modified method for the untouched isolation and separation of LDNs and NDNs from whole blood.
The density gradient medium (1.135 g/mL) is combined at 9:10 with 10x PBS. Specific density gradients of 55%, 70%, and 81% are subsequently made by combining the 100% density gradient medium with 1x phosphate-buffered saline (PBS). Neutrophils isolated from 12 mL of peripheral whole blood obtained from consented donors using a negative selection-based magnetic isolation kit are resuspended in the 55% fraction. A volume of 3 mL of the 81% and 70% fractions is layered into a 15 mL tube, followed by the 55% fraction containing total neutrophils. The density gradients are then centrifuged at 720 x g for 30 min. Two distinct bands are obtained at the 55%/70% interface (LDNs) and 70%/81% interface (NDNs). The cells are carefully pipetted into separate tubes and washed using PBS. The purity of the isolated fractions is determined using flow cytometry. Both LDNs and NDNs were defined as CD14lo CD15+ SSChi by flow cytometry. Isolation purity was calculated at ≥93% of viable cells for both types.
This method provides a reliable and efficient approach for separating LDN and NDNs from peripheral blood, ensuring high purity and viability of the isolated cells. Enhancing the precision of neutrophil isolation facilitates more accurate downstream analyses of these distinct neutrophil subpopulations. These are critical for advancing our understanding of neutrophil heterogeneity and its implications in various physiological and pathological contexts.
Introduction
Neutrophils are granular immune cells and the most abundant leukocyte in peripheral blood, constituting about 50%-70% of leukocytes on average. They develop in the bone marrow from granulocyte-monocyte precursors (GMPs), which in turn develop from hematopoietic progenitor cells (HPCs) in the presence of granulocyte colony-stimulating factor (G-CSF). At homeostasis, they have a lifespan of ~24 h, but studies have shown that their lifespan can be extended under specific physiological conditions and their associated microenvironments such as chronic immune activation1, inflammation1, and even tissue residency in steady state2. Neutrophils have long been considered the first line of defense against pathogens and elicit their anti-microbial effects through 3 major effector functions -- degranulation, phagocytosis, and neutrophil extracellular trap (NET) activation and release (NETosis).
Most studies on neutrophil function and biology examine outcomes for the total neutrophil population. However, from studies in cancer settings delineating N1 (anti-tumor)/ N2 (pro-tumor) subtypes to the classification of neutrophils based on maturity, disease, and physiological state, and even cellular density (low density and normal density neutrophils), it has become increasingly apparent that the human neutrophil population constitutes phenotypically diverse subtypes. Whether the existence of these neutrophil subtypes can be attributed to being completely distinct cell types or due to the complex nature of plasticity, there exists a growing body of literature on atypical neutrophils and presents a compelling opportunity to study low-density neutrophils separate from normal-density neutrophils3.
Described for the first time in SLE patients as a pro-inflammatory neutrophil subset4, LDNs have since been identified in chronic diseases, pregnancy, and even in healthy circulation, in pro-inflammatory as well as suppressive capacities5,6,7,8. LDNs are found concurrently with peripheral blood mononuclear cells (PBMCs) when whole blood is centrifuged over density gradient media. Their specific density corresponds to approximately 1.077 g/mL, compared to NDNs at 1.083 g/mL9. While there is still considerable debate on the subject, there exists speculation that LDNs resemble a more immature granulocyte phenotype (similar to promyelocytes and myelocytes, with a density below 1.080 g/mL)9,10. Others still speculate that there are both mature and immature LDN phenotypes depending on the presence or absence of disease11,12,13. Nevertheless, LDNs have also been detected in healthy individuals; however, their inclusion in some studies is limited due to the difficulty in isolating them in sufficient numbers5.
This study aimed to isolate these two populations in quantities that would allow us to perform downstream in situ metabolic experiments (minimum 0.5 × 106 cells/mL). In doing so, an existing protocol5 was optimized with commonly reported phenotypic markers13,14 that provide the best outcome for isolating and characterizing LDNs and NDNs from whole blood (Figure 1A).
Protocol
Blood samples were collected with informed consent from healthy participants. The study received approval from the Research Ethics Committee of both St. James's Hospital and Tallaght University Hospital.
1. Preparation of density gradient medium, isotonic working solutions fractions and cell separation buffer
- Preparation of isotonic working solutions from density gradient medium
- Preparation of Isotonic 100% working solution (~1.123 g/mL)
- To prepare the isotonic 100% working solution, combine 27 mL of density gradient medium with 3 mL of 10x PBS. This results in an isotonic solution suitable for further dilutions.
- Preparation of isotonic 81% working solution (~1.0996 g/mL)
- Prepare the isotonic 81% working solution by mixing 8.1 mL of the isotonic 100% working solution (prepared in step 1.1.1) with 1.9 mL of 1x PBS. Mix thoroughly to ensure homogeneity.
- Preparation of isotonic 70% working solution (~1.0861 g/mL)
- To prepare the isotonic 70% working solution, combine 7 mL of the isotonic 100% working solution with 3 mL of 1x PBS. Ensure that the solution is well mixed.
- Preparation of isotonic 55% working solution (1.0676 g/mL)
- Make the isotonic 55% working solution by mixing 5.5 mL of the isotonic 100% working solution with 4.5 mL of 1x PBS. Mix thoroughly.
- Preparation of Isotonic 100% working solution (~1.123 g/mL)
- Layering of gradient fractions
- Gradient fraction layering
- Using a 15 mL conical tube, carefully layer 3 mL of the isotonic 81% working solution at the bottom. Then, slowly and gently layer 3 mL of the isotonic 70% working solution on top, avoiding mixing the layers. This gradient will be used for cell separation.
- Gradient fraction layering
- Preparation of cell separation buffer
- Preparation of 500 mL of cell separation buffer
- To prepare the cell separation buffer, combine 2% fetal bovine serum (FBS) and 1 mM ethylenediaminetetraacetic acid(EDTA) with 1x PBS to a total volume of 500 mL. Use this buffer to wash and resuspend cells during the isolation process.
NOTE: Ensure that the layers are well-separated and undisturbed. In this protocol, the isotonic working solutions are referred to as "100%", "81%", "70%", and "55%" based on the method of preparation, consistent with previous studies5. However, it is important to clarify that these labels do not represent the actual final concentrations of the working solutions. For example, the "100%" density gradient medium is prepared by mixing 27 mL of pure density gradient medium with 3 mL of 10x PBS, resulting in a final concentration of 90% Isotonic working solution. The subsequent dilutions (e.g., "81%", "70%", and "55%") similarly reflect preparation ratios rather than true percentage concentrations. This nomenclature was retained to maintain consistency with the terminology used in earlier literature on the subject.
- To prepare the cell separation buffer, combine 2% fetal bovine serum (FBS) and 1 mM ethylenediaminetetraacetic acid(EDTA) with 1x PBS to a total volume of 500 mL. Use this buffer to wash and resuspend cells during the isolation process.
- Preparation of 500 mL of cell separation buffer
2. Isolation of neutrophils from whole blood using negative selection
- Preparation of magnetic beads
- Vortex the magnetic beads thoroughly for 30 s to ensure they are fully resuspended before use.
- Blood preparation
- For each donor, aliquot 4 mL of whole blood into three separate 14 mL round-bottomed tubes.
- Addition of neutrophil isolation cocktail and magnetic beads
- Add 50 µL of neutrophil isolation cocktail and 50 µL of magnetic beads per mL of blood to each tube. For 4 mL of blood, add 200 µL of each reagent.
- Resuspend the mixture well by gently pipetting and incubate at room temperature (RT) for 5 min to allow the reagents to bind to unwanted cells.
- Washing and magnetic separation - first round
- Top up each tube to 12 mL using cell separation buffer and mix thoroughly.
- Place the tubes without lids on a magnet and let them incubate at RT for 10 min to allow the magnetic beads bound to unwanted cells to be drawn to the tube wall.
- Collection of unbound cells
- Carefully transfer the clear cell suspension containing neutrophils from each tube into a new, clean tube using a transfer pipette. Remove the original tube from the magnet.
- Magnetic bead reapplication and incubation - second round
- Vortex the magnetic beads again for 30 s. Add the same volume of magnetic beads to the newly transferred cell suspension as in step 2.3. Resuspend thoroughly and incubate at RT for another 5 min.
- Final magnetic separation and collection
- Place the tubes back onto the magnet without lids and incubate at RT for another 10 min.
- Carefully transfer the clear cell suspension to a new tube. This final suspension represents the isolated total neutrophil population.
3. Separation of low-density neutrophils (LDNs) and normal-density neutrophils (NDNs) from the total neutrophil population
- Pooling and preparation of cell suspensions
- For each donor, pool the isolated neutrophil suspensions into 50 mL tubes. Top up each tube to a total volume of 50 mL using the cell separation buffer.
- Centrifugation
- Centrifuge the tubes at 400 x g for 5 min at RT with the brake on.
- Resuspension and cell counting
- Discard the supernatant and resuspend the neutrophil pellet in 1 mL of cell separation buffer. Perform a cell count to determine the total number of neutrophils.
- Density gradient medium preparation
- Centrifuge the tubes again at 400 x g for 5 min at RT with the brake on. Discard the supernatant and resuspend the neutrophil pellet in 3 mL of the 55% density gradient medium. For optimal resolution, use 3 mL of 55% density gradient medium per 5-6 × 106 total neutrophils.
- If necessary, divide the resuspended cells across multiple pre-made density gradient tubes, ensuring that 3 mL of the cell suspension is layered into each tube.
- Layering onto density gradient tubes
- Slowly layer the 3 mL cell suspension containing the 55% density gradient medium onto the pre-made density gradient tubes (prepared as described in section 1). Ensure careful layering to avoid disturbing the gradient.
- Density gradient centrifugation
- Centrifuge the tubes at 720 x g for 30 min without using the brake. After centrifugation, handle the tubes carefully to avoid disturbing the layers.
- Isolation of neutrophil fractions
- Following centrifugation, observe the neutrophils separate into two distinct layers:
Low-density neutrophils (LDNs) will be located at the 55%/70% interface, forming an upper band at approximately the 3 mL mark, and normal-density neutrophils (NDNs) will be at the 70%/81% interface, appearing as a lower band around the 6 mL mark. - Using a transfer pipette, carefully isolate each layer and transfer them to separate 15 mL tubes.
- Following centrifugation, observe the neutrophils separate into two distinct layers:
- Washing of isolated fractions
- To wash off any residual density gradient medium, add 1x PBS to each 15 mL tube up to the 15 mL mark.
- Centrifuge at 400 × g for 5 min at RT with the brake on. If the cells do not form a firm pellet and instead remain suspended, repeat this wash step, indicating the presence of remaining density gradient medium.
- Cell count and downstream applications
- Count the cells in each fraction and proceed with downstream applications such as metabolic experiments, flow cytometry staining, RNA extraction, or protein lysis as needed.
NOTE: After plating cells out, spinning down the plate at 210 × g for 1 min (brake off) is recommended.
- Count the cells in each fraction and proceed with downstream applications such as metabolic experiments, flow cytometry staining, RNA extraction, or protein lysis as needed.
4. Phenotyping of isolated LDN and NDN
- Isolate ~0.5-1 × 106 LDNs and NDNs. Make a 2x concentrated antibody mastermix using CD14, CD86, CD15, CD16, CD10, Fc block, and viability stain.
- Stain the isolated LDN and NDN populations with an equivalent volume of the antibody mastermix so that the final dilution of antibodies staining the cells is 1:100 (CD14, CD86, CD15, CD16, CD10, Fc block) and 1:50 (viability stain), respectively.
- Incubate the cells for 10 min in the dark at RT.
NOTE: Ensure the cells are thoroughly mixed with the antibody mastermix and Fc block. - Wash the cells once with PBS, then centrifuge at 400 × g for 5 min. Discard the supernatant.
- Fix the cells in 1% paraformaldehyde (PFA) for 15 min at RT in the dark.
- Wash the cells again with PBS, centrifuge at 400 × g for 5 min, and discard the supernatant.
- Resuspend the cells in 200 µL of PBS or as required to be used and acquire the cells on a flow cytometer as soon as possible.
NOTE: The stained and fixed cells can be stored short-term at 4 ◦C in the dark to prevent photobleaching the fluorophores. However, ideally, the phenotyping procedure should be carried out promptly after staining and fixation to ensure optimal cell viability and accurate results.
Results
The successful layering of total neutrophils over the density gradient medium can be observed in Figure 1B. Two distinct bands should be obtained. If mixing of the gradients occurs, or the number of total neutrophils layered per tube is high (greater than roughly 5-6 × 106), the bands will look diffuse (Figure 1C), and the risk of the two neutrophil subtypes mixing increases significantly. To avoid the latter, we recommend layering up to 5-6 × 106 total neutrophils per density-gradient tube. Despite reports of LDNs being at very low numbers in healthy individuals, which therefore makes them unattainable for downstream studies, this method shows good yield of both LDNs (mean 3.28 × 106 cells/mL) and NDNs (mean 10.64 × 106 cells/mL) and purity from healthy participants (Figure 2).
The average isolation purity for LDNs was 93.80% (± 5.80), and for NDNs, it was 96.30% (± 3.30; Figure 2A) after the isolation procedure, with minimal contamination of other cell types (Figure 2B). DAPI staining of nuclei confirmed LDN/NDN presence through nuclei morphology and showed distinctive multi-lobed nuclei in NDNs after isolation (Figure 2C). Absolute counts after isolation showed a common trend of more NDNs being present than LDNs, with mean yields of 10.64 × 106 cells/mL and 3.28 × 106 cells/mL, respectively (Figure 2D). Using flow cytometry, both LDNs and NDNs were defined as CD14lo CD86- CD15+ SSChi cells13 (Figure 2E). CD14 is a common myeloid marker, CD15 is a neutrophil identification marker, and CD10 and CD16 are commonly described neutrophil markers of maturity and activation13. Fluorescence-minus-one controls (FMOs) were used to distinguish CD16 and CD10 positive populations from negative populations. A largely singular population of mature LDNs as well as NDNs (CD16hiCD10+) were observed in healthy individuals, which is consistent with reports in the literature13, as healthy individuals are not likely to have a high percentage of immature neutrophils (CD16lo/intCD10+) in circulation (Figure 2F,G). On average, the frequency of CD16hiCD10+ LDNs was 93.33% (± 5.29), CD16hiCD10+ NDNs was 98.03% (± 1.40), CD16lo/intCD10+ was 0.49% (± 0.38), and CD16lo/intCD10+ was0.17% (± 0.06; Figure 2F,G). When CD16+CD10+ populations were superimposed upon each other, the degree of overlap indicated no noticeable differences in the expression of these markers in LDNs compared to NDNs (Figure 2H). The cells were acquired using a flow cytometer and all data analysis was performed using the appropriate software for flow cytometry.
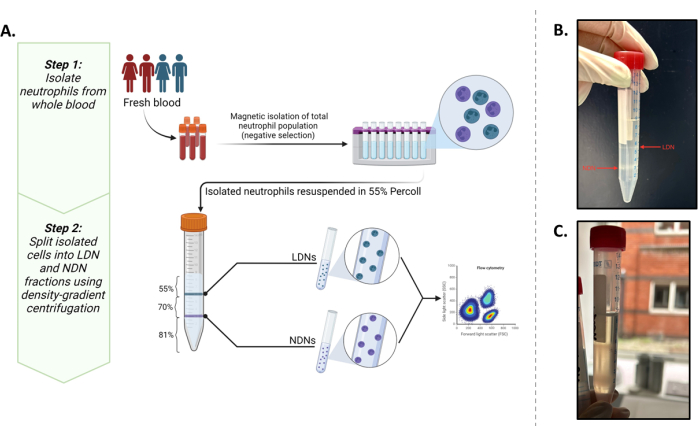
Figure 1: Overview of the protocol. (A) A two-step isolation protocol of LDNs and NDNs from whole blood. The first step consists of total neutrophils isolated from whole blood using negative selection. The second step involves resuspending the total neutrophils in 55% Isotonic working solution and layering it on top of an 81% and 70% gradient. After centrifugations, LDNs will be at the interface of the 55% and 70% layers and NDNs at the interface of the 81% and 70% layers. (B) Visual representation of ideally separated LDNs and NDNs after gradient medium centrifugation. (C) Suboptimal separation of LDNs and NDNs as seen by the diffuse nature of the bands. There is a high likelihood in this case that NDNs contaminate the LDN fraction or vice versa. Graphic created in BioRender. Yennemadi, A. (2025) https://BioRender.com/t03m122. Please click here to view a larger version of this figure.
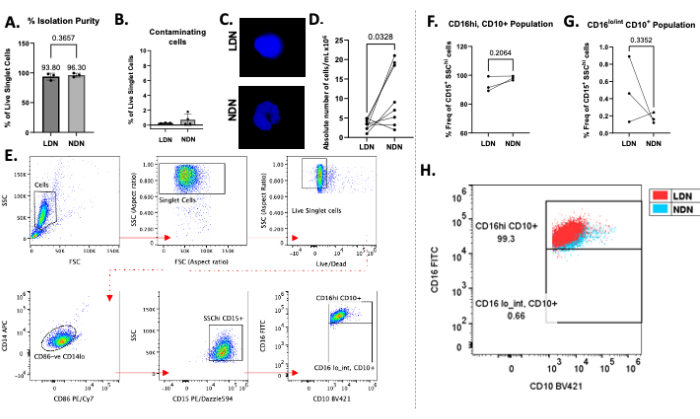
Figure 2: Representative results of the protocol. (A) Percent isolation purity of LDNs and NDNs from whole blood. (B) Percentage of contaminating cells (B cells, T cells, and monocytes) after isolation. (C) DAPI staining of LDNs and NDNs after isolation showing multi-lobed nuclei in NDNs and single-lobed nuclei in LDNs. (D) Absolute number of LDNs and matched NDNs isolated from whole blood. (E) Gating strategy used to define LDNs and NDNs, determine their purity, and calculate their frequency. Debris, doublets, and dead cells were eliminated from analysis (top row), and CD86- CD14lo, CD15+ SSChi cells were gated upon to define neutrophils. (F,G) CD16 and CD10 expression were used to define subpopulations in conjunction with the use of fluorescence-minus-one controls. Frequency of isolated mature (CD16hiCD10+) and immature (CD16lo/intCD10+) LDN and NDN subpopulations, reported as the percentage of total LDNs and NDNs (defined as CD15+SSChi cells). (H) Dot plot showing CD16+ CD10+ LDNs superimposed over CD16+ CD10+ NDNs in healthy individuals. Mean ± SD, Paired t-test, n = 3-8. Please click here to view a larger version of this figure.
Discussion
Here, we present an optimized method of splitting the total neutrophil population into low- density and normal-density neutrophils, together with phenotypic characterization of each cell type using flow cytometry adapted from previous methods5.
This protocol is based on the isolation of LDNs and NDNs from whole blood. A crucial step is that total neutrophils are isolated through negative selection methods. Positive selection methods involve the use of antibodies that bind to surface markers, potentially activating the neutrophils and triggering degranulation5. This activation can interfere with downstream experiments by altering the functional and metabolic state of the neutrophils, leading to inaccurate results and skewing the interpretation of experimental outcomes. Therefore, negative selection ensures the isolation of neutrophils without inducing activation or functional changes. Additionally, the use of negative separation-based magnetic isolation eliminates the need for red blood cell (RBC) lysis. This not only reduces extra wash steps and cell death, thus preserving yield, but it also increases the purity of the isolated neutrophils by minimizing RBC contamination. In comparison to the original protocol, a cell separation buffer was used throughout the entire process, including washing and resuspension, in place of PBS. This contains additives that reduce cell clumping and minimize non-specific binding during magnetic isolation. By using cell separation buffer consistently, we achieved improved cell separation efficiency, higher purity, and a greater yield of viable, untouched neutrophils compared to using PBS. This resulted in enhanced precision for downstream analyses. Additionally, the density gradient was performed over multiple tubes rather than over a single tube to improve the resolution between the two cell types.
The successful separation of the two neutrophil subtypes is unsurprisingly highly dependent on the precise formulation and accurate layering of each gradient15. Equally important is the layering of the 70% over the 81%, as well as the neutrophil suspension (in 55%) over the 70% layer. We highly recommend practicing this method. It is best to use a transfer pipette when layering and positioning the 15 mL tube almost parallel to the bottom of the biosafety cabinet. Mixing of the gradients, even slightly, will cause indistinct and diffuse bands, which may render indistinct populations. Care should be taken to slowly remove only the neutrophil bands using a Pasteur pipette, as the layers above and below should not be aspirated, if possible, due to their toxicity. It is important to minimize contamination of the neutrophil fraction with the Isotonic working solution to avoid any adverse effects on the cells15. Finally, higher cell numbers can result in an overall higher density of the LDN and NDN bands, resulting in poorer resolution of the cell populations. We, therefore, recommend performing a total neutrophil count prior to resuspension in the 55% solution. This is particularly significant if using >12 mL whole blood for neutrophil isolation or if the total neutrophil count exceeds 5-6 × 106.
Alternatives to this method consist of the dextran/Percoll combination, which does not rely on magnetic separation and is essentially a single-step protocol16. The use of dextran sedimentation to remove erythrocytes may not be as efficient or consistent as other methods like direct RBC lysis or density gradients. Dextran sedimentation can leave residual RBCs in the supernatant, which could affect the purity of the neutrophil population. Without a negative selection step, there is a risk that neutrophils could be activated by residual antibodies or other stimuli, which might affect their behavior in downstream assays. The manual removal of PBMC and neutrophil layers from the density gradient medium could result in contamination between the cell populations, particularly if the separation is not clean.
This study presents a relatively simplistic flow cytometry panel to determine the purity of the isolated LDNs and NDNs and perform a minimal characterization of these cells. The markers used here are widely reported in the literature as part of multiple, more complex flow cytometry panels involving neutrophil research. We chose to condense the panel to determine isolation purity, percent frequency of live and dead cells, and examine neutrophil maturation status (based on CD10 and CD16 expression). However, it can be expanded to suit the needs of various end-users' studies. This protocol was specifically designed to separate LDNs and NDNs for individual assessment, as distinguishing these subpopulations together on a flow cytometry panel has proven challenging due to their relative indistinctness (Figure 2H). Consistent with previous reports5, it was found that LDNs and NDNs from healthy individuals cannot be reliably differentiated when analyzed simultaneously. Therefore, isolating them beforehand allows for a more accurate and detailed characterization of each subpopulation.
We suggest that during the optimization phase for this protocol, end-users might choose to include basic lymphocyte markers such as CD3 and CD19 in their flow cytometry panel. This can help with troubleshooting, as suboptimal magnetic isolation can result in the inclusion of lymphocytes in the total neutrophil isolate, which could potentially carry forward into the LDN fraction owing to similar cellular densities. For the purposes of optimization, users may choose to add CD45 to better troubleshoot the contaminants in their isolated fractions, i.e., CD45-ve could be RBCs, and CD45+ve could indicate PBMC contamination. Neutrophil subpopulations were defined based on surface marker expression, which is a dynamic property and can present a high level of variability between donors. Thus, in keeping with good flow cytometry practices, using FMO controls is also suggested, as this can help reduce inter-donor variability and define clearer sub-populations.
We also acknowledge that this protocol was only optimized for fresh whole blood, in accordance with the guidelines from the magnetic isolation kit, which states that only unprocessed, whole blood should be used with this kit and that recovery of the desired isolated cells decreases with samples that are >24 h old. This eliminates the options for the starting material from which neutrophils can be isolated, such as bronchoalveolar lavage fluid (BALF), pleural fluid, ascites, etc. Connelly et al. have shown that delays in processing neutrophils17, even from fresh-drawn whole blood, can alter their phenotype and surface expression. In keeping with this, we recommend the commencement of processing with as little delay as possible from the time of the blood draw. So, while this protocol is ideal for fresh whole blood, compared to single-step protocols like the dextran/Percoll, it can be a relatively long and time-consuming method that does not offer an intermediate stop point. This presents a limitation in cases of clinical studies where samples may be received at later hours in the day. Additionally, this method does not permit the sorting of LDN and NDN subsets, as methods for sorting subsets (by flow cytometry or otherwise) run the risk of unintentionally activating neutrophils.
Neutrophil subpopulations have garnered significant attention in conditions such as systemic lupus erythematosus (SLE)18,19, cancer20, and COVID-1921. The growing interest in these cell types, particularly in the context of inflammatory and autoimmune diseases, underscores the need for reliable methods to accurately assess each population and better understand their individual contributions to pathogenesis.
Disclosures
The authors have no disclosures.
Acknowledgements
This work was funded by the Health Research Board EIA-2024-002 and the Royal City of Dublin Hospital Trust. We would like to thank Dr. Lorraine Thong and Dr. Kevin Brown for their assistance in collecting samples from healthy donors for this manuscript.
Materials
| Name | Company | Catalog Number | Comments |
| 14 mL Polypropylene Round-Bottom Tube (17 x 100 mm) | Corning Science | 352059 | |
| APC anti-human CD14 (63D3) | BioLegend | 367118 | |
| Brilliant Violet 421 anti-human CD10 (25 tests) | BioLegend | 312217 | |
| Dulbecco's Phosphate-Buffered Saline | Sigma | D8537-1L | |
| EasyEights EasySep Magnet | StemCell Technologies | #18103 | |
| EasySep Buffer (cell separation buffer) | StemCell Technologies | #20144 | |
| EasySep Direct Human Neutrophil Isolation Kit | StemCell Technologies | #19666 | |
| FcR Blocking Reagent, human | Miltenyi Biotec | 130-059-901 | |
| FITC anti-human CD16 (3G8) | BioLegend | 302005 | |
| OneComp eBeads Compensation Beads | eBioscience Inc. | 01-1111-42 | |
| PE/Cy7 anti-human CD86 (BU63) | BioLegend | 374209 | |
| PE/Dazzle 594 anti-human CD15 (SSEA-1) | BioLegend | 323037 | |
| Phosphate-Buffered Saline Tablets | Gibco | 18912-014 | |
| Zombie NIR Fixable Viability Kit | BioLegend | 423105 |
References
- Tracchi, I., et al. Increased neutrophil lifespan in patients with congestive heart failure. Eur J Heart Fail. 11 (4), 378-385 (2009).
- Ballesteros, I., et al. Co-option of neutrophil fates by tissue environments. Cell. 183 (5), 1282-1297.e18 (2020).
- Mckenna, E., et al. Neutrophils: Need for standardized nomenclature. Front Immunol. 12, 602963(2021).
- Hacbarth, E., Kajdacsy-Balla, A. Low density neutrophils in patients with systemic lupus erythematosus, rheumatoid arthritis, and acute rheumatic fever. Arthritis Rheumatol. 29 (11), 1334-1342 (1986).
- Hardisty, G. R., et al. High purity isolation of low density neutrophils casts doubt on their exceptionality in health and disease. Front Immunol. 12, 625922(2021).
- Scapini, P., Marini, O., Tecchio, C., Cassatella, M. A. Human neutrophils in the saga of cellular heterogeneity: Insights and open questions. Immunol Rev. 273 (1), 48-60 (2016).
- Ssemaganda, A., et al. Characterization of neutrophil subsets in healthy human pregnancies. PLoS One. 9 (2), e85696(2014).
- Tay, S. H., Celhar, T., Fairhurst, A. M. Low-density neutrophils in systemic lupus erythematosus. Arthritis Rheumatol. 72 (10), 1587-1595 (2020).
- Hassani, M., et al. On the origin of low-density neutrophils. J Leukoc Biol. 107 (5), 809-818 (2020).
- Cowland, J. B., Borregaard, N. Isolation of neutrophil precursors from bone marrow for biochemical and transcriptional analysis. J Immunol Methods. 232 (1-2), 191-200 (1999).
- Blanco-Camarillo, C., Aleman, O. R., Rosales, C. Low-density neutrophils in healthy individuals display a mature primed phenotype. Front Immunol. 12, 672520(2021).
- Park, J., et al. Low-density granulocytes display immature cells with enhanced NET formation in people living with HIV. Sci Rep. 13 (1), 13282(2023).
- Denny, M. F., et al. A distinct subset of proinflammatory neutrophils isolated from patients with systemic lupus erythematosus induces vascular damage and synthesizes type I IFNs. J Immunol. 184 (6), 3284-3297 (2010).
- Ui Mhaonaigh, A., et al. Low density granulocytes in anca vasculitis are heterogenous and hypo-responsive to anti-myeloperoxidase antibodies. Front Immunol. 10, 2603(2019).
- Mosca, T., Forte, W. C. Comparative efficiency and impact on the activity of blood neutrophils isolated by Percoll, Ficoll and spontaneous sedimentation methods. Immunol Invest. 45 (1), 29-37 (2016).
- Ren, Y., et al. Increased apoptotic neutrophils and macrophages and impaired macrophage phagocytic clearance of apoptotic neutrophils in systemic lupus erythematosus. Arthritis Rheum. 48 (10), 2888-2897 (2003).
- Connelly, A. N., et al. Optimization of methods for the accurate characterization of whole blood neutrophils. Sci Rep. 12 (1), 3667(2022).
- Carmona-Rivera, C., Kaplan, M. J. Low-density granulocytes in systemic autoimmunity and autoinflammation. Immunol Rev. 314 (1), 313-325 (2023).
- Yennemadi, A. S., Keane, J., Leisching, G. Mitochondrial bioenergetic changes in systemic lupus erythematosus immune cell subsets: Contributions to pathogenesis and clinical applications. Lupus. 32 (5), 603-611 (2023).
- Futoh, Y., et al. Peripheral low-density granulocytes after colorectal cancer surgery in predicting recurrence. BJS Open. 7 (1), zrac154(2023).
- Dwivedi, A., et al. Emergence of dysfunctional neutrophils with a defect in arginase-1 release in severe COVID-19. JCI Insight. 9 (17), e171659(2024).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved