Method Article
Adjunctive Diode Laser Therapy and Probiotic Lactobacillus Therapy in the Treatment of Periodontitis and Peri-Implant Disease
In This Article
Summary
This article describes two protocols: 1) adjunctive diode laser therapy for treating periodontitis and 2) probiotic Lactobacillus therapy for treating peri-implant disease, with emphasis on the laser usage mode (inside or outside pocket), laser application regimen (single or multiple sessions), and a probiotic protocol of professional and home administration.
Abstract
Periodontal and peri-implant diseases are plaque-induced infections with a high prevalence, seriously impairing people's quality of life. The diode laser has long been recommended as adjunctive therapy in treating periodontitis. However, the optimal combination of usage mode (inside or outside periodontal pocket) and application regimen (single or multiple sessions of appointment) has not been described in detail. Meanwhile, probiotic Lactobacillus is regarded as a potential adjuvant in the management of the peri-implant disease. Nonetheless, a detailed protocol for an effective probiotic application is lacking. This article aims to summarize two clinical protocols. For periodontitis, the optimal collaboration of laser usage mode and application regimen was identified. Regarding peri-implant mucositis, a combined therapy containing professional topical use and home administration of probiotic Lactobacillus was established. This updated laser protocol clarifies the relationship between the treatment mode (inside or outside the periodontal pocket) and the number of laser appointments, further refining the existing diode laser therapy. For inside pocket irradiation, a single session of laser treatment is suggested whereas, for outside pocket irradiation, multiple sessions of laser treatment provide better effects. The improved probiotic Lactobacillus therapy resulted in the disappearance of swelling of the peri-implant mucosa, a reduced bleeding on probing (BOP), and an obvious reduction and good control of plaque and pigmentation; however, probing pocket depth (PPD) had limited improvement. The current protocol should be regarded as preliminary and could be further enhanced.
Introduction
Periodontal disease is a chronic multifactorial infection resulting in progressive destruction of periodontium1. Its severe form, periodontitis, affects up to 50% population worldwide2 and is regarded as a major cause of tooth loss in adults3. Replacement of missing teeth with dental implants has been extensively favored over traditional options4. The implants show prominent functional and aesthetic performances with a long-term survival rate of 96.1% after 10 years5,6. The implants, however, can suffer from peri-implant disease leading to mucosal inflammation (peri-implant mucositis) or surrounding bone loss (peri-implantitis)7, which may cause the implant failure8. Therefore, it is of utmost necessity to manage the periodontal and peri-implant diseases effectively, in order to preserve natural teeth or improve the survival rate of dental implants.
Periodontal and peri-implant diseases share similar etiology9, i.e., both are initiated by exposure to dental plaque, consisting mainly of anaerobic and microaerophilic bacteria10. Mechanical debridement is considered a reliable modality to achieve efficient disruption of pathogenic deposits on root or implant surfaces11. Nevertheless, it has restricted accessibility using instruments when there is complex tooth anatomy (i.e., root furcation and groove), leading to insufficient decontamination12. Under this context, the application of lasers and probiotics has emerged to supplement mechanical debridement13,14.
A variety of lasers have been proposed for periodontal treatment, such as Nd:YAG; CO2; Er:YAG; Er,Cr:YSGG; and diode laser15. Among these, the diode laser is the most popular choice for clinical treatment due to its portability and low cost16. The diode laser has been recommended as an ideal adjunct in destroying biofilms, eliminating inflammation, and facilitating wound healing due to its photobiomodulation and photothermal effects12,13. The diversity of laser usages, nonetheless, leads to significant clinical heterogeneity among current studies. Thus, in our recent publication, we evaluated 30 clinical trials and summarized the optimal combination of laser usage mode and application regimen12. However, few studies report the detailed procedure of the combination protocol. On the other hand, probiotic Lactobacillus has been drawing increasing attention as a potential adjuvant in treating peri-implant disease, due to its antimicrobial and anti-inflammatory performances17,18. The clinical benefits, however, have not reached an agreeable consensus. One critical account referred to the variety of probiotic administration protocols17.
Based on the current evidence, this article describes two modified clinical protocols: the existing protocol for the use of adjunctive diode laser in treating periodontitis is improved based on two laser usage modes (inside or outside pocket) and two application regimens (single or multiple sessions of appointment)12. For the adjunctive probiotic Lactobacillus therapy in treating peri-implant disease, a combination of professional local use and home administration of probiotic is described17.
Protocol
This study was approved by the Institutional Review Board of the College of Stomatology, Xi'an Jiaotong University (xjkqll[2022]NO.034). Informed consent was available from the patients involved in this study.
1. Adjunctive diode laser therapy in the non-surgical treatment of periodontitis
- Eligibility criteria
- Use the following inclusion criteria: age ≥ 18 years; probing pocket depth (PPD) ≥ 5 mm; detectable clinical attachment loss (CAL) and radiographic bone loss (RBL).
- Use the following exclusion criteria: patients with systematic diseases or under medication that may affect the inflammation and healing process; patients who had received periodontal treatment within 6 months; smokers or alcoholics; pregnant or lactating patients; periodontal-endodontic combined lesions; class III tooth mobility.
- Clinical examination
- Measure full-mouth PPD, bleeding on probing (BOP) at six sites per tooth (i.e., mesiobuccal, buccal, distobuccal, distolingual, lingual, and mesiolingual), and mobility of each tooth, excluding third molars. Measure CAL for teeth with PPD ≥ 5 mm. Detect the cementoenamel junction by probing to calculate the CAL.
- Record the baseline parameters on a periodontal chart (Supplementary File 1).
- Disinfection and anesthesia
- Gargle with 3% hydrogen peroxide for 1 min, followed by pure water. Disinfect the operating area with 1% iodophor. Administer a local injection of primacaine adrenaline for anesthesia.
- Mechanical debridement by scaling and root planing (SRP)
NOTE: SRP is necessary for treating periodontitis including ultrasonic or manual cleaning. Dental scaling removes the plaque and calculus from the surface of the tooth above and below the gumline. Root planing usually follows dental scaling and attempts to smooth out the rough surface of the root and helps the gums to reattach to the teeth.- Firstly, use an ultrasonic device to perform SRP for teeth with PPD > 3 mm. Secondly, use hand-held instruments (Gracey curettes 5/6, 7/8, 11/12, and 13/14) to perform SRP for teeth with PPD > 3 mm (Figure 1). Thirdly, use an ultrasonic device to perform SRP for these teeth a second time.
NOTE: The diode laser alone is not effective in eliminating calculus, so a thorough SRP cannot be omitted. If the patient has a large number of affected teeth, only one quadrant can be treated at each appointment. The ultrasonic and manual SRP can be performed within 1-4 appointments. - Rinse the periodontal pockets with 3% hydrogen peroxide and gargle with pure water.
- Apply polishing paste to the teeth surface, polish the full-mouth teeth surfaces using a low-speed handpiece with a rubber cap, and then gargle with pure water.
- Firstly, use an ultrasonic device to perform SRP for teeth with PPD > 3 mm. Secondly, use hand-held instruments (Gracey curettes 5/6, 7/8, 11/12, and 13/14) to perform SRP for teeth with PPD > 3 mm (Figure 1). Thirdly, use an ultrasonic device to perform SRP for these teeth a second time.
- Adjunctive diode laser therapy
NOTE: Choose teeth with PPD ≥ 5 mm for laser treatment. Choose one of the following two usage modes (i.e., steps 1.5.1 or 1.5.2).- Inside pocket laser irradiation (only one session of laser appointment per tooth is suggested) (Figure 2A).
- Ensure that both the operator and patient wear protective goggles to protect the eyes from laser damage.
- Prepare the diode laser device (wavelength = 980 nm, output power = 1 W, power density = 1414.7 W/cm2, continuous wave, 300 µm fiber optic delivery system).
NOTE: Improperly increasing the power may cause thermal damage; please consult the laser manufacturer and follow the manufacturer's instructions before use. - Calibrate the length of the fiber tip exposed to 1 mm less than the measured PPD (Figure 2B).
NOTE: To prevent bleeding due to the insertion of the fiber tip too deep into the periodontal pocket, it is generally recommended that the exposed fiber tip length is 1 mm less than the measured periodontal pocket depth. - Gently insert the fiber tip into the periodontal pocket 1 mm less than the measured PPD, and slowly sweep the tip both in mesial-distal and apical-coronal directions for 30 s per tooth (Figure 2B,C).
NOTE: During this process, inserting the fiber too deeply into the periodontal pocket can irritate periodontal bleeding and thus prevent the laser from acting. Remove the granulation attached to the fiber tip using a cotton ball containing 75% alcohol to avoid jeopardizing the laser effectiveness.
- Outside pocket laser irradiation (a total of 3-5 sessions of laser appointments per tooth is suggested) (Figure 3A).
- Ensure that both the operator and patient wear protective goggles to protect the eyes from laser damage.
- Prepare the diode laser device (wavelength = 980 nm, output power = 0.4 W, power density = 566.2 W/cm2, continuous wave, 300 µm fiber optic delivery system).
- Irradiate the gingival surface of the pocket for approximately 15 s per pocket, with the laser fiber tip 5 mm (2-10 mm) away from the gingival surface and directed at an angle of 90° (Figure 3B,C).
- Repeat the same outside pocket laser treatment after 1, 3, 5, and 7 days.
- Inside pocket laser irradiation (only one session of laser appointment per tooth is suggested) (Figure 2A).
- Give oral hygiene instruction (OHI) to each patient, including Bass brushing technique, interdental floss, and brush19.
- Clinical examination
- Measure the PPD, CAL, and BOP for each tooth 4-6 weeks, 3 months, and 6 months after periodontal treatment. Record the postoperative parameters on a new periodontal chart (Supplementary File 1).
- Compare the baseline and postoperative parameters.
2. Adjunctive probiotic therapy in the nonsurgical treatment of peri-implant mucositis (Figure 4A)
- Eligibility criteria
- Use the following inclusion criteria: age ≥ 18 years; at least one implant with erythema, swelling, suppuration, or BOP in the peri-implant mucosa; radiographic bone loss (RBL) < 2 mm.
- Use the following exclusion criteria: peri-implantitis (RBL ≥ 2 mm); implants with mobility; patients with systematic diseases or under medication that may affect the inflammation and healing process; patients who had received periodontal treatment within 6 months; smokers or alcoholics; pregnant or lactating patients.
- Clinical examination
- Measure the clinical parameters PPD, BOP at six sites per implant (i.e., mesiobuccal, buccal, distobuccal, distolingual, lingual, and mesiolingual), and plaque index (PI) at four sites per implant (i.e., mesial, buccal, distal, and lingual).
- Record the baseline parameters on a periodontal chart (Supplementary File 1).
- For disinfection, gargle with 3% hydrogen peroxide for 1 min, followed by pure water.
- Mechanical debridement by supragingival scaling
- Use a titanium ultrasound tip to perform supragingival scaling for the mucositis implants, adjusting the mode to medium power (Figure 4B). Rinse the pockets with 3% hydrogen peroxide, and then gargle with pure water.
NOTE: Supragingival scaling removes the plaque and calculus from the surface of the implant denture above the gumline.
- Use a titanium ultrasound tip to perform supragingival scaling for the mucositis implants, adjusting the mode to medium power (Figure 4B). Rinse the pockets with 3% hydrogen peroxide, and then gargle with pure water.
- Professional administration of probiotic
- Grind the probiotic tablet into powder using a sterilized mortar (Figure 4C).
NOTE: If there are lumpy particles in the powder, they can easily clog the syringe. - Make a solution of probiotic powder and sterile saline in a 1:3 ratio (Figure 4D). Deliver the probiotic solution into the peri-implant sulcus using a 5 mL syringe with a blunted and soft tip (Figure 4E).
- Grind the probiotic tablet into powder using a sterilized mortar (Figure 4C).
- Home administration of probiotics
- Instruct the patients to dissolve one tablet for approximately 10 min in the oral cavity every 12 h, twice a day for 1 month (Figure 4A).
- Give OHI to each patient, including Bass brushing technique, interdental floss, and brush19.
- Clinical examination
- Measure the PPD, PI, and BOP for each implant 4-6 weeks, 3 months, and 6 months after treatment. Record the postoperative parameters on a new periodontal chart (Supplementary File 1).
- Compare the baseline and postoperative parameters.
Results
Periodontal pockets with PPD ≥ 5 mm require laser irradiation after SRP, as it is difficult to obtain complete debridement by SRP alone (Figure 1A,B). After SRP, if the periodontal pockets bleed profusely and clot on the tooth surface, the operator needs to stop the bleeding and remove the clot by rinsing and gargling several times. This is because a large amount of blood will prevent the laser from working (Figure 1C,D).
The exposed fiber tip is calibrated to be 1 mm less than the measured PPD. Laser parameters were set at 1 W, using continuous wave (Figure 2B). For the recommended laser parameters, one should refer to the manufacturer's guidelines and adjust the parameters appropriately for different clinical scenarios. The fiber tip was 5 mm away from the gingival surface and directed at an angle of 90° (Figure 3B).
The fully ground probiotic powder has no lumpy particles (Figure 4C). Dissolving it in saline (1:3) results in a green solution (Figure 4D).
Compared to pre-operation (Figure 5A,C), diode laser-assisted SRP therapy (inside or outside mode) effectively removed pathogenic plaque biofilm and eliminated inflammation in patients with periodontitis, achieving significant improvements (i.e., with regards to erythema, swelling, PPD, BOP) (Figure 5B,D). Compared to pre-operation (Figure 5E), probiotic therapy resulted in the disappearance of swelling of the peri-implant mucosa, reduced BOP, and an obvious reduction and good control of plaque and pigmentation; however, there was no significant change in PPD (Figure 5F).
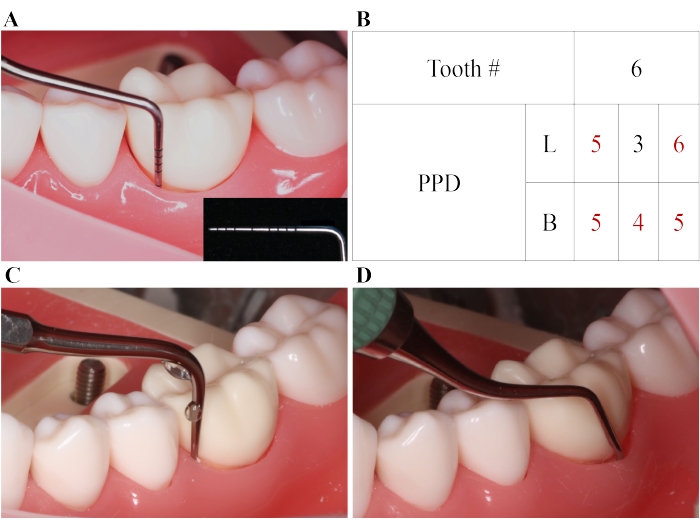
Figure 1: SRP for periodontitis. (A) A periodontal pocket with PPD = 5 mm. (B) PPD numbers of the tested tooth (mm). SRP is performed for the pocket with PPD ≥ 4mm, and an adjunctive diode laser treatment is conducted for the pocket with PPD ≥ 5 mm. (C) SRP with ultrasound instruments. (D) SRP with hand-held instruments. B: buccal; L: lingual; SRP: scaling and root planing. Please click here to view a larger version of this figure.

Figure 2: Adjunctive laser therapy inside the periodontal pocket. (A) Phases of the laser treatment with inside mode. (B) Calibration of fiber tip exposed (4 mm) to measure 1 mm less than PPD (5 mm). The fiber tip with a diameter of 300 µm is inserted into the periodontal pocket (1 mm shorter than the measured PPD). (C) The fiber tip is swept in the pocket in mesial-distal and apical-coronal directions (the green curve indicates the path of the fiber tip). Please click here to view a larger version of this figure.
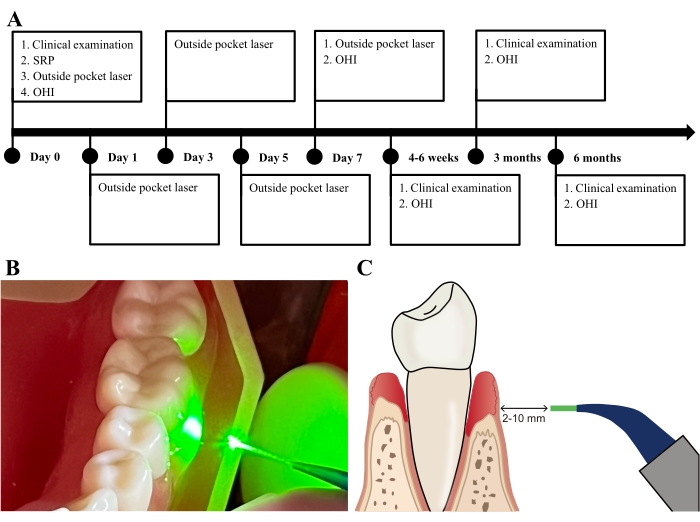
Figure 3: Adjunctive laser therapy outside the periodontal pocket. (A) Phases of the laser treatment with outside mode. (B) The fiber tip irradiates the pocket at a 5 mm distance away from the gingival surface. (C) The distance between the fiber tip and gingival surface ranges from 2-10 mm, and the tip is directed at an angle of 90°. Please click here to view a larger version of this figure.
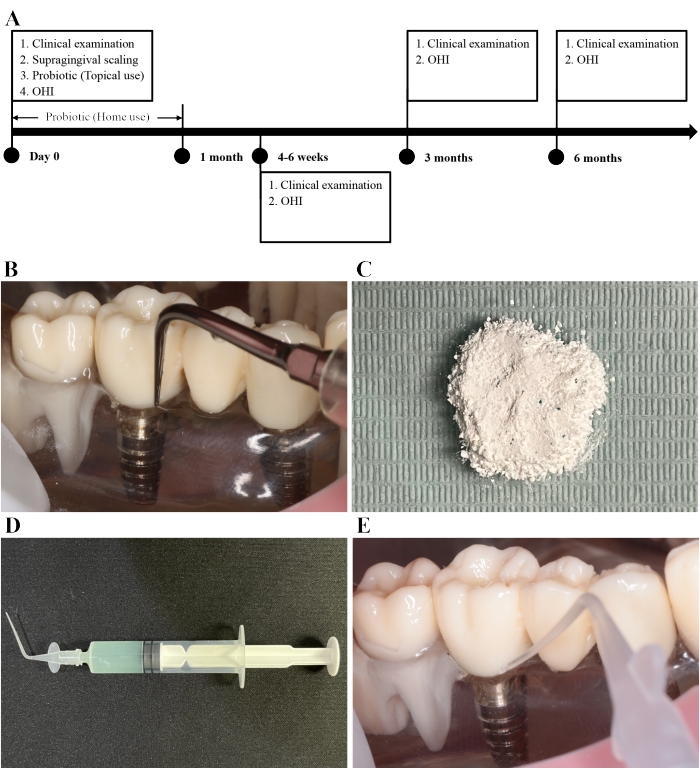
Figure 4: Supragingival scaling and adjunctive probiotic Lactobacillus therapy. (A) Phases of the probiotic Lactobacillus therapy. (B) A titanium ultrasound tip is used to perform supragingival scaling for mucositis implants. (C) The probiotic powder. (D) The probiotic solution with a green color. (E) The probiotic solution is delivered into the peri-implant sulcus with a blunted and soft tip. Please click here to view a larger version of this figure.

Figure 5: Clinical examination before and 1 month after treatment. (A) One month after laser treatment in the inside usage mode, gingival erythema and swelling disappeared, and PPD and BOP were greatly reduced compared to the (B) pre-operation condition. (C) One month after laser treatment in the outside usage mode, gingival erythema and swelling improved, and PPD and BOP reduced as compared with the (D) pre-operation condition. (E) After 1 month of probiotic therapy, peri-implant mucosal swelling disappeared, pigmentation and plaque were reduced and well-controlled, and BOP was reduced as compared with the (F) pre-operation condition; however, there was no significant change in PPD relative to the pre-operation condition (F). Please click here to view a larger version of this figure.
Supplementary File 1: Periodontal chart. B: buccal; BOP: bleeding on probing; CAL: clinical attachment loss; L: lingual; PI: plaque index; PPD: probing pocket depth. Please click here to download this File.
Supplementary File 2: Characteristics of studies referring to adjunctive laser therapy. Please click here to download this File.
Supplementary File 3: Characteristics of studies referring to adjunctive probiotic Lactobacillus therapy. Please click here to download this File.
Discussion
Although diode laser has been widely utilized in periodontal therapy, the clinical effectiveness remains controversial among current clinical trials15,20. As demonstrated, the laser usage mode and application regimen have significant impacts on the efficacy of periodontal laser therapy12. Most researchers, however, ignore the potential role, eliciting results that are hard to explain. Under different usage modes, excessive or insufficient laser action can lead to negative result21. Only with an optimal combination of usage mode and application regimen can the laser maximize its therapeutic benefits12. There seems to be an urgent need to improve the existing protocol for diode laser therapy. Therefore, in this article, two periodontal laser usage modes (inside pocket or outside pocket) are described in view of the photobiomodulation and photothermal effects. Furthermore, based on published randomized controlled trials, the suggested number of laser appointment sessions under the two usage modes was proposed; for inside pocket use, a single session of laser application is suggested whereas, for outside pocket use, multiple (3-5) sessions of laser application provide a better clinical effect.
It is important to note that laser parameters also affect laser efficacy. Common periodontal laser parameters relate to wavelength, energy density, and output power22. Most studies used diode lasers with 808-980 nm wavelength and reported good clinical results23. The range of 600-1,100 nm diode laser was confirmed to penetrate deep into the tissue, acting on the epithelial and connective tissues of the gingiva20. The energy density ranged from 1.6-24.84 J/cm2 (inside mode) and 3-10 J/cm2 (outside mode). It was reported that lasers with a density of 1.5-16 J/cm2 had good anti-inflammatory properties24. The output power was reported to range between 0.5-2.5 W for the inside application, while it was much lower (0.01-0.5 W) for the outside use. Given that the recommended parameters vary in part among different laser manufacturers, improperly increasing the laser power may cause thermal damage to the periodontal tissues during inside-pocket sweeping25,26. It is strongly recommended to consult the laser manufacturer and determine the appropriate parameters before use. In this protocol, a safe power level of 1 W was chosen for the inside mode.
Considering that probiotic Lactobacillus administration gives reliable results in the management of gingivitis and periodontitis, it is expected to exert similar therapeutic benefits in peri-implant disease. Nevertheless, the limited number of studies makes it difficult to evaluate the effectiveness of probiotic Lactobacillus17,27, especially for the advanced form of the disease, peri-implantitis17,28,29. Thus, the probiotic administration protocol was offered only for patients with peri-implant mucositis. Given the obvious clinical diversity in the published studies, it is crucial to establish a rational protocol with reproducible effect. As a result, we systematically reviewed related studies and suggested a refined protocol, including a combination of professional topical use and home administration of probiotic Lactobacillus and enhancing the OHI.
According to the authors' experience, when choosing the inside pocket mode of laser therapy, it is critical to achieve gentle insertion and continuous movement of the fiber tip. Heavy or deep insertion of the fiber will cause periodontal bleeding, which can weaken the laser effect. Hence, the insertion depth is suggested to be 1 mm shorter than the measured PPD. Prolonged action of a particular site in the pocket can cause excessive photothermal effects (over 10 °C), leading to periodontal pain and even permanent damage to the periodontal ligament and bone30. During this session, the operator should check the fiber tip regularly. Once a blood clot is attached to the tip, it needs to be wiped off with a cotton ball moistened with 75% alcohol to avoid impeding the laser action. Regarding the professional use of probiotic therapy, the concentration of the probiotic solution should not be too high; otherwise, it will easily block the injection needle.
As a modified protocol for existing studies, the probiotic therapy in this paper aims to reduce clinical heterogeneity and contribute to the implementation of future clinical studies in this field. The current result showed that probiotic Lactobacillus therapy led to the disappearance of the peri-implant mucosal swelling, reduced BOP, and obvious reduction and excellent control of plaque and pigmentation after a 1-month follow-up. This demonstrated the effectiveness of probiotics in controlling peri-implant mucosal inflammation. However, PPD improvement was limited. As peri-implant mucositis does not cause bone loss, it only presents as mucosal erythema, swelling, or bleeding, and there is usually no deep peri-implant pocket31. The patient, in this case, had only mild swelling of the mucosa prior to treatment, and therefore, the pre- and post-operative changes in PPD were not significant. Moreover, this protocol should be regarded as preliminary and could be further enhanced (e.g., by using chlorhexidine gargle, increasing the frequency of topical probiotic use, improving the active ingredients of probiotic tablets, etc.)32,33.
Some limitations still exist in the protocol of probiotic therapy. In daily clinical practice, patients' oral hygiene behavior is influenced by various factors. Some have difficulty performing adequate oral hygiene management, despite being professionally instructed. Insufficient home oral cleaning undermines the benefits of professional periodontal treatment and even worsens the condition, especially for probiotic therapy. Thus, it is recommended to reinforce OHI at each follow-up visit and ask patients to practice the procedure again. Besides, the probiotic therapy in this article is not introduced to patients with peri-implantitis, due to its more complex pathology and controversial clinical trial results17. Further studies are required to explore a protocol of probiotic therapy for peri-implantitis.
To facilitate access to the extant studies related to diode laser treatment for periodontitis, and probiotic therapy for peri-implant diseases, a summary of these studies14,28,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,
48,49,50,51,52,53,54 is presented in Supplementary File 2 and Supplementary File 3, respectively.
Disclosures
The authors have no conflicts of interest.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (grant numbers 82071078, 81870798, and 82170927).
Materials
| Name | Company | Catalog Number | Comments |
| 1% iodophor | ADF, China | 21031051 | 100 mL |
| 3% hydrogen peroxide | Hebei Jianning, China | 210910 | 500 mL |
| 75% alcohol | Shandong Anjie, China | 2021100227 | 500 mL |
| Diode laser (FOX 980) | A.R.C, Germany | PS01013 | 300-μm fiber tip |
| Gracey curettes | Hu-Friedy, USA | 5/6, 7/8, 11/12, 13/14 | |
| Low-speed handpiece | NSK, Japan | 0BB81855 | |
| Periodontal probe | Shanghai Kangqiao Dental Instruments Factory, China | 44759.00 | |
| Periodontal ultrasonic device (PT3) | Guilin zhuomuniao Medical Instrument, China | P2090028PT3 | |
| Polishing paste | Datsing, China | 21010701 | |
| Primacaine adrenaline | Produits Dentaires Pierre Rolland, France | S-52 | 1.7 mL |
| Probiotic | Biogaia, Sweden | Prodentis | 30 probiotic tablets (24 g) |
| Titanium ultrasound tip (P59) | Guilin Zhuomuniao Medical Instrument, China | 200805 |
References
- Papapanou, P. N., et al. Periodontitis: consensus report of workgroup 2 of the 2017 world workshop on the classification of periodontal and peri-implant diseases and conditions. Journal of Clinical Periodontology. 45, 162-170 (2018).
- Peres, M. A., et al. Oral diseases: a global public health challenge. Lancet. 394 (10194), 249-260 (2019).
- Nazir, M. A. Prevalence of periodontal disease, its association with systemic diseases and prevention. International Journal of Health Sciences (Qassim). 11 (2), 72-80 (2017).
- Khoury-Ribas, L., Ayuso-Montero, R., Willaert, E., Peraire, M., Martinez-Gomis, J. Do implant-supported fixed partial prostheses improve masticatory performance in patients with unilateral posterior missing teeth. Clinical Oral Implants Research. 30 (5), 420-428 (2019).
- Bohner, L., Hanisch, M., Kleinheinz, J., Jung, S. Dental implants in growing patients: a systematic review. The British Journal of Oral & Maxillofacial Surgery. 57 (5), 397-406 (2019).
- Jemt, T. Implant survival in the edentulous jaw: 30 years of experience. Part ii: a retro-prospective multivariate regression analysis related to treated arch and implant surface roughness. The International Journal of Prosthodontics. 31 (6), 531-539 (2018).
- Muñoz, V., Duque, A., Giraldo, A., Manrique, R. Prevalence of peri-implant disease according to periodontal probing depth and bleeding on probing: a systematic review and meta-analysis. The International Journal of Oral & Maxillofacial Implants. 33 (4), 89-105 (2018).
- Larsson, L., et al. Regenerative medicine for periodontal and peri-implant diseases. Journal of Dental Research. 95 (3), 255-266 (2016).
- Salvi, G. E., Cosgarea, R., Sculean, A. Prevalence and mechanisms of peri-implant diseases. Journal of Dental Research. 96 (1), 31-37 (2017).
- Asa'ad, F., Garaicoa-Pazmiño, C., Dahlin, C., Larsson, L. Expression of micrornas in periodontal and peri-implant diseases: a systematic review and meta-analysis. International Journal of Molecular Sciences. 21 (11), 4147 (2020).
- Sculean, A., et al. Effectiveness of photodynamic therapy in the treatment of periodontal and peri-implant diseases. Monographs in Oral Science. 29, 133-143 (2021).
- Yu, S., et al. Clinical effectiveness of adjunctive diode laser on scaling and root planing in the treatment of periodontitis: is there an optimal combination of usage mode and application regimen? A systematic review and meta-analysis. Lasers in Medical Science. 37 (2), 759-769 (2022).
- Cobb, C. M., Low, S. B., Coluzzi, D. J. Lasers and the treatment of chronic periodontitis. Dental Clinics of North America. 54 (1), 35-53 (2010).
- Mongardini, C., Pilloni, A., Farina, R., Di Tanna, G., Zeza, B. Adjunctive efficacy of probiotics in the treatment of experimental peri-implant mucositis with mechanical and photodynamic therapy: a randomized, cross-over clinical trial. Journal of Clinical Periodontology. 44 (4), 410-417 (2017).
- Cobb, C. M. Lasers and the treatment of periodontitis: the essence and the noise. Periodontology 2000. 75 (1), 205-295 (2017).
- Slot, D. E., Jorritsma, K. H., Cobb, C. M., Vander Weijden, F. A. The effect of the thermal diode laser (wavelength 808-980 nm) in non-surgical periodontal therapy: a systematic review and meta-analysis. Journal of Clinical Periodontology. 41 (7), 681-692 (2014).
- Gao, J., et al. Does probiotic lactobacillus have an adjunctive effect in the nonsurgical treatment of peri-implant diseases? A systematic review and meta-analysis. Journal of Evidence Based Dental Practice. 20 (1), 101398 (2020).
- Staab, B., Eick, S., Knöfler, G., Jentsch, H. The influence of a probiotic milk drink on the development of gingivitis: a pilot study. Journal of Clinical Periodontology. 36 (10), 850-856 (2009).
- . Oral hygiene instruction online Available from: https://www.oralhygiene-instruction.com/en/ (2022)
- Zhao, P., et al. Effect of adjunctive diode laser in the non-surgical periodontal treatment in patients with diabetes mellitus: a systematic review and meta-analysis. Lasers in Medical Science. 36 (5), 939-950 (2021).
- Huang, Y. Y., Sharma, S. K., Carroll, J., Hamblin, M. R. Biphasic dose response in low level light therapy-an update. Dose-Response. 9 (4), 602-618 (2011).
- Passanezi, E., Damante, C. A., de Rezende, M. L., Greghi, S. L. Lasers in periodontal therapy. Periodontology 2000. 67 (1), 268-291 (2015).
- Qadri, T., Javed, F., Johannsen, G., Gustafsson, A. Role of diode lasers (800-980 nm) as adjuncts to scaling and root planing in the treatment of chronic periodontitis: a systematic review. Photomedicine and Laser Surgery. 33 (11), 568-575 (2015).
- Ren, C., McGrath, C., Jin, L., Zhang, C., Yang, Y. Effect of diode low-level lasers on fibroblasts derived from human periodontal tissue: a systematic review of in vitro studies. Lasers in Medical Science. 31 (7), 1493-1510 (2016).
- Angiero, F., Parma, L., Crippa, R., Benedicenti, S. Diode laser (808 nm) applied to oral soft tissue lesions: a retrospective study to assess histopathological diagnosis and evaluate physical damage. Lasers in Medical Science. 27 (2), 383-388 (2012).
- Gutiérrez-Corrales, A., et al. Comparison of diode laser - Oral tissue interaction to different wavelengths. In vitro study of porcine periodontal pockets and oral mucosa. Medicina Oral, Patología Oral y Cirugía Bucal. 25 (2), 224-232 (2020).
- Zhao, R., Hu, H., Wang, Y., Lai, W., Jian, F. Efficacy of probiotics as adjunctive therapy to nonsurgical treatment of peri-implant mucositis: a systematic review and meta-analysis. Frontiers in Pharmacology. 11, 541752 (2020).
- Galofré, M., Palao, D., Vicario, M., Nart, J., Violant, D. Clinical and microbiological evaluation of the effect of Lactobacillus reuteri in the treatment of mucositis and peri-implantitis: A triple-blind randomized clinical trial. Journal of Periodontal Research. 53 (3), 378-390 (2018).
- Tada, H., et al. The effects of Lactobacillus reuteri probiotics combined with azithromycin on peri-implantitis: A randomized placebo-controlled study. Journal of Prosthodontic Research. 62 (1), 89-96 (2018).
- Kwon, S. J., et al. Thermal irritation of teeth during dental treatment procedures. Restorative Dentistry and Endodontics. 38 (3), 105-112 (2013).
- Berglundh, T., et al. Peri-implant diseases and conditions: consensus report of workgroup 4 of the 2017 world workshop on the classification of periodontal and peri-implant diseases and conditions. Journal of Clinical Periodontology. 45, 286-291 (2018).
- Hallström, H., Lindgren, S., Widén, C., Renvert, S., Twetman, S. Probiotic supplements and debridement of peri-implant mucositis: a randomized controlled trial. Acta Odontologica Scandinavica. 74 (1), 60-66 (2016).
- Peña, M., et al. Evaluation of the effect of probiotics in the treatment of peri-implant mucositis: a triple-blind randomized clinical trial. Clinical Oral Investigations. 23 (4), 1673-1683 (2019).
- Alzoman, H. A., Diab, H. M. Effect of gallium aluminium arsenide diode laser therapy on Porphyromonas gingivalis in chronic periodontitis: a randomized controlled trial. International Journal of Dental Hygiene. 14 (4), 261-266 (2016).
- Angiero, F., et al. Evaluation of bradykinin, VEGF, and EGF biomarkers in gingival crevicular fluid and comparison of photobiomodulation with conventional techniques in periodontitis: a split-mouth randomized clinical trial. Lasers in Medical Science. 35 (4), 965-970 (2019).
- Balasubramaniam, A. S., Thomas, L. J., Ramakrishnanan, T., Ambalavanan, N. Short-term effects of nonsurgical periodontal treatment with and without use of diode laser (980 nm) on serum levels of reactive oxygen metabolites and clinical periodontal parameters in patients with chronic periodontitis: a randomized controlled trial. Quintessence International. 45 (3), 193-201 (2014).
- De Micheli, G., et al. Efficacy of high intensity diode laser as an adjunct to non-surgical periodontal treatment: a randomized controlled trial. Lasers in Medical Science. 26 (1), 43-48 (2011).
- Dukić, W., Bago, I., Aurer, A., Roguljić, M. Clinical effectiveness of diode laser therapy as an adjunct to non-surgical periodontal treatment: A randomized clinical study. Journal of Periodontology. 84 (8), 1111-1117 (2013).
- Euzebio Alves, V. T., et al. Clinical and microbiological evaluation of high intensity diode laser adjutant to non-surgical periodontal treatment: A 6-month clinical trial. Clinical Oral Investigations. 17 (1), 87-95 (2013).
- Gündoğar, H., Şenyurt, S. Z., Erciyas, K., Yalım, M., Üstün, K. The effect of low-level laser therapy on non-surgical periodontal treatment: a randomized controlled, single-blind, split-mouth clinical trial. Lasers in Medical Science. 31 (9), 1767-1773 (2016).
- Jose, K. A., et al. Management of chronic periodontitis using chlorhexidine chip and diode laser-a clinical study. Journal of Clinical and Diagnostic Research. 10 (4), (2016).
- Lin, J., Bi, L., Song, Y., Ma, W., Wang, N. Gingival curettage with diode laser: clinical study. Zhong Guo Ji Guang Yi Xue Za Zhi/Chinese Journal of Laser Medicine & Surgery (in Chinese. 18 (06), 353-357 (2009).
- Makhlouf, M., Dahaba, M. M., Tuner, J., Eissa, S. A., Harhash, T. A. Effect of adjunctive low level laser therapy (LLLT) on nonsurgical treatment of chronic periodontitis. Photomedicine and Laser Surgery. 30 (3), 160-166 (2012).
- Manjunath, S., Singla, D., Singh, R. Clinical and microbiological evaluation of the synergistic effects of diode laser with nonsurgical periodontal therapy: A randomized clinical trial. Journal of Indian Society of Periodontology. 24 (2), 145-149 (2020).
- Matarese, G., Ramaglia, L., Cicciù, M., Cordasco, G., Isola, G. The effects of diode laser therapy as an adjunct to scaling and root planing in the treatment of aggressive periodontitis: a 1-year randomized controlled clinical trial. Photomedicine and Laser Surgery. 35 (12), 702-709 (2017).
- Pamuk, F., et al. The effect of low-level laser therapy as an adjunct to non-surgical periodontal treatment on gingival crevicular fluid levels of transforming growth factor-beta 1, tissue plasminogen activator and plasminogen activator inhibitor 1 in smoking and non-smoking chronic periodontitis patients: a split-mouth, randomized control study. Journal of Periodontal Research. 52 (5), 872-882 (2017).
- Pejcic, A., Mirkovic, D. Anti-inflammatory effect of low level laser treatment on chronic periodontitis. Medical Laser Application. 26 (1), 27-34 (2011).
- Saglam, M., Kantarci, A., Dundar, N., Hakki, S. S. Clinical and biochemical effects of diode laser as an adjunct to nonsurgical treatment of chronic periodontitis: a randomized, controlled clinical trial. Lasers in Medical Science. 29 (1), 37-46 (2014).
- Shi, Z., Jiang, C., Xu, Y., Sun, Y. Effects of diode laser on the treatment for moderate to severe chronic periodontitis. Kou Qiang Yi Xue/Stomatology. 34 (4), 245-248 (2014).
- Üstün, K., et al. Clinical and biochemical effects of 810 nm diode laser as an adjunct to periodontal therapy: a randomized split-mouth clinical trial). Photomedicine and Laser Surgery. 32 (2), 61-66 (2014).
- Zhang, L., Shi, J., Guo, J., Zhang, N. Clinical evaluation of diode laser assisted treatment of chronic periodontitis. Shi Yong Kou Qiang Yi Xue Za Zhi/Journal of Practical Stomatology. 34 (3), 404-406 (2018).
- Alqahtani, F., et al. Efficacy of mechanical debridement with adjunctive probiotic therapy in the treatment of peri-implant mucositis in cigarette-smokers and never-smokers. Clinical Implant Dentistry and Related Research. 21 (4), 734-740 (2019).
- Flichy-Fernández, A. J., et al. The effect of orally administered probiotic Lactobacillus reuteri-containing tablets in peri-implant mucositis: a double-blind randomized controlled trial. Journal of Periodontal Research. 50 (6), 775-785 (2015).
- Calderín, S., García-Núñez, J. A., Gómez, C. Short-term clinical and osteoimmunological effects of scaling and root planing complemented by simple or repeated laser phototherapy in chronic periodontitis. Lasers in Medical Science. 28 (1), 157-166 (2013).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionExplore More Articles
This article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved