Method Article
Isolation of microRNAs from Tick Ex Vivo Salivary Gland Cultures and Extracellular Vesicles
In This Article
Summary
The present protocol describes the isolation of microRNAs from tick salivary glands and purified extracellular vesicles. This is a universal procedure that combines commonly used reagents and supplies. The method also allows the use of a small number of ticks, resulting in quality microRNAs that can be readily sequenced.
Abstract
Ticks are important ectoparasites that can vector multiple pathogens. The salivary glands of ticks are essential for feeding as their saliva contains many effectors with pharmaceutical properties that can diminish host immune responses and enhance pathogen transmission. One group of such effectors are microRNAs (miRNAs). miRNAs are short non-coding sequences that regulate host gene expression at the tick-host interface and within the organs of the tick. These small RNAs are transported in the tick saliva via extracellular vesicles (EVs), which serve inter-and intracellular communication. Vesicles containing miRNAs have been identified in the saliva of ticks. However, little is known about the roles and profiles of the miRNAs in tick salivary vesicles and glands. Furthermore, the study of vesicles and miRNAs in tick saliva requires tedious procedures to collect tick saliva. This protocol aims to develop and validate a method for isolating miRNAs from purified extracellular vesicles produced by ex vivo organ cultures. The materials and methodology needed to extract miRNAs from extracellular vesicles and tick salivary glands are described herein.
Introduction
Ticks are ectoparasites that vector many pathogens to wildlife, livestock, humans, and their pets1,2. Tick feeding results in significant economic loss by causing damage to hide, reducing weight and milk production due to severe anemia, and the transmission of potentially deadly disease-causing pathogens1,3,4,5. Current control practices for managing tick populations are focused on the use of acaricides. Nevertheless, the continuous emergence of acaricide resistance in ticks parasitizing livestock5,6, the increased incidence of tick bites7, and pathogen transmission within residential areas8,9, have led to a need for unique tick control alternatives.
The tick salivary glands are essential organs that ensure a tick's biological success. They are formed by different acinus types (I, II, III, and IV) with various physiological functions. The salivary glands are responsible for osmoregulation, both off and on the host, by returning water excess and iron content to the host via salivation2,10. Type I acini are also involved in the uptake of water from the atmosphere by the secretion of hygroscopic saliva10,11. Salivary effector proteins, such as cement and cystatins, are produced within secretory cells in type II and III acini10,12. Type I acini do not affect tick feeding, indicating that the bloodmeal intake does not trigger morphological and physiological changes in these acini type13,14. On the other hand, Acini type II and III are activated during feeding and present very little activity pre-attachment. Thus, feeding is necessary to trigger the enlargement of the secretory cells within type II acini and the production of bioactive compounds. Type III acini are reduced in size during feeding due to the secretion within the secretory granules12.
The salivary glands are also the site of pathogen infection in the tick and route of transmission. During feeding, ticks secrete several compounds with pharmaceutical effects that are needed for successful completion of the bloodmeal10,15,16. These compounds have anti-inflammatory, immunosuppressive, and vasodilatory properties10,15,17. Recent studies have shown that extracellular vesicles (EVs) derived from tick salivary glands harbor several of these compounds, inducing anti-inflammatory and immuno-modulatory effects18,19,20. "Extracellular vesicles" is an umbrella term used to describe vesicles classified as exosomes and microvesicles based on their size and biogenesis. Overall, EVs are lipid blebs with bilayer membranes that are ~40 nm-1 µm in size21; generally, exosomes are described as being 40-150 nm in size, whereas microvesicles are between 150 nm-1 µm in size21,22,23. However, the size is not indicative of the EVs biogenesis pathway22.
The biogenesis of exosomes starts with the sequential invagination of the plasma membrane. This invagination leads to the formation of multivesicular bodies and finally results in the deformation of the vesicular membrane by the action of ESCRT complexes or sphingomyelinases (sMases)24,25. The exosomes can either be lysed within the lysosomes to maintain cellular homeostasis or exit via vesicular fusion to the plasma membrane to deliver cellular constituents to the recipient cells21,24. On the other hand, microvesicles are formed by the action of flopasses and flipasses, changing the conformation of lipids in the plasma membrane26. EVs are essential for cell-to-cell communication, serving as a transport system for intracellular cargo, such as lipids, proteins, nucleic acids, and microRNAs (miRNAs)21,27,28. Once transported, these vesicles deliver their cargo into the cytoplasm of the recipient cells, generating phenotypic changes in the receiving cell22,29. Due to the importance of extracellular vesicles in tick feeding and the manipulation of host immune and wound healing responses18,20, the cargo within extracellular vesicles presents potential targets for the development of anti-tick therapeutics and a unique mechanism to disrupt tick feeding. This includes miRNAs within tick salivary glands and salivary gland-derived extracellular vesicles.
miRNAs are short non-coding sequences, ~18-22 nucleotide (nt) in length, that can post-transcriptionally regulate, degrade, or silence mRNA sequences30,31. During transcription, the pri-miRNAs are cleaved by Dicer (RNA polymerase III) to form a distinctive hairpin-like structure, becoming a pre-miRNA. The pre-miRNA is cut once again by Drosha (RNA polymerase III) to form a mature miRNA duplex. The mature sequence becomes integrated into the RNA-induced silencing complex (RISC) complementary to the mRNA sequence, causing translation repression or mRNA degradation28,30,32. During host feeding, miRNAs within the tick saliva can modulate host gene expression to suppress immune responses and enhance pathogen transmission33,34,35,36,37. Although extensive studies on EVs and miRNAs exist, their roles during feeding at the tick-host interface are still poorly understood. Optimizing protocols that can easily result in the isolation and purification of high-quality miRNAs is crucial for advancing our knowledge on these topics.
Multiple options can be utilized to isolate EVs, such as ultracentrifugation, exosome precipitation, polymer precipitation, immunoaffinity chromatography, and size-based exclusion techniques38. However, these techniques cannot distinguish between exosomes or microvesicles. Thus, as mentioned previously, EV is used as an umbrella term when isolating EVs from different samples. The vesicles isolated in the experiments described herein represent a mixture of vesicles derived from different biogenesis pathways. Further purification of a specific population of extracellular vesicles can be achieved by immunoprecipitation using beads coated with antibodies against markers (i.e., exosomal markers, tumor markers) unique to the vesicle population of interest39,40. miRNAs can also be extracted via different commercially available isolation kits7,41,42.
The objective of this project was to develop a protocol that combines commonly applied methods to isolate EVs and extract miRNA from both EVs and fed-tick salivary glands. Because the secretion of bioactive compounds is activated by feeding12, ticks should be allowed to feed to identify miRNAs that may be important for manipulating host immune and wound healing responses. The present protocol requires a small number of ticks (20 ticks) to isolate EVs and their respective miRNAs, compared to other previously described studies that required 2000 ticks43. Further, it avoids the contamination of salivary secretions with pilocarpine44, which could influence experiments studying the effect of EVs and their miRNAs on host cells.
Protocol
All animal experiments were performed following animal usage protocol (AUP#2020-0026) approved by the institutional animal care and use committee (AICUC) at Texas A&M University. The tick species, Ixodes scapularis and Rhipicephalus (Boophilus) microplus, and New Zealand Male White Rabbits, 42-72 days of age, were used for the present study. I. scapularis was received from the Center for Disease Control (CDC) and Oklahoma State University, certified as pathogen-free. R. microplus was reared at the Cattle Fever Tick Research Laboratory in Edinburg, Tx. The rabbits were obtained from commercial sources (see Table of Materials). This protocol can universally isolate extracellular vesicles and miRNAs from different tick species, life stages, and tissues.
1. Rearing of female I. scapularis and capsule preparation
- Prepare an ethylene-vinyl acetate foam capsule following the procedures for hard ticks-feeding rabbits45. Place one capsule on each shoulder blade of the rabbit for a total of two capsules per infestation.
NOTE: Briefly, these capsules consist of two squares of ethylene-vinyl acetate foam with an inside empty space. One square is glued to the back of the animal (in this case, rabbits) with a tissue adhesive (see Table of Materials). Fine mesh is glued to the second square to avoid the escape of ticks. The two squares are closed using self-adhesive hook tape. - Allow the capsules to set for 24 h before adhering the capsule on the rabbits. Store the foam capsules at room temperature.
NOTE: The capsules can be stored indefinitely at room temperature. - Adhere the capsule to shaved-to-skin rabbits and leave for 24 h before tick infestation.
2. Preparation of vesicle-free media
- To prepare vesicle-free media13, combine 0.5 mL of fetal bovine serum, 0.5 mL of tryptose phosphate broth, 0.1 mL of 10% lipoprotein-cholesterol concentrate, 0.5 mL of 5% sodium bicarbonate (NaHCO3), 0.25 mL of 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES), and 8.125 mL of L15C300 medium (see Table of Materials). Adjust the volume of the medium as needed. Place the medium into a 26.3 mL polycarbonate centrifuge bottle.
- Balance the bottles by a weight difference of 0.01 g maximum to ensure the proper functioning of the ultracentrifuge.
- Ultracentrifuge the medium for 18 h at 100,000 x g at 4 °C. Remove the supernatant via pipette, carefully ensuring not to disturb any pellet formed.
- Transfer the supernatant to a new centrifuge tube and ultracentrifuge a second time for 18 h at 100,000 x g at 4 °C, with proper balance.
- Isolate the remaining supernatant and pass through a 0.22 µm syringe filter to remove contaminants. Pipette the supernatant into a 50 mL centrifuge tube.
- Store the supernatant in a -20 °C freezer until needed or up to 3 years.
3. Rabbit infestation
- Cut the top off a 10 mL syringe and load adult I. scapularis females using a standard paintbrush.
- Cover the syringe opening using sterile gauze (Figure 1).
- Place the rabbit on a table-top or a working surface horizontally; wrap the rabbit tightly with a towel and cover both eyes, leaving the prepared capsule (step 1.1) exposed to infest the rabbit with ticks.
- Open the capsule and insert ticks by pushing the syringe handle. Deposit the ticks close to the rabbit skin. Quickly close capsules to prevent the ticks from escaping.
- According to the experimental design, allow the female ticks to feed for as long as needed. Do not allow male Ixodes scapularis to remain in the capsule for more than 24 h to avoid desiccation.
NOTE: The number of ticks to be infested into the animals is at the investigator's discretion. This protocol used ~100 ticks per infestation to account for mortality and enough material for replicates. The preliminary experiments determined that at least 20 ticks/replicate were needed to obtain enough material for sequencing the miRNAs.
4. Removal of the fed females
- Place the infested rabbits under 2% isoflurane in oxygen using a gas diffuser. Set the oxygen rate between 700-1000 L/min.
NOTE: Other anesthetics can be used to avoid any distress or pain. - Remove the fed females by gripping the ticks by their capitulum, using fine forceps, as close to the skin as possible. Ensure that the mouthparts are completely removed to avoid bacterial infection.
- Place the ticks in a 15 mL centrifuge tube.
- Clean the bite site with 70% ethanol and add a small amount of triple antibiotic (fingertip worth, see Table of Materials) to prevent infection at the tick bite site.
- Carry the ticks to the laboratory for dissections (step 5). Perform these dissections within 24-48 h of removing the ticks to avoid degradation of the salivary glands15,43.
5. Salivary gland dissection and extracellular vesicle secretion
- Add 500 µL of vesicle-free medium (step 2) with 5 µL of 100x penicillin, 5 µL of 100x streptomycin, 5 µL of 10 mg/mL of rifampicin, and 5 µL of 100x amphotericin to each well (see Table of Materials). Add 1x PBS with rifampicin to any wells that do not contain vesicle-free medium to prevent bacterial growth.
- Place the ticks on a clear microscopic glass slide with double-sided carpet tape under a dissecting microscope. To prevent organ desiccation, add 10 µL of 1x PBS to each tick prior to dissection.
- Using 4 mm vannas scissors (see Table of Materials), make a small incision of ~1 mm on the side of each female. Completely remove the dorsal side of the tick (Figure 2) and remove both salivary glands.
- Put 20-40 tick salivary glands in 500 µL of vesicle-free medium added to a single well in a 24-well tissue culture-treated plate (Figure 3).
- Incubate the salivary gland samples at 32 °C for 24 h to allow the secretion of the EVs.
6. Isolation of extracellular vesicles
- After 24 h incubation, pipette all the medium containing the salivary glands into a 1.5 mL microcentrifuge tube.
- Centrifuge the sample at 300 x g at 4 °C for 10 min to isolate the salivary glands. Two phases will be separated in this step: (1) the supernatant containing the EVs and (2) the salivary glands (pellet).
- To continue the isolation of the EVs, pipette the supernatant into a new 1.5 mL microcentrifuge tube. Resuspend the pellet containing the salivary glands (step 6.2) in 500 µL of RNAlater (see Table of Materials) and store at -80 °C until used or indefinitely.
NOTE: These salivary glands will be used for miRNA isolation in step 8. - Centrifuge the supernatant at 2000 x g at 4 °C for 10 min to remove cellular debris. Pipette the supernatant into a new 1.5 mL microcentrifuge tube. Discard the pellet.
- Centrifuge the supernatant at 10,000 x g for 30 min at 4 °C to remove apoptotic bodies and larger EVs. Toss the pellet, and pipette the supernatant into a new 1.5 mL microcentrifuge tube.
- Attach a 10 mL syringe to a 1 µm nylon syringe filter. Place the syringe and filter above an ultracentrifuge tube (Figure 4A). Then add the sample (Figure 4B) and fill the syringe with 10 mL of 1x PBS (Figure 4C), and balance the tube accordingly (Figure 4D).
- Place tubes into a 70Ti rotor (see Table of Materials) and spin at 100,000 x g for 18 h at 4 °C.
- After 18 h of ultracentrifugation, observe a pellet at the lower end of the tube (Figure 5). The pellet is the concentrated extracellular vesicles.
- Remove 90%-100% of the supernatant without resuspending the EV pellet. Resuspend the pellet with 1x PBS. If not all the supernatant could be removed, use the remaining supernatant to resuspend the pellet.
- Pipette 500 µL of the EV pellet/supernatant into a 300 K centrifugal filter (see Table of Materials).
NOTE: This will remove any non-vesicle-associated proteins, miRNAs, and other molecules, while the vesicles will not pass through the filter. - Centrifuge at 8,000 x g for 10 min at ambient temperature. Repeat step 6.10 until all EV pellets/supernatant have passed through the filter.
- Add 400 µL of sterilized 1x PBS to the column and mix thoroughly via pipette to remove EVs attached to the membrane. Put the sample into a 1.5 mL DNase-/RNase-free tube. The samples can be stored at -80 °C indefinitely or until used. Avoid excessive sample thawing to prevent sample degradation.
NOTE: Place tubes on ice when taken out of ultracentrifuge to prevent EV degradation. The final sample will be the EV pellet in 400 µL of 1x PBS. 25 µL of this sample will be used for nanoparticle tracking analysis (NTA, step 7), and 375 µL for miRNA isolation (step 8).
7. Nanoparticle tracking analysis (NTA)
- Take 25 µL of the final sample (step 6.12) and add 375 µL of 1x PBS.
- Load the diluted sample into a 1 mL needle-less syringe and screw tightly onto the optical lens of the NTA.
- Adjust the settings accordingly and capture videos based on setting preferences.
NOTE: In the present study, three readings were taken of 60 s each. Every NTA reading represents a technical replicate. Different samples from tick feeding for the same length of time represent individual biological replicates. The camera was set at level 7 and the detection threshold was set at level 5. - Estimate the final EV numbers by the following formula:
((starting EVs concentration (dilution factor)) * (total volume left in sample tube)/1000) = total EVs in the sample
NOTE: The volume must be divided by 1000 because the NTA reads the samples as concentration/mL.
8. miRNA extraction from salivary glands and extracellular vesicles
- Add 100 µL of the lysis reagent (see Table of Materials) to the remaining sample from step 6.12 and homogenize with sterilized pestles (Figure 6).
- Add the remaining 600 µL of the lysis reagent and incubate for 5 min at room temperature.
- Add 140 µL of chloroform, shake vigorously for 15 s, and incubate for 3 min at room temperature.
- Spin at 12,000 x g for 15 min at 4 °C.
- Pipette the clear top phase, avoiding interphase, into a new 1.5 mL tube. This will result in ~525 µL of expected sample volume. Next, add a 1:1 volume of 100% molecular grade ethanol.
- Add 700 µL of the sample into an RNA isolation spin column (see Table of Materials).
- Spin at 8,000 x g for 30 s at ambient temperature, discard flow-through.
- Wash the sample with 700 µL of RTE buffer (see Table of Materials), spin at 8,000 x g for 30 s, discard flow-through.
- Wash the sample with 500 µL of RPE buffer (see Table of Materials), spin at 8,000 x g for 30 s, discard flow-through.
- Add 500 µL of 80% molecular grade ethanol and spin at 8,000 x g for 2 min, discard flow-through.
- Spin the column at the maximum velocity for 5 min to dry the membrane. Discard the collection tube and place the column onto a new 1.5 mL microfuge tube.
- Add 14 µL of RNase-/DNase-free water to the membrane and incubate for 5 min at room temperature.
- Centrifuge at 21,000 x g for 1 min at room temperature.
- Add 1 µL of RNase inhibitor (see Table of Materials) to the eluted miRNAs and mix well via pipette.
NOTE: Before miRNA extraction from salivary glands, remove any RNAlater by adding 1 ml of 1x PBS (1:1 volume) and spin down the salivary glands at maximum velocity for 15 min at 4 °C. This needs to be repeated three times or until the salivary glands have subsided enough to remove the supernatant. At this point, the sample represents a mix of small RNAs of ~20-150 bp in size (Supplementary Figure 1).
9. Measuring miRNA concentration
- Measure the concentration of small RNA via a commercially available RNA assay kit41 (see Table of Materials).
- In each tube, mix 199 µL of dilution buffer (Component A and B provided in the kit per sample), 1 µL of fluorescent dye specific to the detection of miRNAs (measurement wavelength 260 nm) (per sample), and 2 µL of small RNA (step 8) for each sample, according to the manufacturer's instructions.
- Before reading the sample tubes, read pre-diluted miRNA standard 1 and standard 2 (provided in the kit) to create a standard curve prior to sample measurement.
NOTE: The standards are used as a comparison tool to determine the measured miRNA concentration in the samples. - Place each standard and sample tube in the dedicated fluorometer (see Table of Materials) to measure the concentration as ng/µL.
10. Determining the miRNA quality
- Determine the quality of miRNA and other small RNAs via gel electrophoresis using a bioanalyzer46, following the manufacturer's instructions (see Table of Materials).
- Prior to sample reading, normalize the samples to be the same concentration.
- Read the samples through a small RNA chip41,46, following the manufacturer's instructions (see Table of Materials).
NOTE: To determine the miRNA quality, the gel-like images (bands) and electropherograms (peaks) are indicators of the sample quality. The darker the band in the gel, the better is the miRNA quality. The lighter the band (or if no band is present), the poorer the quality or proves sample degradation46,47.
11. microRNA enrichment
- Enrich the miRNA using a small RNA sequencing kit, following the manufacturer's instructions of the small RNA sample preparation kit (see Table of Materials).
- Ligate each sample with a 3' adenylated adapter and remove the excess adapter via bead cleanup. Next, add a 5' adapter and remove the excess adapter by a bead cleanup48.
NOTE: Both adapters and beads for cleanup were provided in the small RNA sequencing kit from section 11.1 (see Table of Materials). - To synthesize the first strand, prepare a master mix of the samples ligated with both adapters, RT buffer, and M-MuLV Reverse transcriptase provided in the kit (see Table of Materials). Next, incubate the mix for 30 min at 42 °C and 10 min at 90 °C. Follow-up with a sample cleanup as stated in the instructions.
- Amplify the 1st strand using the RT forward primer and reverse universal primer provided in the kit (see Table of Materials) via a conventional polymerase chain reaction (PCR) for 2 min at 95 °C, then for 12-25 cycles for 20 s at 95 °C, 30 s at 60 °C, and 15 s at 72 °C. Lastly, 2 min at 72 °C.
NOTE: The PCR product size is expected to be ~150 bp (Figure 7). - Measure the quality of the PCR product via tape station following the manufacturer's instructions (see Table of Materials).
- Sequence the samples using a next-generation sequencing system (75 cycles), following the manufacturer's instructions (see Table of Materials).
NOTE: For miRNA enrichment, sample quantity and quality must be checked (steps 9-10) before the library preparation.
12. Bioinformatic analysis
- Identify the conserved and unique miRNAs using an integrated application tool for miRNA identification.
NOTE: In the present study, miRNA identification was performed via miRDeep249,50. miRDeep2 is a free online catalog of all identified conserved and novel miRNAs found in various species49,50 (see Table of Materials). - Align the reads to the corresponding genome using the mapper integrated with the software.
- Input the following parameters into the mapper module.
- Trim the adapter sequences added from step 11.2 and remove sequences less than 18 nucleotides long. Remove the redundant read sequences.
- Convert the files using the parse to FASTA format parameter, only if the file is not in FASTA format49.
- Align the filtered and trimmed sequences to the corresponding genome. If there is no genome for the species of interest, align to the closest homologous species.
- Show the output files as "reads.fa" and "reads_vs_genome.arf". The "read.fa" will contain only the non-redundant reads, and the "reads_vs_genome.arf" will include the mapped reads aligned to the genome.
- Using both output files from step 12.2, perform the miRNA expression profiling using the quantifier integrated with the software.
- Download the species of interest miRNA precursor sequences and the mature miRNA sequences from miRBase51,52,53,54,55,56. If there are no precursor or mature sequences for the species of interest, download the "hairpin.fa.gz" and "mature.fa.gz" that contain all the precursors and mature sequences for all the organisms available on miRBase.
- Uncompress the "hairpin.fa.gz" and "mature.fa.gz" files.
- Input the "reads.fa" and "read_vs_genome.arf" files from section 12.3.4 and the uncompressed files from 12.4.2.
- Read the output files as "outputname.csv","outputname.html", and "outputname.pdf". The output file name can be set according to the investigator.
NOTE: The "outputname.csv" file will contain the expression profiles. Specifically, the expression profile will have the miRNA ID, the sum of reads for all samples mapped to the miRNA, the number of reads mapped to a specific miRNA for each sample, and the precursor ID corresponding to the mature miRNAs. The "outputname.html" from step 12.4.4 will contain the links to the University of Santa Cruz Genome Browser (USCS), National Center for Biotechnology Information (NCBI), and miRBase. The USCS and NCBI will contain the query sequences of the current precursors against the non-redundant nucleotide database, and miRBase will have the information of the current miRNA precursor. The "outputname.pdf" will have the RNA secondary structure of the miRNAs that are expressed and the alignment of the precursor sequence.
- To identify unique and conserved miRNAs, use miRDeep2.
- Use the output files from step 12.3.4 and step 12.4.2 as the input files.
NOTE: The output files that read as "outputname.html" will contain the predictions of the unique and conserved miRNAs in the sample. - Use scores >1 for miRNA profiling and scores >4 for further experimental analysis, such as miRNA inhibition and mimicking endogenous miRNAs34,36,57.
- Select and identify the unique miRNAs from the miRbase based on the following: miRDeep2 score (step 12.5.3), estimation of true probability (>90%), significant randfold p-value (<0.05)49,50,56.
NOTE: The bioinformatics analysis was done via miRDeep2 in Cyverse Discovery Environment58,59, a free online platform for bioinformatics analysis. The conserved and unique miRNAs identified through miRDeep2 were compared against all species from the miRbase. Future miRDeep2 identification can be compared against the corresponding genome of the species of interest.
- Use the output files from step 12.3.4 and step 12.4.2 as the input files.
Results
The present protocol provides a detailed methodology to extract miRNAs from salivary glands and EVs. According to the results, this protocol is effective for the isolation of miRNA from adults of two different tick species, I. scapularis and R. microplus, and can potentially be used in other tick species as well. The EVs concentration (particles/mL) was measured via NTA. For R. microplus, each gender and life stage contained three biological replicates measured in three technical replicates. The samples were separated by gender and life stage (Figure 8) to show the variation within each sample. Next, the samples were combined to display the variation and statistical differences using a two-way ANOVA and Tukey's multiple comparison tests20 (p-value = < 0.05) (Figure 9). Each sample consisted of ~40 salivary glands dissected from 20 females, males, and nymphs. After the isolation and quantification of the EVs, the samples were used for small RNA purification.
The concentration of the small RNA within each sample was measured via Qubit (Table 1), and the quality was measured via a bioanalyzer using standard gel electrophoresis46,47. The concentrations are in nanograms (Table 1) in a 14 µL volume for both the tick species. The average total of small RNA concentrations from I. scapularis organs ranged from 45.92-6,356 ng. The RNA concentration of I. scapularis vesicles ranged from 72.24-2,128 ng. For R. microplus, the organ concentration ranged from 259-2,142 ng, and the vesicles ranged from 59.22-1,848 ng. The samples were normalized to the same concentration, and their quality was then measured via a bioanalyzer (Figure 10). A reference ladder was used in the gel as a marker for quality assessment. The band integrity, intensity, and peaks were representatives of potential degradation or total concentration in each sample. Bands corresponding to 5 s and 5.8 s ribosomal RNAs were only present in the salivary gland organ samples (Figure 10, lanes A-D) and absent in the vesicle from the salivary gland samples (Figure 10, lanes E,F), demonstrating the differences in small RNA profiles between organs and extracellular vesicles. Sample degradation was inferred by the absence of bands in the gel; this signified that there was significant sample degradation. It is recommended that any samples having significant degradation be discarded.
To demonstrate the presence of miRNA samples within the preparations, small RNA cDNA libraries were prepared from RNA samples that have been stored for 3-4 months after small RNA isolation. Curiously, higher sample degradation was found in these samples. The lanes A-D and G showed signs of degradation; on the contrary, E, F, and H-K showed minimal degradation and sufficient concentration for miRNA cDNA library prep (Supplementary Figure 1). Only one sample was degraded when the samples were used immediately after purification (Figure 10), suggesting that miRNA samples were more prone to degradation once they were purified from EVs. Thus, samples E, F, H, and I were selected for enrichment analysis. The asterisks show the band sizes of ~150 bp, which were the expected sizes after cDNA library preparation (Figure 7). The lower faint bands indicate the primer dimer.
During EV isolation, the centrifugation velocity and time can affect the final EV concentration. When pipetting the EV pellet into the 300 k columns, as mentioned previously in step 6.10, a low volume and velocity are essential to prevent EVs from passing through the membrane. Previous studies showed that 700 µL at 12,000 x g for 20 min using a different centrifugal concentrator was sufficient for properly separating EVs from the soluble proteins20; however, low EV concentrations were displayed in the NTA using a different filter. Thus, optimizing the filters and other available materials is essential. When first using the 300 k columns, different velocity intervals were tested to determine which gave the highest EV concentration. The number of vesicles being lost during centrifugation was measured by NTA analysis; it was concluded that lower speeds resulted in higher vesicle concentration in the flow-through (not shown). It was concluded that 500 µL at 6,000-8,000 x g was satisfactory to isolate the EVs. Once this was determined, this protocol was used to isolate vesicles from I. scapularis and R. microplus. The concentration of the vesicles isolated from each tick species was measured via NTA (Figure 8). The EV concentrations varied between 7.07 x 107 to 7.94 x 109 particles/mL. The EV quantity correlated with the miRNA concentrations, where the greater amount of EV resulted in a higher concentration of miRNA extracted.
Figure 1: 1 mL needle-less syringes loaded with female adult I. scapularis and covered with sterilized gauze. Please click here to view a larger version of this figure.

Figure 2: The dorsal side of an engorged female was removed with 4 mm vannas scissors. The female was submersed in 1x PBS to prevent organ desiccation. The yellow arrows point to the exposed salivary glands. The figure was taken at 50x magnification with a high-resolution objective lens. Please click here to view a larger version of this figure.

Figure 3: A cell culture plate after a 24 h incubation at 32 ˚C. The first row of wells contains the vesicle-free media and dissected salivary glands. The rest of the wells contain 1x PBS with Rifampicin. Please click here to view a larger version of this figure.

Figure 4: A sample preparation process for an ultracentrifuge cycle. (A) A 10 mL needle-less syringe topped with a 1 µm syringe filter. (B) The supernatant after three rounds of centrifugation is pipetted into the syringe. (C) The rest of the syringe is filled to 10 mL with 1x sterilized PBS. (D) The syringe is covered, and the supernatant with 1x PBS is filtered into the ultracentrifuge tube. Please click here to view a larger version of this figure.
Figure 5: After 18 h of ultracentrifugation, a pellet of extracellular vesicles forms at the bottom of the ultracentrifuge tube. Please click here to view a larger version of this figure.

Figure 6: A sterilized pestle is used to homogenize salivary gland organs and the extracellular vesicles. Please click here to view a larger version of this figure.
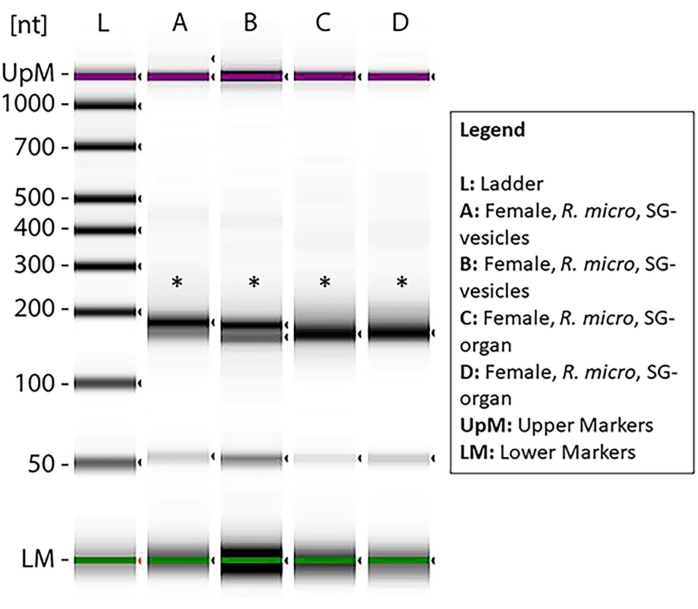
Figure 7: A gel electrophoresis measured via tape station displaying the miRNA prepped libraries after an enrichment analysis. The samples from female R. microplus salivary gland organs and EVs were based on minimal degradation and sufficient miRNA concentration for cDNA library synthesis. The ladder (L) shows the reference points in nucleotides and the upper and lower markers. The asterisks indicate the bands of size product ~150 bp, which signify the small RNA cDNA libraries. The lower bands show primer dimer. Please click here to view a larger version of this figure.
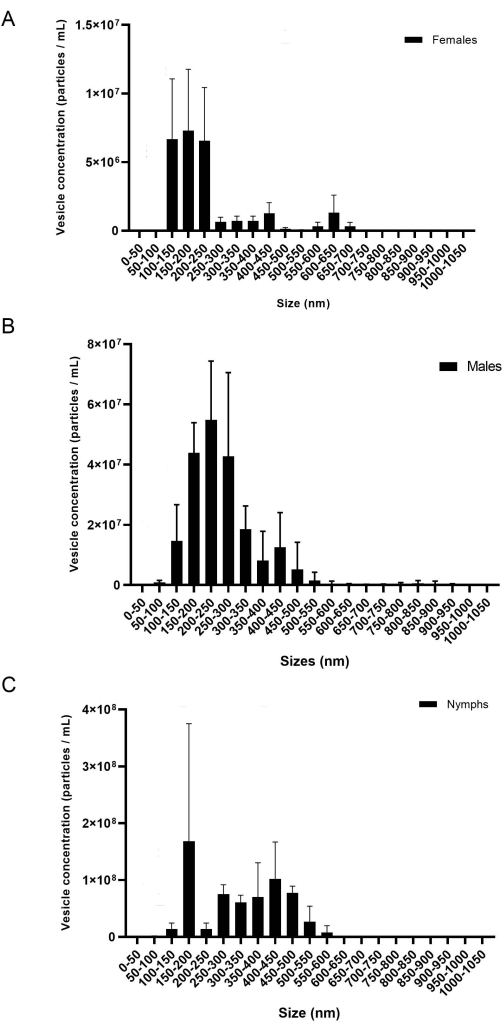
Figure 8: A representative figure of the variation in between the biological replicates (A) females, (B) males, and (C) nymphs. The x-axis shows the EV size (nm), and the y-axis shows the EV concentration (particles/mL). A two-way ANOVA and Tukey's comparison test displayed statistical differences (P-value = < 0.05). The error bars represent the standard error to account for variation. Each sample contained 20 ticks with three biological replicates. The samples were recorded for 60 s, three times each. The camera was set at level 7, and the detection threshold was set at level 5. Please click here to view a larger version of this figure.

Figure 9: A representative figure of the variation for all combined biological replicates for nymphs (red), females (blue), and males (black). The x-axis shows the EV size (nm), and the y-axis displays the EV concentration (particles/mL). A two-way ANOVA and Tukey's comparison test displayed statistical differences (P-value = < 0.05). The error bars represent the standard error to account for variation. Each sample contained 20 ticks with three biological replicates. The asterisks symbolize a significant difference of p < 0.05. Each recording was done for 60 s, three times each. The camera was set at level 7, and the detection threshold was set at level 5. Please click here to view a larger version of this figure.
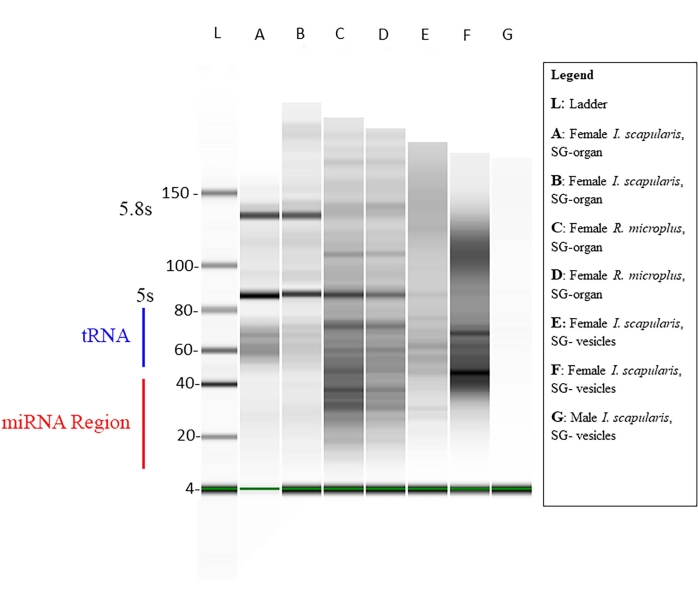
Figure 10: A representative gel via the bioanalyzer. The gel electrophoresis was performed using a base ladder as a reference. A mature miRNA sequence is ~18-22 nt long, where faint bands are shown in the designated size range. Other small RNAs, such as small nuclear RNAs, transfer-messenger RNAs, and regulatory RNAs, are also present in the samples. The sizes of the small RNAs ranged from ~20-150 nt. The samples vary from tick species, tissues, and gender. For lane G, an example of sample degradation shows no bands. Please click here to view a larger version of this figure.
Supplementary Figure 1: A gel electrophoresis was measured via the bioanalyzer, displaying the extracted miRNAs' quality from R. microplus female salivary gland organs and EVs. The ladder (L) shows the reference sizes in nucleotides, where mature miRNAs measure ~18-22 nt in length. The lanes A-D and G show degradation, and lanes E, F, and H-K show minimal degradation. Please click here to download this File.
| Species | Gender | Organ/ Vesicle | EV concentration | miRNA concentration (ng) |
| R. microplus | Female | Organ | N/A | 259 |
| R. microplus | Female | Organ | N/A | 2,142 |
| R. microplus | Female | Vesicle | 1.64 E+08 | 59.22 |
| R. microplus | Female | Vesicle | 1.64 E+09 | 1,848 |
| I. scapularis | Female | Organ | N/A | 45.92 |
| I. scapularis | Female | Organ | N/A | 6,356 |
| I. scapularis | Female | Vesicle | 1.73E+08 | 65.66 |
| I. scapularis | Female | Vesicle | 3.14 E+09 | 2,128 |
Table 1: An example of the miRNA concentrations for both salivary glands and extracellular vesicles. The column of miRNA concentrations represents the lowest to highest concentrations for each tick species.
| microRNA | I. scapularis | I. ricinus | R. microplus | H. longicornis | References |
| miR-8 | A | P | P | P | 33, 36, 37, 43 |
| miR-71 | P | P | P | A | 33, 36, 37, 43 |
| miR-279 | P | A | A | P | 33, 36, 37, 43 |
| let-7 | A | P | P | P | 33, 36, 37, 43 |
Table 2: The conserved miRNAs in different tick species. (P) indicates the miRNA were commonly expressed, or present, and (A) signifies the miRNAs were not commonly expressed or detected.
| Sample Type | Number of Scores >1* | Number of Scores >4γ | Number of Conserved | Number of Novel |
| Male | 17 | 0 | 48,885 | 23 |
| Female | 25 | 0 | 48,885 | 21 |
| Nymphs | 38 | 0 | 48,885 | 38 |
Table 3. The conserved and unique miRNAs were detected in the EVs for R. microplus females, males, and nymphs. *miRNA score used for profiling. γmiRNA score used for functional experiments.
Supplementary Table 1: A table showing next-generation sequencingresults for R. microplus males EVs secreted from salivary glands. Please click here to download this Table.
Supplementary Table 2: A table showing next-generation sequencingresults for R. microplus female EVs secreted from salivary glands. Please click here to download this Table.
Supplementary Table 3: A table showing next-generation sequencingresults for R. microplus nymph EVs secreted from salivary glands. Please click here to download this Table.
Discussion
The current protocol provides a detailed methodology for extracting miRNA from salivary glands and EVs. However, there are important considerations, all of which are detailed in the notes for each section of this protocol. The capsule and mesh netting must be secured during tick feeding to prevent ticks from escaping. The capsule preparation and placement are described in Koga et al.40. Several replicates of the tick dissections need to be done if an unsuitable sample is discarded. Additionally, several challenges can be present when utilizing EV isolation techniques from tick tissue18,20,43. For example, tissues should be kept moist during dissection to avoid desiccation. This is done by adding PBS throughout the dissection. Both the PBS and media used for the dissection and culture of the organs should be maintained with antibiotics to avoid bacterial contamination from the tick microbiome. These should include antibiotics that target Gram-negative and Gram-positive bacteria as both can be found within tick tissues60. Likewise, care must be taken during dissection to diminish contamination with tissues from other organs. Thus, dissections need to be done slowly, and as the user gains experience, more ticks can be dissected. Lastly, because EVs are secreted from all cells, including pathogen-infected cells, tick studies conducting EV isolation need to use EV-free media and buffers to avoid cross-EV contamination25,61.
Nevertheless, this protocol allows for the reduction of the number of ticks that are needed to produce tick salivary EVs. Previous protocols required the salivation of a significantly larger number of ticks. For example, miRNAs secreted within salivary EVs in Haemaphysalis longicornis needed the salivation of 2,000 adult ticks43. This can be extremely expensive for laboratories lacking the capacity to rear ticks. Similarly, Amblyomma maculatum tissues used for EV isolation were partially frozen before isolation and treated with 75 U/mL of collagenase type 3, which could affect the authenticity of the EV secretion19. Further, these studies required 80-100 pairs of salivary glands18.
Comparatively, this protocol can be applied to multiple tick species, and requires a low number of ticks to isolate EVs and extract quality conserved and novel miRNAs (Table 2)33,36,37,43. The miRNA concentrations varied greatly but were sufficient for next-generation sequencing (Table 3 and Supplementary Tables 1-3). This protocol can be adjusted to accommodate a larger sample size if larger miRNA concentrations are needed. Also, materials used in this protocol are substitutable depending on material availability. However, sample volumes and centrifugation velocity must be adjusted following the manufacturer's instructions for the kits and columns used.
A disadvantage of this method is that miRNAs and EVs can easily degrade throughout the steps of extraction and isolation. Therefore, the protocol must be accomplished quickly and efficiently. When stated, the samples must be kept on ice, and the miRNA extraction must be conducted in a sterilized environment. Additionally, an RNase treatment can be done on the isolated EVs to eliminate large RNAs attached to the EV membrane. This can prevent large RNAs from contaminating the sample during miRNA extractions. Lastly, adding an RNAse inhibitor to the miRNA sample after isolation from EVs or organs is an important preventive measure for degradation. This protocol can be altered and applied in parallel to the experiment's objectives being conducted.
Future applications for this protocol may include the study of pathogen-vector interactions to understand how pathogens affect the miRNA and other genomic cargo within tick salivary EVs. Likewise, this protocol can define specific proteins and cellular processes involved in the packaging of miRNAs into tick EVs, and the specific effect these EVs and miRNAs have on wound healing and immune responses. Due to the increasing tick resistance to acaricides, there is a desperate need for unique control methods. EVs have the potential for an alternative control method compared to acaricides. EVs can be used as nano-transporters in therapeutic applications18,23,61. In humans, EVs transporting miRNAs have been used to suppress tumor growth during cancer immunotherapy62,63. Similarly, in ticks, EVs can carry genetically modified miRNAs that have been shown to affect vital tick biological functions and pathogen transmission36,57,64. The future direction is to use this protocol to determine the miRNA profiles of multiple tick species to identify miRNAs of interest for functional studies.
Disclosures
The authors declare no conflict of interest.
Acknowledgements
We are greatly appreciative for the assistance from the Cattle Fever tick Laboratory in Edinburg, Texas. We would like to thank Michael Moses, Jason Tidwell, James Hellums, Cesario Agado, and Homer Vasquez. We would also like to acknowledge the assistance of Sarah Sharpton, Elizabeth Lohstroh, Amy Filip, Kelsey Johnson, Kelli Kochcan, Andrew Hillhouse, Charluz Arocho Rosario, and Stephanie Guzman Valencia throughout the project. We would like to thank the Texas A&M Aggie Women in Entomology (AWE) Writing Group for their help and advice during the writing of this manuscript. The following reagents were provided by Centers for Disease Control and Prevention for distribution by BEI Resources, NIAID, NIH: Ixodes scapularis Adult (Live), NR-42510. Female I. scapularis ticks were also received from the Tick Rearing Facility at Oklahoma State University. This project was funded by Texas A&M University T3: triads for transformation grant and the cooperative agreement #58-3094-1-003 by the USDA-ARS to AOC.
Materials
| Name | Company | Catalog Number | Comments |
| 0.22 µm syringe filter | GenClone | 25-240 | |
| 1 µm nylon syringe filter | Tisch Scientific | 283129028 | |
| 1 inch black adhesive | Amazon | B00FQ937NM | Capsule |
| 10 mL needeless syringe | Exelint | 26265 | |
| 3' and 5' Adapters | Illumina | 20024906 | NEXTFLEX Small RNA-Seq Kit |
| 4 mm vannas scissors | Fine Science Tools | 15000-08 | |
| 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid | Sigma-Aldrich | 1.1523 | |
| 70Ti rotor | Beckman Coulter | 337922 | |
| Amphotericin | Corning | 30-003-CF | |
| Beads | Illumina | 20024906 | NEXTFLEX Small RNA-Seq Kit |
| Bioanalyzer | Agilent | G2939BA | |
| Bioanalyzer kit | Agilent | 5067-1513 | |
| Centrifuge 5425 | Eppendorf | ||
| Chloroform | Macron | UN1888 | |
| Cyverse Discovery Enviornment | https://cyverse.org/discovery-environment | ||
| Dissecting microscope | Nikon | SMZ745 | |
| Double-sideded carpet tape | amazon | 286373 | |
| Falcon Tubes, 50 mL | VWR | 21008-940 | |
| Fetal Bovine Serum | Gibco | FBS-02-0050 | |
| fine forceps | Excelta | 5-S-SE | |
| Foamies, 2 mm | Amazon | B004M5QGBQ | Capsule |
| Isoflurane | Phoenix Pharmaceuticals manfactured | 193.33165.3 | |
| Ixodes scaplaris | CDC, Oklahoma State University | ||
| L15C300 medium | In-lab | ||
| lipoprotein-cholesterol concentrate | MPI | 02191476-CF | |
| Microscope slide | VWR | 10118-596 | |
| miRDeep2 | https://github.com/rajewsky-lab/mirdeep2 | ||
| M-MuLV Reverse Transcriptase | Illumina | 20024906 | NEXTFLEX Small RNA-Seq Kit |
| molecular grade ethanol | Fischer Bioreagents | UN1170 | |
| multi-well 24 well tissue culture treated plate | Corning | 353047 | |
| Nanopaticle Tracking Analyzer machine | Malvern Panalytical | ||
| Nanosep with 300K Omega filter | Pall Corporation | OD3003C33 | |
| NEXTFLEX Small RNA-Seq Kit v3 | PerkinElmer | ||
| NextSeq 500/550 High Output Kit (75 cycles) | Illumina | 20024906 | |
| Optima XPN 90 Ultracentrifuge | Beckman Coulter | ||
| Penicillin | Thermofischer Scientific | ICN19453780 | |
| Pippettes | Ependorff | ||
| polycarbonate centrifuge bottle | Beckman Coulter | 355618 | |
| Qiagen miRNeasy kit | Qiagen | 217084 | |
| QIAzol lysis reagent | Qiagen | 79306 | |
| Qubit | Thermofisher | Q32880 | |
| Qubit kit | Thermofisher | Q10212 | |
| Rabbits | Charles River | ||
| Reverse Universal Primer | Illumina | 20024906 | NEXTFLEX Small RNA-Seq Kit |
| Rhipicephalus microplus | Cattle Fever Tick Research Labratoty | ||
| Rifampicin | Fischer Bioreagents | 215544 | |
| RNAlater | Invitrogen | 833280 | |
| RNAse free tubes | VWR | 87003294 | |
| RNAse inhibitor | Thermo Fischer | 11111729 | |
| RNAse/DNAse free water | Qiagen | 217084 | |
| RNeasy Minelute spin column | Qiagen | 217084 | Qiagen miRNeasy kit |
| RPE Buffer | Qiagen | 217084 | Qiagen miRNeasy kit |
| RT Buffer | Illumina | 20024906 | NEXTFLEX Small RNA-seq kit |
| RT Forward Primer | Illumina | 20024906 | NEXTFLEX Small RNA-seq kit |
| RTE Buffer | Qiagen | 217084 | Qiagen miRNeasy kit |
| Sodium bicarbonate | Sigma-Aldrich | S6014-25G | |
| Sorvall ST16 | Thermo Fischer | 75004380 | |
| Sterilized Gauze sponges | Covidien | 2187 | |
| Sterilized PBS | Sigma | RNBK0694 | |
| streptomycin | thermofischer Scientific | 15240062 | |
| TapeStation | Aligent | G2991BA | |
| Tear Mender Instant Fabric and Leather Adhesive | Amazon | 7.42836E+11 | Capsule |
| Tissue Adhesive | 3M VetBond | ||
| Triple Antibiotics | dechra | 17033-122-75 | |
| Tryptose phosphate broth | BD | BD 260300 |
References
- Jongejan, F., Uilenberg, G. The global importance of ticks. Parasitology. 129, 3-14 (2004).
- Anderson, J. F., Magnarelli, L. A. Biology of ticks. Infectious Disease Clinics of North America. 22 (2), 195-215 (2008).
- de la Fuente, J. Controlling ticks and tick-borne diseases… looking forward. Ticks and Tick-Borne Diseases. 9 (5), 1354-1357 (2018).
- Nicholson, W. L., Sonenshine, D. E., Noden, B. H., Brown, R. N. Ticks (Ixodia). Medical and Veterinary Entomology. , 603-672 (2019).
- Guerrero, F. D., Lovis, L., Martins, J. R. Acaricide resistance mechanisms in Rhipicephalus (Boophilus) microplus. Revista Brasileira de Parasitologia Veterinária. 21 (1), 1-6 (2012).
- Abbas, R. Z., Zaman, M. A., Colwell, D. D., Gilleard, J., Iqbal, Z. Acaricide resistance in cattle ticks and approaches to its management: the state of play. Veterinary Parasitology. 203 (1-2), 6-20 (2014).
- Redshaw, N., et al. A comparison of miRNA isolation and RT-qPCR technologies and their effects on quantification accuracy and repeatability. Biotechniques. 54 (3), 155-164 (2013).
- Estrada-Peña, A., Jongejan, F. Ticks feeding on humans: a review of records on human-biting Ixodoidea with special reference to pathogen transmission. Experimental and Applied Acarology. 23 (9), 685-715 (1999).
- Eisen, R. J., Eisen, L. The blacklegged tick, Ixodes scapularis: an increasing public health concern. Trends in Parasitology. 34 (4), 295-309 (2018).
- Bowman, A. S., Sauer, J. R. Tick salivary glands: function, physiology and future. Parasitology. 129, 67 (2004).
- Kim, D., Maldonado-Ruiz, P., Zurek, L., Park, Y. Water absorption through salivary gland type I acini in the blacklegged tick, Ixodes scapularis. PeerJ. 5, 3984 (2017).
- Nunes, P. H., Bechara, G. H., Camargo-Mathias, M. I. Morphological changes in the salivary glands of Amblyomma cajennense females (Acari: Ixodidae) in different feeding stages on rabbits at first infestation. Experimental and Applied Acarology. 45 (3), 199-209 (2008).
- Bishop, R., et al. A cement protein of the tick Rhipicephalusappendiculatus, located in the secretory e cell granules of the type III salivary gland acini, induces strong antibody responses in cattle. International Journal for Parasitology. 32 (7), 833-842 (2002).
- Yamaji, K., et al. A salivary cystatin, HlSC-1, from the ixodid tick Haemaphysalis longicornis play roles in the blood-feeding processes. Parasitology Research. 106 (1), 61-68 (2009).
- Simo, L., Kazimirova, M., Richardson, J., Bonnet, S. I. The essential role of tick salivary glands and saliva in tick feeding and pathogen transmission. Frontiers in Cellular and Infection Microbiology. 7, 281 (2017).
- Perner, J., Kropáčková, S., Kopáček, P., Ribeiro, J. M. C. Sialome diversity of ticks revealed by RNAseq of single tick salivary glands. PLoS Neglected Tropical Diseases. 12 (4), 0006410 (2018).
- Madden, R. D., Sauer, J. R., Dillwith, J. W. A proteomics approach to characterizing tick salivary secretions. Experimental and Applied Acarology. 32 (1), 131-141 (2004).
- Zhou, W., et al. Discovery of exosomes from tick saliva and salivary glands reveals therapeutic roles for CXCL12 and IL-8 in wound healing at the tick-human skin interface. Frontiers in Cell and Developmental Biology. 8, 554 (2020).
- Zhou, W., et al. Exosomes serve as novel modes of tick-borne flavivirus transmission from arthropod to human cells and facilitates dissemination of viral RNA and proteins to the vertebrate neuronal cells. PLoS Pathogens. 14 (1), 1006764 (2018).
- Chávez, A. S. O., et al. Tick extracellular vesicles enable arthropod feeding and promote distinct outcomes of bacterial infection. Nature Communications. 12 (1), 1-17 (2021).
- Pegtel, D. M., Gould, S. J. Exosomes. Annual Review of Biochemistry. 88, 487-514 (2019).
- Mathieu, M., Martin-Jaular, L., Lavieu, G., Théry, C. Specificities of secretion and uptake of exosomes and other extracellular vesicles for cell-to-cell communication. Nature Cell Biology. 21 (1), 9-17 (2019).
- Andaloussi, S. E. L., Mäger, I., Breakefield, X. O., Wood, M. J. A. Extracellular vesicles: biology and emerging therapeutic opportunities. Nature Reviews Drug Discovery. 12 (5), 347-357 (2013).
- Van Niel, G., D'Angelo, G., Raposo, G. Shedding light on the cell biology of extracellular vesicles. Nature Reviews Molecular Cell Biology. 19 (4), 213 (2018).
- Gioseffi, A., Edelmann, M. J., Kima, P. E. Intravacuolar pathogens hijack host extracellular vesicle biogenesis to secrete virulence factors. Frontiers in Immunology. 12, 662944 (2021).
- Chávez, A. S. O., O'Neal, A. J., Santambrogio, L., Kotsyfakis, M., Pedra, J. H. F. Message in a vesicle-trans-kingdom intercommunication at the vector-host interface. Journal of Cell Science. 132 (6), 224212 (2019).
- Janas, T., Janas, M. M., Sapoń, K., Janas, T. Mechanisms of RNA loading into exosomes. FEBS Letters. 589 (13), 1391-1398 (2015).
- Lu, T. X., Rothenberg, M. E. MicroRNA. Journal of Allergy and Clinical Immunology. 141 (4), 1202-1207 (2018).
- Pegtel, D. M., et al. Functional delivery of viral miRNAs via exosomes. Proceedings of the National Academy of Sciences. 107 (14), 6328-6333 (2010).
- Bartel, D. P. MicroRNAs: target recognition and regulatory functions. Cell. 136 (2), 215-233 (2009).
- Ambros, V. MicroRNAs and developmental timing. Current Opinion in Genetics and Development. 21 (4), 511-517 (2011).
- Bushati, N., Cohen, S. M. microRNA functions. Annual Review of Cell and Developmental Biology. 23, 175-205 (2007).
- Hackenberg, M., Langenberger, D., Schwarz, A., Erhart, J., Kotsyfakis, M. In silico target network analysis of de novo-discovered, tick saliva-specific microRNAs reveals important combinatorial effects in their interference with vertebrate host physiology. RNA. 23 (8), 1259-1269 (2017).
- Luo, J., et al. MicroRNA-1 promotes the development of and prolongs engorgement time in Hyalomma anatolicum anatolicum (Acari: Ixodidae) ticks. Biorxiv. , (2020).
- Zhou, J., Zhou, Y., Cao, J., Zhang, H., Yu, Y. Distinctive microRNA profiles in the salivary glands of Haemaphysalis longicornis related to tick blood-feeding. Experimental and Applied Acarology. 59 (3), 339-349 (2013).
- Hermance, M. E., Widen, S. G., Wood, T. G., Thangamani, S. Ixodes scapularis salivary gland microRNAs are differentially expressed during Powassan virus transmission. Scientific Reports. 9 (1), 1-17 (2019).
- Barrero, R. A., et al. Evolutionary conserved microRNAs are ubiquitously expressed compared to tick-specific miRNAs in the cattle tick Rhipicephalus (Boophilus) microplus. BMC Genomics. 12 (1), 1-17 (2011).
- Colombo, M., et al. Analysis of ESCRT functions in exosome biogenesis, composition and secretion highlights the heterogeneity of extracellular vesicles. Journal of Cell Science. 126 (24), 5553-5565 (2013).
- Greening, D. W., Xu, R., Ji, H., Tauro, B. J., Simpson, R. J. . Proteomic Profiling. , 179-209 (2015).
- Koga, K., et al. Purification, characterization and biological significance of tumor-derived exosomes. Anticancer Research. 25, 3703-3707 (2005).
- Wright, K., de Silva, K., Purdie, A. C., Plain, K. M. Comparison of methods for miRNA isolation and quantification from ovine plasma. Scientific Reports. 10 (1), 1-11 (2020).
- Mráz, M., Malinova, K., Mayer, J., Pospisilova, S. MicroRNA isolation and stability in stored RNA samples. Biochemical and Biophysical Research Communications. 390 (1), 1-4 (2009).
- Nawaz, M., et al. miRNA profile of extracellular vesicles isolated from saliva of Haemaphysalis longicornis tick. Acta Tropica. 212, 105718 (2020).
- Ribeiro, J. M. C., Zeidner, N. S., Ledin, K., Dolan, M. C., Mather, T. N. How much pilocarpine contaminates pilocarpine-induced tick saliva. Medical and Veterinary Entomology. 18 (1), 20-24 (2004).
- Almazán, C., et al. A versatile model of hard tick infestation on laboratory rabbits. Journal of Visualized Experiments. (140), e57994 (2018).
- Masotti, A., Preckel, T. Analysis of small RNAs with the Agilent 2100 Bioanalyzer. Nature Methods. 3 (8), 658 (2006).
- Benesova, S., Kubista, M., Valihrach, L. Small RNA-sequencing: approaches and considerations for miRNA analysis. Diagnostics. 11 (6), 964 (2021).
- Mackowiak, S. D. Identification of novel and known miRNAs in deep-sequencing data with miRDeep2. Current Protocols in Bioinformatics. 36 (1), 12 (2011).
- Friedländer, M. R., Mackowiak, S. D., Li, N., Chen, W., Rajewsky, N. miRDeep2 accurately identifies known and hundreds of novel microRNA genes in seven animal clades. Nucleic Acids Research. 40 (1), 37-52 (2012).
- Griffiths-Jones, S. The microRNA registry. Nucleic Acids Research. 32, 109-111 (2004).
- Griffiths-Jones, S., Grocock, R. J., Van Dongen, S., Bateman, A., Enright, A. J. miRBase: microRNA sequences, targets and gene nomenclature. Nucleic Acids Research. 34, 140-144 (2006).
- Griffiths-Jones, S., Saini, H. K., Van Dongen, S., Enright, A. J. miRBase: tools for microRNA genomics. Nucleic Acids Research. 36, 154-158 (2007).
- Kozomara, A., Birgaoanu, M., Griffiths-Jones, S. miRBase: from microRNA sequences to function. Nucleic Acids Research. 47, 155-162 (2019).
- Kozomara, A., Griffiths-Jones, S. miRBase: integrating microRNA annotation and deep-sequencing data. Nucleic Acids Research. 39, 152-157 (2011).
- Kozomara, A., Griffiths-Jones, S. miRBase: annotating high confidence microRNAs using deep sequencing data. Nucleic Acids Research. 42, 68-73 (2014).
- Wu, F., et al. MicroRNA let-7 regulates the expression of ecdysteroid receptor (ECR) in Hyalomma asiaticum (Acari: Ixodidae) ticks. Parasites and Vectors. 12 (1), 1-13 (2019).
- Goff, S. A., et al. The iPlant collaborative: cyberinfrastructure for plant biology. Frontiers in Plant Science. 2, 34 (2011).
- Merchant, N., et al. The iPlant collaborative: cyberinfrastructure for enabling data to discovery for the life sciences. PLoS Biology. 14 (1), 1002342 (2016).
- Kumar, D., et al. An exploratory study on the microbiome of northern and southern populations of Ixodes scapularis ticks predicts changes and unique bacterial interactions. Pathogens. 11 (2), 130 (2022).
- Zhang, Y., et al. Exosome: a review of its classification, isolation techniques, storage, diagnostic and targeted therapy applications. International Journal of Nanomedicine. 15, 6917 (2020).
- Di Leva, G., Croce, C. M. miRNA profiling of cancer. Current Opinion in Genetics and Development. 23 (1), 3-11 (2013).
- Ganju, A., et al. miRNA nanotherapeutics for cancer. Drug Discovery Today. 22 (2), 424-432 (2017).
- Luo, J., et al. MicroRNA-1 Expression and Function in Hyalomma Anatolicum anatolicum (Acari: Ixodidae) Ticks. Frontiers in Physiology. 12, 596289 (2021).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved