Method Article
Robot-assisted Total Mesorectal Excision and Lateral Pelvic Lymph Node Dissection for Locally Advanced Middle-low Rectal Cancer
* These authors contributed equally
In This Article
Summary
The robotic technique described herein aims to detail a stepwise approach to robot-assisted total mesorectal excision and lateral pelvic lymph node dissection for locally advanced (T3/T4) rectal cancer located below the peritoneal reflection.
Abstract
Since their approval for clinical use, da Vinci surgical robots have shown great advantages in gastrointestinal surgical operations, especially in complex procedures. The high-quality 3-D visual, multijoint arm and natural tremor filtration allow the surgeon to expose and dissect more accurately with minimal invasion. Total mesorectal excision is the standard surgical technique for the treatment of resectable rectal cancer. To reduce the lateral recurrence rate, lateral pelvic lymph node dissection can be performed, as it is a safe and feasible procedure for locally advanced middle-low rectal cancer with a high possibility of metastasis to the lateral lymph nodes. However, the complexity of the anatomic structures and the high postoperative complication rate limit its application. Recently, several surgeons have increasingly used robotic techniques for total mesorectal excision and lateral pelvic lymph node dissection. Compared with open and laparoscopic surgery, the robotic technique has several advantages, such as less blood loss, fewer blood transfusions, minimal trauma, shorter postoperative hospitalization, and quicker recovery. A robotic approach is generally regarded as a reasonable alternative for complicated procedures such as lateral pelvic lymph node dissection, although there are a limited number of high-quality prospective randomized controlled studies reporting direct evidence. Here, we provide the detailed steps of robot-assisted total mesorectal excision and lateral pelvic lymph node dissection performed at the First Affiliated Hospital of Xi'an Jiaotong University.
Introduction
Since their approval for clinical use by the United States Food and Drug Administration in 2000, da Vinci surgical robots have been increasingly utilized across different surgical specialties1. The robotic surgical system has the advantages of using flexible multijoint arms, a high-quality three-dimensional camera, tremor filtration, and greatly improved ergonomics, which can minimize the invasiveness of the operation and thus making it ideal for complex procedures.
For decades, total mesorectal excision (TME) has been the standard for the treatment of resectable rectal cancer. However, for advanced (T3/T4) rectal cancer located below the peritoneal reflection, lateral pelvic lymph node (LPLN) metastasis is a major cause of local recurrence after surgery2. Clinical evidence clearly shows that lateral pelvic lymph node dissection (LPLND) could significantly reduce the local recurrence rate3. Compared with the open procedure, robot-assisted TME and LPLND have been associated with less blood loss, fewer blood transfusions and fewer postoperative complications4. In addition, the long-term outcomes are not significantly different between the two procedures5. The results of these reports indicate that robot-assisted LPLND may be a feasible modality for locally advanced rectal cancer. However, it should be noted that robot-assisted TME and LPLND are complex procedures and should be performed by an experienced surgeon.
Herein, a standard systematic approach to robot-assisted TME and LPLND is described step by step. This procedure was developed at the center having experience in performing more than three thousand robotic procedures6. In addition, this approach was based on normal anatomical characteristics; rare anatomical variations should be noted.
We present the case of a 64-year-old male patient who had intermittent hematochezia for approximately 3 months. Digital rectal examination revealed that a mass was located on the anterior and right lateral wall of the rectum, 5 cm from the anus. An enhanced computed tomography (CT) scan and endoscopic ultrasound (EUS) revealed lower rectal cancer with internal iliac lymph node metastasis. Colonoscopic biopsy confirmed the presence of moderately differentiated adenocarcinoma. The preoperative evaluation suggested that the clinical stage was cT3N+M0. Accordingly, we decided to perform robot-assisted TME and LPLND. Patient consent was obtained prior to performing these procedures.
Protocol
This protocol complies with the guidelines of the Ethics Committee of the First Affiliated Hospital of Xi 'an Jiaotong University (No. 2019ZD04).
1. Preoperative preparation, patient position, and anesthesia
- Ensure appropriate dietary management before operation.
- Prescribe a preoperative oral carbohydrate drink to be consumed at bedtime and 4 h prior to surgery.
NOTE: This was allowed based on the enhanced recovery after surgery (ERAS) protocol. - Do not prescribe any additional oral antibiotics.
NOTE: The implementation of these measures should be carefully considered according to the experience of each center.
- Prescribe a preoperative oral carbohydrate drink to be consumed at bedtime and 4 h prior to surgery.
- Administer preoperative antibiotics and venous thromboembolism prophylaxis.
- Inject preventive antibiotics (2 g of cefmetazole, 4.5 g of piperacillin/tazobactam, or 300 mg of clindamycin based on the drug allergy test) within 1 h prior to the incision.
- Administer deep venous thrombosis prophylaxis in the form of a subcutaneous low-molecular-weight heparin injection (Nadroparin calcium, 2850 IU) 2-4 h before anesthesia.
- Induce general anesthesia.
NOTE: This should be performed based on the experience of each center.- Administer multimodal analgesia to minimize narcotic administration, including regional nerve blockade and nonsteroidal analgesia.
- Perform appropriate restrictive intraoperative fluid management according to base requirements, blood loss and hemodynamic monitoring results.
- In addition, during the operation, dynamically monitor the patient's electrocardiogram, radial arterial pressure, pulse oximetry, capnography, urinary volumes, temperature, and blood gas analysis 7.
- Place a 16-18 Fr sterile urethral catheter.
- Place the patient in a Lloyd Davis position and secure them carefully to the operating table. Ensure that the legs are carefully padded in stirrups and that both arms are tucked at the side.
2. Operation settings and port placement
NOTE: These measures can be appropriately adapted according to the experience of each surgeon.
- Ensure that the primary surgeon operates from the robotic console and customizes their settings (Figure 1).
- Have an assistant laparoscopic surgeon stand on the right side of the patient (Figure 1).
- Have a nurse stand on the left side of the patient (Figure 1).
- Before the operation, confirm the white balance, adjust the focus and 3D calibration of the da Vinci Si robot lens following the operation system guidance, and heat the lens in warm water (not more than 55 °C) to prevent fog.
- Establish the pneumoperitoneum and port placement.
- Make a 12-mm incision 2-3 cm above the umbilicus and slightly to the left.
- Elevate the umbilicus and abdominal wall with a towel clamp and pierce into the abdominal cavity using a Veress needle.
- Attach a disposable syringe filled with 5 mL of normal saline to the Veress needle with the flow tap open. Aspirate the syringe and ensure that no blood or fecal matter was aspirated. Inject 5 mL of saline.
- Remove the syringe from the Veress needle with the flow tap left open. Observe the movement of the fluid column at the top of the Veress needle to confirm that the Veress needle pierced the appropriate location.
NOTE: Free movement without any resistance of the fluid from the needle into the abdomen indicates a positive result for the saline drop test and the Veress needle has pierced the abdominal cavity. - Connect the insufflation tube to the Veress needle. Then, start the CO2 insufflation apparatus at a pressure setting of 12 mmHg.
NOTE: The pressure should be set between 8 and 15 mmHg. Confirm that the Veress needle is in the appropriate location by noting the pressure. If the pressure increases over the set pressure rapidly, this usually indicates that the Veress needle has not pierced the abdominal cavity. - After the measured pressure reaches the setting pressure, remove the Veress needle and set a 12-mm trocar as a visual port (Figure 2).
- Insert the robotic camera lens and fully inspect the abdominal cavity. Search and biopsy suspicious metastatic nodules and send them for frozen section histology8.
- If the adhesions disturb the trocar setting, release them first using the laparoscopic instrument.
- Set the three robotic arm ports. Place the trocar after making transverse 8 mm skin incisions for each trocar. Place arm 1 (R1) in the right McBurney's point (Figure 2). Place arm 2 (R2) in the left midclavicular line at the level of the visual port (Figure 2). Place arm 3 (R3) in the left anterior axillary line at the level of the visual port (Figure 2).
NOTE: Ensure the distance between adjacent ports is 8-10 cm. - Place a 12 mm assistant port 1 (A1) in the right midclavicular line at the level of the visual port (Figure 2).
- Place an 8 mm assistant port 2 (A2) approximately 1-2 cm above the pubic symphysis (Figure 2).
- Place the patient in a 30° Trendelenburg position with a 15° right down.
- After setting these ports, place the da Vinci robot between the patient's legs and attach the camera arm and three operation arms to the trocar using system guidance.
- Place the robotic instruments. Place monopolar scissors in R1, bipolar grasper in R2, and Cadiere grasping forceps in R3.
NOTE: The instrument in R1 can be replaced depending on the actions of the primary surgeon. The most commonly used instruments are monopolar scissors and harmonic scalpels.
3. Total mesorectal excision
- Mobilize the left colon.
- Retract the descending and sigmoid colon medially by Cadiere grasping forceps in R3 to expose the left paracolic sulci.
- Release the physiologic adhesions of the descending and sigmoid colon along the paracolic sulci with monopolar scissors in R1. Incise the peritoneum along paracolic sulci and dissect the descending colon from superior to inferior with monopolar scissors in R1 until the ureter is exposed to mobilize the lateral side of the descending and splenic flexure colon. Place a piece of sterile gauze near the ureter as an indicator.
- After mobilizing the lateral side of the descending and splenic flexure colon, grasp and keep elevating the sigmoid colon with its mesentery forward using Cadiere grasping forceps in R3. Create tension in the mesentery with a bipolar grasper in R2 and forceps in the assistant's hand. Then, recognize the "white line" of Toldt's fascia, an avascular plane. Incise the peritoneum along the "white line".
- Separate along this plane toward the lateral paracolic sulci with monopolar scissors in R1 to mobilize the sigmoid colon. Afterward, create a tunnel between the medial and lateral compartments under the guidance of the indicator gauze that was set previously. Continue to develop this plane downward to the sacral promontory using electrocautery and combine sharp and blunt spreading. Perform the dissection rapidly to completely mobilize the descending and sigmoid colon.
- Transect the inferior mesenteric artery (IMA) and inferior mesenteric vein (IMV).
- After mobilizing the sigmoid colon, grasp and keep elevating the sigmoid colon with forceps in R3 to expose the aorta. Dissect along the aorta superiorly with monopolar scissors in R1 to expose the IMA.
- Change the instrument in R1 from monopolar scissors to a harmonic scalpel.
- From the root of the IMA, separate the lymphatic tissue from the vessels with an ultrasonic scalpel in R1 until the left colic artery appears. Have the assistant surgeon clip the IMA below the origin of the left colic artery with a large locking clip. Then, transect with the harmonic scalpel to minimize bleeding.
- Continue to separate the lymphatic tissue from the left colic artery with a harmonic scalpel in R1. Recognize the inferior mesenteric vein (IMV) and the descending branch of the left colic artery. Have the assistant surgeon clip and transect these 2 vessels.
- Perform pelvic dissection of the rectum.
- Use a ribbon retractor to lift the rectum. Place the grasping forceps in A2 by the assistant surgeon and control the movement of the rectum by griping the ribbon retractor. Change the instrument in R1 to monopolar scissors.
- Lift the rectum forward with Cadiere grasping forceps inserted through the posterior margin of the sigmoid colon to expose the sacral promontory. Then, dissect into the retrorectal plane between mesorectal fascia and prehypogastric nerve fascia with monopolar scissors in R1. Develop along this plane and separate the mesorectal fascia from prehypogastric nerve fascia using monopolar scissors in R1 until the level of the levator ani muscle is reached (Figure 3A).
NOTE: The integrity of the mesorectal fascia should be retained. - Incise the peritoneum and open the lateral mesorectal plane close to the rectum with monopolar scissors in R1. Have the assistant surgeon move the rectum to the other side.
- Change the instrument in R1 to a harmonic scalpel. Carefully dissect and develop this plane until the level of the levator ani muscle is reached. Repeat this step for the contralateral side.
NOTE: In fact, because anterior dissection has still not been performed, the lateral mesorectal plane is difficult to develop completely. If it seems difficult to operate, consider dealing with the anterior plane first. - Incise the peritoneum 1 cm above the reflection of the visceral peritoneum with the harmonic scalpel in R1. After incising the reflection of the visceral peritoneum, identify the seminal vesicles and Denonvilliers' fascia that cover the posterior wall of the seminal vesicle.
NOTE: In women, dissection should be performed between the vaginal posterior wall and mesorectal fascia. Surgeons should avoid damaging the thin vaginal posterior wall. - Continue to develop the plane between Denonvilliers' fascia and mesorectal fascia until the level of the levator ani muscle is reached with the harmonic scalpel in R3.
- At this time, conduct a digital rectal examination transanally to confirm that the dissection has proceeded past the distal margin of the tumor and that there are appropriate margins for resection.
- Separate the adipose tissue surrounding the rectum at this level. Transect the rectum using a laparoscopic linear cutting stapler.
NOTE: Ensure that the rectum is transected below the distal border of the tumor with a distal margin of 2 cm or greater whenever possible. - Take the rectum out from the pelvic cavity and copiously irrigate the pelvic cavity with distilled water. Perform hemostasis using electrocautery with bipolar forceps in R2.
4. Lateral pelvic lymph node dissection
NOTE: Bilateral LPLND can commence on either the left or the right side. The current technique guideline suggests starting on the left. After releasing and mobilizing the sigmoid colon and rectum, the left common/external iliac artery and left ureter can be identified clearly, which facilitates starting the lymphadenectomy on this side. The lateral pelvic lymph nodes involve the common iliac area (No. 273), external iliac area (No. 293), obturator area (No. 283), and internal iliac area (No. 263). However, previous studies indicate that common iliac and external iliac lymph node metastases are rare9. Therefore, treatment guidelines for colorectal cancer recommend primarily focusing on the obturator area (No. 283) and internal iliac area (No. 263) for dissection9.
- Starting on the left, incise the peritoneum just lateral to the ureter with the harmonic scalpel in R1. Extend the incision up to the vas deferens.
NOTE: In women, the incision should be extended up to the round ligament. Use a harmonic scalpel to minimize vascular injury. - Identify the left ureter at the level of its crossing with the iliac vessels. Then mobilize the ureter and move it to the medial side with forceps in R3. Let the ureter and prehypogastric nerve fascia become the medial plane of the lateral node dissection.
NOTE: Complete skeletonization of the ureter may damage the blood supply of the ureter, which should be avoided if possible. In addition, keep the dissection lateral to the ureter and prehypogastric nerve fascia to avoid damaging the pelvic autonomic nerve located medial to this fascia. - From the lateral to the external iliac artery, separate the lymphatic tissue surrounding the external iliac artery and vein with the harmonic scalpel in R1.
- Retract the external iliac vein laterally with the aspirator in the assistant's hand. At the bifurcation of the internal and external iliac artery, separate lymphatic tissue with a harmonic scalpel in R1 and identify the obturator nerve and umbilical artery. At the lateral wall, completely release the lymphatic tissue from the surface of the psoas and internal obturator muscles (Figure 3C).
NOTE: The bifurcation of the internal and external iliac arteries is at the proximal end of the lateral node dissection. - Retract the umbilical artery and vesicohypogastric fascia medially with the aspirator in the assistant's hand and separate lymphatic tissue from vesicohypogastric fascia. Let the umbilical artery and vesicohypogastric fascia become the medial wall of dissection of obturator nodes (#283). Carefully separate the lymphatic tissue from fascia and nerve along the obturator nerve with the harmonic scalpel in R1 and identify the obturator artery and vein, which are the branches of the internal iliac artery and vein. Carefully isolate the obturator artery and vein to avoid injury.
NOTE: Some patients may have two or more obturator nerve branches according to our experience. Injury or transection of one of these branches may not lead to severe dysfunction. However, complete transection of all the branches of one side of the obturator nerve should be avoided as much as possible. - Retract the ureter and prehypogastric nerve fascia medially with the aspirator in the assistant's hand (Figure 3D). Completely release the lymphatic tissue from fascia with a harmonic scalpel in R1. Identify and isolate the 2-3 superior vesical arteries - these are the branches of the umbilical artery.
NOTE: Avoid ligating all superior vesical artery branches to minimize urinary dysfunction. At least one superior vesical artery should be preserved, especially when bilateral LPLND is performed. Otherwise, severe urinary dysfunction can occur. - Continue to dissect the lymphatic and fatty tissue distally with the harmonic scalpel in R1 until meeting the vas deferens.
NOTE: In women, dissection should be performed until the round ligament is reached. - Remove the lymphatic adipose tissue as a single specimen from the fossa using a sterile specimen bag (Figure 3E,F). Check and ensure that there is no residual lymphatic tissue and no bleeding.
- If necessary, repeat steps listed in this section on the right to complete right-side lymphadenectomy.
5. Reconstruction of the digestive tract
NOTE: Here, depending on the experience and preference of the primary surgeon, either a stapled colorectal or handsewn anastomosis can be chosen via open or robotic laparoscopic methods. Methods of anastomosis include straight end-to-end anastomosis, small reservoir end-to-side colorectal anastomosis, or colonic J-pouch anastomosis10. Here, we provide a basic, open, straight end-to-end stapled colorectal anastomosis technique.
- Make a vertical midline incision below umbilical. Place a wound protector.
NOTE: One can also select a Pfannenstiel incision or other type of incision depending on the experience and preference of the primary surgeon. - Determine the transection level according to the position of the tumor and the length of the colon. The transection level should be at least 10 cm proximal to the proximal border of the tumor. Attempt to attach the proximal colon toward the rectal stump and ensure that there is no undue tension.
- Separate the proximal and distal mesentery. Ligate the vascular arch of colon. Release the fat tissue surrounding the transection level colon.
- Clamp the colon using purse-string forceps at the level determined previously. Make a purse string using a purse-string needle. Transect the colon.
- Insert the anvil into the colon lumen and secure the purse string onto the anvil shaft with 0 silk sutures. Return the proximal colon to the abdominal cavity. Now, the specimen has been completely removed.
- Introduce a circular stapler transanally under laparoscopic guidance, gently rotating the adjusting knob counterclockwise. Fully extend the trocar and pierce the tissue.
- Slide the anvil shaft over the trocar until the anvil snaps into a fully seated position. Close by turning adjusting the knob clockwise. Start the stapler complete the anastomosis.
6. Diverting loop ileostomy
NOTE: Whether a diverting loop ileostomy is performed depends on the height and quality of the anastomosis and whether the patient was treated with radiation preoperatively. If ileostomy is not chosen, please skip steps 6.1.1-6.1.7.
- Make an incision in the right lower quadrant away from any skin creases, bony prominences, and other incisions.
NOTE: If diverting loop ileostomy is expected preoperatively, attempt to place R1 through the lateral aspect of the marked area to minimize the number of incisions. - Evert a length of mobilized, well-vascular supplied small bowel loop through the abdominal wall while avoiding any twisting of the mesentery.
- Make a mesenteric defect at the avascular area. Place a drain in the mesentery to aid in the externalization of the loop for maturation as a stoma.
- Divide the antimesenteric wall of the ileum close to the distal limb at the level of the stoma bridge using electrocautery.
NOTE: This opening should be created from one mesenteric edge to the other. - Perform interrupted suturing at the edge of the stoma and the distal third of the abdominal incision.
- Attach the edge of the stoma to the seromuscularity of the proximal ileum wall by reversing the ileum wall. Interrupted suture the edge of the stoma, the seromuscularity of the proximal ileum and the proximal subcuticular layer of the abdominal opening. Create an everted bud.
- Complete the mucocutaneous junction.
- Close the fascia and incision.
- Copiously irrigate all wounds with saline.
- Place a 19-Fr round channel drain beside the anastomotic stoma through the R3 incision.
- Interrupted suture the peritoneum and subcutaneous layer. Intradermal suture the skin layer.
Results
The detailed perioperative information of the case presented in the video is shown in Table 1 and Figure 3. The procedure was performed in April 2019 by the corresponding author using the da Vinci Si Robot system. The estimated blood loss during the operation was 90 mL, and no transfusions were required. Postoperative management adhered to the principles of ERAS. After the first defecation on the 6th day after the operation, we administered a meglumine diatrizoate enema and performed X-ray radiography to determine whether anastomotic leakage occurred. We then removed the drain after confirming no evidence of leakage. The patient did not report any urinary or sexual dysfunction during follow-up.
The pathologic examination of the specimen indicated adenocarcinoma with moderate differentiation (Figure 4). No positive lymph nodes were detected in any of the 19 mesorectal nodes or 18 lateral lymph nodes. The final pathologic stage was T3N0M0. There was no evidence of lymphatic, venous, or perineural invasion. We recommended that the patient receive adjuvant chemotherapy with FOLFOX. Until January 2021, the patient still remained without any evidence of recurrence or metastasis.
At our center, robot-assisted TME and LPLND have been performed in 89 patients. All procedures were successfully completed under robotic assistance without conversion to open surgery. The detailed information is shown in Table 2. The mean operative time was 173.5 min. Postoperative complications developed in 14.6% of the patients. The median number of lymph nodes detected was 32. The total lateral pelvic lymph node metastasis rate reached 22.5%. As of April 2021, there were 3 patients who presented local recurrence in the pelvic lateral wall and anastomotic stoma, with a median follow-up time of 1.9 years. Urinary dysfunction was defined as ≥50 mL of residual urine occurring at the 3rd month after the operation. A total of 74 patients accepted the evaluation, and 5 patients met the criterion. Sexual dysfunction in men was measured using the International Index of Erectile Function, a 5-item version (IIEF-5) questionnaire, and the Female Sexual Function Index (FSFI) questionnaire was used for women. A total of 49 patients accepted the postoperative evaluation. A score of less than 17 on the IIEF-5 questionnaire or less than 28 on the FSFI questionnaire was considered indicative of sexual dysfunction. Two patients reported sexual dysfunction.
Table 1: Representative results. This table shows the detailed baseline clinical characteristics, intraoperative and postoperative outcomes and pathology results of the representative case. BMI: body mass index; ASA: American Society of Anesthesiologists; LPLND: lateral pelvic lymph node dissection; TME: total mesorectal excision. *Here, we counted only complications requiring additional therapeutic intervention. Please click here to download this Table.

Figure 1: Operation setting. This figure has been adapted from Napoli, N., Kauffmann, E. F., Menonna, F., Iacopi, S., Cacace, C., Boggi, U. Robot-Assisted Radical Antegrade Modular Pancreatosplenectomy Including Resection and Reconstruction of the Spleno-Mesenteric Junction. J. Vis. Exp. (155), e60370, doi:10.3791/60370 (2020)11. Please click here to view a larger version of this figure.
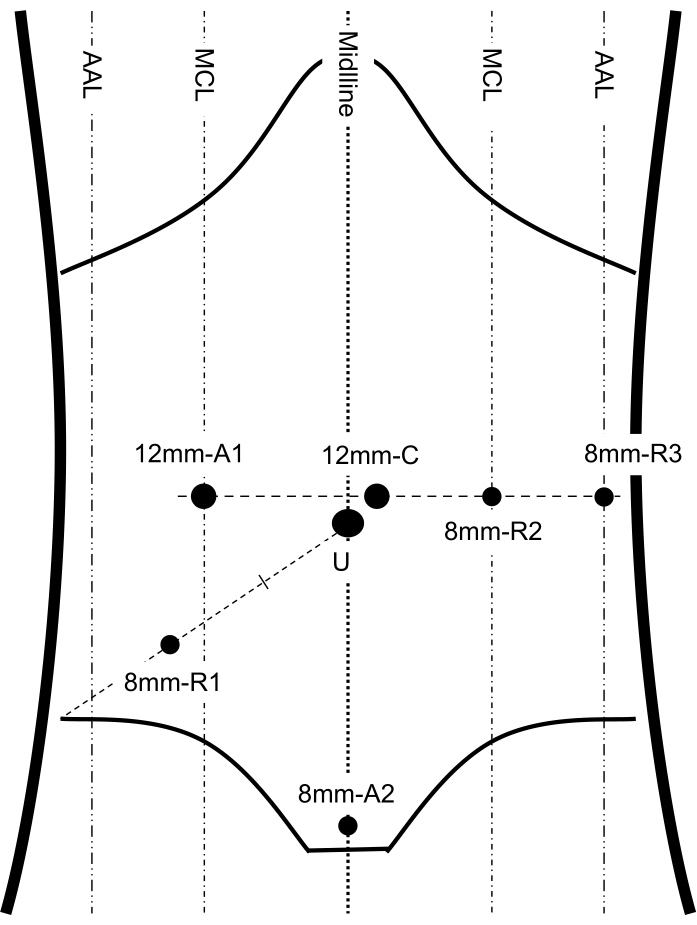
Figure 2: Port placement. This figure shows the important anatomic landmarks of the abdomen and port placement, including 3 robotic arms, 1 camera and 2 assistant ports. MCL: midclavicular line; AAL: anterior axillary line; C: camera port; U: umbilicus; R1, 2, 3: robotic arm 1, 2, 3; A1, 2: assistant port 1, 2. This figure has been modified from Shi F, Li Y, Pan Y, et al. Clinical feasibility and safety of third space robotic and endoscopic cooperative surgery for gastric gastrointestinal stromal tumors dissection: A new surgical technique for treating gastric GISTs. Surg Endosc. 2019;33(12):4192-4200. doi:10.1007/s00464-019-07223-w12. Please click here to view a larger version of this figure.

Figure 3: Robotic TME and LPLND. (A) Dissection of the retrorectal plane was performed between mesorectal fascia and prehypogastric nerve fascia. The yellow dashed line indicates the sacral promontory. (B) Incision along the yellow dashed line to open the anterior plane between Denonvilliers' fascia and the mesorectal fascia. (C) Dissection of the obturator nodes. The yellow dashed line indicates the range of obturator nodes (#283). The blue dashed line indicates the umbilical artery. (D) Dissection of the internal iliac lymph node. The yellow dashed line indicates the range of internal iliac lymph nodes (#263). (E) The LPLND was completed. (F) Whole specimen of resected lateral lymphatic and adipose tissue. MRF: mesorectal fascia; PHNF: prehypogastric nerve fascia. Please click here to view a larger version of this figure.
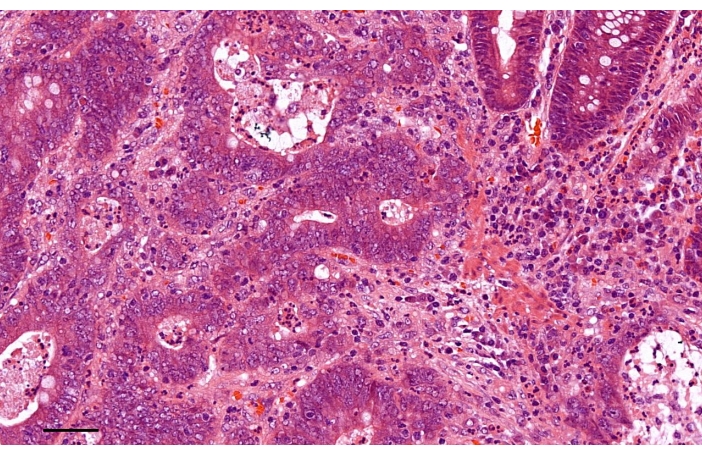
Figure 4: The pathologic examination of the specimen indicated adenocarcinoma with moderate differentiation (Hematoxylin-Eosin staining). Scale bar, 50 µm. Please click here to view a larger version of this figure.
Table 2: Results of 89 consecutive robot-assisted TME and LPLND. This table shows the detailed baseline clinical characteristics, intraoperative and postoperative outcomes and pathology results. BMI: body mass index; ASA: American Society of Anesthesiologists; LPLND: lateral pelvic lymph node dissection; TME: total mesorectal excision. *Here, we counted only complications requiring additional therapeutic intervention. Please click here to download this Table.
Discussion
Colorectal cancer (CRC) is one of the most common cancers worldwide13. Among them, more than a third are rectal cancer. Due to the higher postoperative functional requirement and the sophisticated neuro- and fascial anatomy of the pelvis and perineum, the best surgical approach for rectal cancer, especially low or ultralow rectal cancer, is still under great debate. Since its first report in 1979, total mesorectal excision (TME) has been the standard surgical technique for the treatment of resectable rectal cancer14. With complete excision of the mesorectum, the local recurrence rate decreases significantly. However, this approach is still challenging to perform in low rectal cancer patients, and a high conversion rate and positive resection margins remain concerns15,16. Sylla et al. developed the transanal total mesorectal excision (TaTME) strategy as a novel approach to the surgical treatment of rectal cancer17. Indeed, it has been proposed that TaTME has the advantages of fewer abdominal incisions, better visualization of the mesorectal plane and distal resection margin and better feasibility in the narrow pelvis space15. However, some controversy over long-term oncological outcomes and postoperative quality of life remains. Nationwide data show that TaTME has a higher local recurrence rate than laparoscopic TME18. In addition, due to a long period of intraoperative anal traction, patients who undergo TaTME may endure long-term (over 6 months) anal pain19. This emerging technique may require improvements to the procedure itself, standardized guidelines and structured training programs to be applied widely.
Another technique has emerged and has been increasingly accepted among colorectal surgeons as a popular option. A hospital in Seoul performed and reported the first da Vinci robotic-assisted TME in 200720. Robot-assisted surgical procedures overcome the limitations of the open (limited visual field and narrow operating space) and laparoscopic approaches (reduction in manual dexterity, a counterintuitive motion mode, magnified natural tremors of the hand and flat visuals). Compared with laparoscopic procedures, the da Vinci surgical robot system trades a flat, 2-dimensional misplaced visual that must be obtained through additional personnel for a 3-dimensional high-quality visual field that can display more detailed anatomical structures. In addition, the da Vinci system adopts a multijoint arm with 7 degrees of freedom to perfectly copy the motion of a natural human hand instead of awkward straight "chopstick" motions. Moreover, several ergonomic inventions have greatly reduced natural tremors to ensure the stability of the surgical instruments and to minimize unanticipated injury. However, the loss of tactile sense and force feedback has still not been addressed. Recent systematic reviews and meta-analyses have shown that the robotic TME has a significantly lower conversion to open surgery rate than laparoscopic procedures, although they included patients with a higher body mass index and lower tumor location as well as a higher proportion of patents receiving neoadjuvant therapy, which are all adverse factors for surgical procedures21,22. The long-term oncological outcomes of robotic and laparoscopic procedures are equivalent23. A robotic approach is generally regarded as a reasonable alternative for complicated procedures such as TME and LPLND. However, it should be acknowledged that robotic TME still has several limitations, such as a higher cost for both patients and departments and additional training requirements16.
The standard TME procedure does not include dissection of the lateral pelvic lymph nodes (LPLNs). However, according to the results of previous studies from the Japanese Society for Cancer of the Colon and Rectum (JSCCR), the total metastasis rate of LPLN in patients whose lower tumor border was distal to the peritoneal reflection and whose cancer invaded beyond the muscularis propria was 20.1%9. A multicenter, randomized controlled trial (RCT) for clinical stage II/III lower rectal cancer (JCOG0212) showed that a high-quality TME procedure with LPLN dissection (LPLND) can reduce the local recurrence rate after surgery (12.6% in TME alone vs. 7.4% in TME with LPLND, P=0.024)3. In Western countries, neoadjuvant radiotherapy/chemoradiotherapy (NART/CRT) has become standard treatment for clinical stage II/III rectal cancer rather than LPLND24. However, a recent multicenter study showed that NART/CRT followed by TME alone is not sufficient to prevent local recurrence in rectal cancer patients with enlarged LPLNs. The addition of LPLND can significantly reduce the recurrence rate (19.5% in TME alone versus 5.7% in TME with LPLND, P=0.042)25. Therefore, a standard TME procedure selectively combined with LPLND according to the clinical and imaging features of the patient should be a standard surgical treatment for locally advanced middle-low rectal cancer. However, the major factor limiting the development and wide use of LPLND is the high incidence rates of postoperative urinary and sexual dysfunction. Two meta-analyses reported that compared with TME alone, additional LPLND markedly increased the incidence of urinary dysfunction, while only one meta-analysis reported a higher incidence of sexual dysfunction26,27.
Currently, we use the robotic surgical system to perform TME and additional LPLND. According to our preliminary results, the use of robotic TME and LPLND leads to favorable perioperative outcomes and equivalent medium-term oncological outcomes. As the da Vinci robot system has several characteristics, as we described previously, the system shows inherent advantages in the identification and dissection of important nerves and vessels to possibly reduce the risk of postoperative complications. However, it should be noted that the decision to combine LPLND should depend on clinical features and patient factors. Until now, widely preventive LPLND has not been recommended for patients without any evidence of lateral lymph node metastasis due to its high risk of injury, the low metastasis rate and high rates of postoperative urinary and sexual dysfunction28. In addition, some patient factors should be considered. One question arises: For older rectal cancer patients, are they going to die with cancer or of cancer?29 For older rectal cancer patients, surgical procedures should be more circumspectly decided. In general, older patients have various comorbidities and frailty, leading to higher rates of intra- and postoperative complications. In addition, unlike younger patients, maintaining function and quality of life are more important for older patients rather than achieving optimal oncological benefits. Thus, comprehensive preoperative evaluation of the benefits and risk of harm is absolutely necessary.
Based on our experience, several key points of this technique should be emphasized to ensure a successful procedure. The most important is adequate familiarity with anatomical structures. During the TME phase, the major consideration is how to effectively protect autonomic pelvic nerves. It should be noted that the dissection was performed by a harmonic scalpel instead of electrocautery, which could reduce the risk of thermal injury. In addition, a radical understanding of the fascia and planes surrounding the rectum is needed30. There are three planes to consider when performing dissection and mobilization of the rectum. The first is the classical TME plane between the mesorectal fascia and prehypogastric nerve fascia (posterior and lateral to the rectum) or Denonvilliers' fascia (anterior to the rectum). By developing this plane, the surrounding autonomic nerves can be safeguarded. Outside the TME plane, there is a second plane between the prehypogastric nerve fascia and presacral fascia (posterior) or vesicohypogastric fascia (lateral) and anterior to Denonvilliers' fascia, which carries a higher risk of injury to the pelvic plexus. The third plane is close to the endopelvic fascia and is rarely adopted. In addition, during the LPLND phase, the anatomical structure of the lateral pelvic region is complex, especially the obturator and internal iliac artery regions. We should clearly identify three planes: the lateral wall plane, composed of the psoas and internal obturator muscles; the medial plane, composed of the ureter and the hypogastric nerve fascia and pelvic plexus; and the dorsal plane, composed of the internal iliac vessels and the sciatic nerve. These three planes define the boundaries of dissection. In addition, the vesicohypogastric fascia divides the area into the obturator and internal iliac compartments, with the easily identifiable umbilical artery as its superior border. Selective ligation of the vessels can control bleeding and expose the anatomic structures. However, it should be noted that the obturator nerve and superior vesical artery should be preserved carefully. If the bleeding is not controlled, a quick and safe open conversion should be performed while the assistant temporarily applies pressure. In addition, the R3 arm plays an important role in retraction of the organ and tissue to allow for optimal exposure. An experienced surgeon can properly place the R3 arm to expose the target structures more clearly and ensure accurate dissection.
In conclusion, the robotic TME and LPLND technique is safe and feasible for patients with locally advanced middle-low rectal cancer. This technique enables better exposure of complicated anatomic structures and can reduce unanticipated injury, following the development trend of minimally invasive surgery. An appropriate selection of surgical indications and a radical understanding of anatomic structures are critical factors of successful procedures. In addition, we suggest appropriately individualized adjustments based on the preferences and experiences of individual surgeons.
Disclosures
Nothing to disclose.
Acknowledgements
This project was supported by the National Natural Science Foundation of China (No. 81870380) and the Shaanxi Province Science Foundation (2020ZDLSF01-03 and 2020KWZ-020).
Materials
| Name | Company | Catalog Number | Comments |
| 0 Silk suture | N/A | N/A | Secure the anvil |
| 12mm Trocar | Medtronic (Minneapolis, MN) | NONB12STF | Assistant port 1 |
| 19 Fr drain | N/A | N/A | Pelvic drain |
| 2-0 Silk suture | N/A | N/A | Close skin incisions |
| 2-0 V-Loc sutures | Covidien (Dublin, Ireland) | VLOCL0315 | Barbed Absorable Suture |
| 4-0 PDS | Ethicon (Somerville, NJ) | SXPP1A400 | Synthetic Absorbable Suture |
| 8mm Trocar | Medtronic (Minneapolis, MN) | NONB8STF | Assistant port 2 |
| Bipolar forceps | Intuitive (Sunnyvale, CA) | 470172 | Operation |
| Cadiere grasping forceps | Intuitive (Sunnyvale, CA) | 470049 | Operation |
| Circular stapler | EzisurgMed (Shanghai, China) | CS2535 | Laparoscopic Surgical Stapler |
| Da Vinci Si | Intuitive (Sunnyvale, CA) | N/A | Surgical Robot |
| Da Vinci Xi | Intuitive (Sunnyvale, CA) | N/A | Surgical Robot |
| Hem-o-lok ligation clip | Weck (Morrisville, NC) | 544995 | Ligation of vessel |
| Laparoscopic single use linear cutting stapler | EzisurgMed (Shanghai, China) | U12M45 | Laparoscopic Surgical Stapler |
| Large needle driver | Intuitive (Sunnyvale, CA) | 470006 | Operation |
| Monopolar scissors | Intuitive (Sunnyvale, CA) | 470179 | Operation |
| Ribbon retractor | N/A | N/A | Control movement of rectum |
| Specimen Bags | N/A | N/A | Extract specimen |
| Veress needle | N/A | N/A | Saline drop test |
References
- Fantus, R. J., et al. Facility-level analysis of robot utilization across disciplines in the National Cancer Database. Journal of Robotic Surgery. 13 (2), 293-299 (2019).
- Akiyoshi, T., et al. Results of a japanese nationwide multi-institutional study on lateral pelvic lymph node metastasis in low rectal cancer is it regional or distant disease. Annals of Surgery. 255 (6), 1129-1134 (2012).
- Fujita, S., et al. Mesorectal excision with or without lateral lymph node dissection for clinical stage II/III lower rectal cancer (JCOG0212): A multicenter, randomized controlled, noninferiority trial. Annals of Surgery. 266 (2), 201-207 (2017).
- Yamaguchi, T., Kinugasa, Y., Shiomi, A., Tomioka, H., Kagawa, H. Robotic-assisted laparoscopic versus open lateral lymph node dissection for advanced lower rectal cancer (vol 30, pg 721). Surgical Endoscopy and Other Interventional Techniques. 30 (2), 729 (2016).
- Yamaguchi, T., et al. Oncological outcomes of robotic-assisted laparoscopic versus open lateral lymph node dissection for locally advanced low rectal cancer. Surgical Endoscopy and Other Interventional Techniques. 32 (11), 4498-4505 (2018).
- Gustafsson, U. O., et al. Guidelines for perioperative care in elective colorectal surgery: enhanced recovery after surgery (ERAS((R))) society recommendations: 2018. World Journal of Surgery. 43 (3), 659-695 (2019).
- Brind'Amour, A., et al. Canadian guidelines on the management of colorectal peritoneal metastases. Current Oncology. 27 (6), 621-631 (2020).
- Watanabe, T., et al. Japanese society for cancer of the colon and rectum (JSCCR) guidelines 2016 for the treatment of colorectal cancer. International Journal of Clinical Oncology. 23 (1), 1-34 (2018).
- Professional Committee of Robotic Surgery, C.C.C.o.C.M.D.A. Robotic and Laparoscopic Surgery Committee of Chinese Research Hospital, A. [Chinese expert consensus on robotic surgery for colorectal cancer (2020 edition)]. Zhonghua Wei Chang Wai Ke Za Zhi. 24 (1), 14-22 (2021).
- Napoli, N., et al. Robot-Assisted Radical Antegrade Modular Pancreatosplenectomy Including Resection and Reconstruction of the Spleno-Mesenteric Junction. Journal of Visualized Experiments. (155), e60370 (2020).
- Shi, F., et al. Clinical feasibility and safety of third space robotic and endoscopic cooperative surgery for gastric gastrointestinal stromal tumors dissection : A new surgical technique for treating gastric GISTs. Surgical Endoscopy and Other Interventional Techniques. 33 (12), 4192-4200 (2019).
- Sung, H., et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer Journal for Clinicians. 71 (3), 209-249 (2021).
- Heald, R. J. A new approach to rectal cancer. British Journal of Hospital Medication. 22 (3), 277-281 (1979).
- Jiang, T. Y., Ma, J. J., Zheng, M. H. Controversies and consensus in transanal total mesorectal excision (taTME): Is it a valid choice for rectal cancer. Journal of Surgical Oncology. 123, 59-64 (2021).
- Di Saverio, S., Stupalkowska, W., Hussein, A., Fearnhead, N., Wheeler, J. Laparoscopic ultralow anterior resection with intracorporeal coloanal stapled anastomosis for low rectal cancer - is robotic surgery or transanal total mesorectal excision always needed to achieve a good oncological and sphincter-sparing dissection - a video vignette. Colorectal Diseases. 21 (7), 848-849 (2019).
- Sylla, P., Rattner, D. W., Delgado, S., Lacy, A. M. NOTES transanal rectal cancer resection using transanal endoscopic microsurgery and laparoscopic assistance. Surgical Endoscopy and Other Interventional Techniques. 24 (5), 1205-1210 (2010).
- Larsen, S. G., Pfeffer, F., Korner, H. Norwegian moratorium on transanal total mesorectal excision. British Journal of Surgery. 106 (9), 1120-1121 (2019).
- Koedam, T. W., et al. Transanal total mesorectal excision (TaTME) for rectal cancer: effects on patient-reported quality of life and functional outcome. Techniques in Coloproctology. 21 (1), 25-33 (2017).
- Baik, S. H., et al. Robotic total mesorectal excision for the treatment of rectal cancer. Journal of Robotic Surgery. 1 (1), 99-102 (2007).
- Gavriilidis, P., et al. Robotic vs laparoscopic total mesorectal excision for rectal cancers: has a paradigm change occurred? A systematic review by updated meta-analysis. Colorectal Diseases. 22 (11), 1506-1517 (2020).
- Prete, F. P., et al. Robotic versus laparoscopic minimally invasive surgery for rectal cancer: A Systematic review and meta-analysis of randomized controlled trials. Annals of Surgery. 267 (6), 1034-1046 (2018).
- Qiu, H., et al. Long-term oncological outcomes in robotic versus laparoscopic approach for rectal cancer: A systematic review and meta-analysis. International Journal of Surgery. 80, 225-230 (2020).
- Kapiteijn, E., et al. Preoperative radiotherapy combined with total mesorectal excision for resectable rectal cancer. New England Journal of Medicine. 345 (9), 638-646 (2001).
- Ogura, A., et al. Neoadjuvant (Chemo)radiotherapy with total mesorectal excision only is not sufficient to prevent lateral local recurrence in enlarged nodes: Results of the multicenter lateral node study of patients with low cT3/4 rectal cancer. Journal of Clinical Oncology. 37 (1), 33-43 (2019).
- Emile, S. H., Elfeki, H., Shalaby, M., Sakr, A., Kim, N. K. Outcome of lateral pelvic lymph node dissection with total mesorectal excision in treatment of rectal cancer: A systematic review and meta-analysis. Surgery. 169 (5), 1005-1015 (2021).
- Hajibandeh, S., Hajibandeh, S., Matthews, J., Palmer, L., Maw, A. Meta-analysis of survival and functional outcomes after total mesorectal excision with or without lateral pelvic lymph node dissection in rectal cancer surgery. Surgery. 168 (3), 486-496 (2020).
- Laparoscopic surgery committee of the endoscopist branch in the chinese medical doctor, A. Laparoscopic surgery committee of colorectal cancer committee of chinese medical doctor, A. and Colorectal surgery group of the surgery branch in the chinese medical, A. [Chinese expert consensus on the diagnosis and treatment for lateral lymph node metastasis of rectal cancer (2019 edition)]. Zhonghua Wei Chang Wai Ke Za Zhi. 22 (10), 901-912 (2019).
- Podda, M., et al. Multidisciplinary management of elderly patients with rectal cancer: recommendations from the SICG (Italian Society of Geriatric Surgery), SIFIPAC (Italian Society of Surgical Pathophysiology), SICE (Italian Society of Endoscopic Surgery and new technologies), and the WSES (World Society of Emergency Surgery) International Consensus Project. World Journal of Emergency Surgery. 16 (1), 35 (2021).
- Fung, T. L. D., Tsukada, Y., Ito, M. Essential anatomy for total mesorectal excision and lateral lymph node dissection, in both trans-abdominal and trans-anal perspective. Surgeon. , (2020).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved