Method Article
In Vitro Three-Dimensional Sprouting Assay of Angiogenesis Using Mouse Embryonic Stem Cells for Vascular Disease Modeling and Drug Testing
In This Article
Summary
This assay utilizes mouse embryonic stem cells differentiated into embryoid bodies cultured in 3D-collagen gel to analyze the biological processes that control sprouting angiogenesis in vitro. The technique can be applied for testing drugs, modeling diseases, and for studying specific genes in the context of deletions that are embryonically lethal.
Abstract
Recent advances in induced pluripotent stem cells (iPSC) and gene editing technologies enable the development of novel human cell-based disease models for phenotypic drug discovery (PDD) programs. Although these novel devices could predict the safety and efficacy of investigational drugs in humans more accurately, their development to the clinic still strongly rely on mammalian data, notably the use of mouse disease models. In parallel to human organoid or organ-on-chip disease models, the development of relevant in vitro mouse models is therefore an unmet need for evaluating direct drug efficacy and safety comparisons between species and in vivo and in vitro conditions. Here, a vascular sprouting assay that utilizes mouse embryonic stem cells differentiated into embryoid bodies (EBs) is described. Vascularized EBs cultured onto 3D-collagen gel develop new blood vessels that expand, a process called sprouting angiogenesis. This model recapitulates key features of in vivo sprouting angiogenesis-formation of blood vessels from a pre-existing vascular network-including endothelial tip cell selection, endothelial cell migration and proliferation, cell guidance, tube formation, and mural cell recruitment. It is amenable to screening for drugs and genes modulating angiogenesis and shows similarities with recently described three-dimensional (3D) vascular assays based on human iPSC technologies.
Introduction
In the past three decades, target-based drug discovery (TDD) has been widely employed in drug discovery by the pharmaceutical industry. TDD incorporates a defined molecular target playing an important role in a disease and relies on the development of relatively simple cell culture systems and readouts for drug screening1. Most typical disease models used in TDD programs include traditional cell culture methods such as cancer cells or immortalized cell lines grown within artificial environments and non-physiological substrates. Although many of these models have provided viable tools for identifying successful drug candidates, the use of such systems can be questionable owing to their poor disease relevance2.
For most diseases, the underlying mechanisms are indeed complex and various cell types, independent signaling pathways, and multiple sets of genes are often found to contribute to a specific disease phenotype. This is also true for inherited diseases where the primary cause is a mutation in one single gene. With the recent advent of human induced pluripotent stem cell (iPSC) technologies and gene editing tools, it is now possible to generate 3D organoids and organ-on-chip disease models that could better recapitulate the in vivo human complexity3,4. The development of such technologies is associated with a resurgence in interest in phenotypic drug discovery (PDD) programs1. PDD can be compared to empirical screening, as they do not rely on knowledge of the identity of a specific drug target or a hypothesis about its role in disease. The PDD approach is now increasingly recognized to strongly contribute to the discovery of first-in-class drugs5. Because the development of human organoid and organ-on-chip technologies is still in its infancy, it is expected that iPSC models (complemented with innovative imaging and machine-learning tools6,7) will provide, in the near future, multiple novel complex cell-based disease models for drug screening and associated PDD programs to overcome the poor productivity of the TDD approach8,9.
While human organoid and organ-on-chip models can provide important insights into disease complexity and to the identification of novel drugs, bringing drugs into new clinical practice also strongly relies on data from animal models to assess their efficacy and safety. Among them, genetically modified mice are certainly the most preferred mammalian models. They have many advantages as they have a relatively short generation time for mammals, have many similar phenotypes to human diseases, and can be easily genetically manipulated. They are therefore extensively used in drug discovery programs10. However, bridging the gap between mice and humans remains an important challenge11. The development of in vitro mouse models equivalent to human organoid and organ-on-chip models could at least partially fill this gap as it will allow direct drug efficacy and safety comparisons between in vivo mouse and in vitro human data.
Here, a vascular sprouting assay in mouse embryoid bodies (EBs) is described. Blood vessels are composed of endothelial cells (inner lining of vessel walls), mural cells (vascular smooth muscle cells and pericytes)12. This protocol is based on the differentiation of mouse embryonic stem cells (mESCs) into vascularized EBs using hanging droplets that recapitulate de novo endothelial cell and mural cell differentiation13,14. Mouse ESCs can be easily established in culture from isolated day 3.5 mouse blastocysts having different genetic backgrounds15. They also provide possibilities for clonal analysis, lineage tracing, and can be easily genetically manipulated to generate disease models13,16.
As blood vessels nourish all organs, it is not surprising that many diseases if not all, are associated with changes in the microvasculature. In pathological conditions, endothelial cells can adopt an activated state or can become dysfunctional resulting in mural cell death or migration away from blood vessels. These can result in excessive angiogenesis or in vessel rarefaction, can induce abnormal blood flow and defective blood vessel barrier leading to immune cell extravasation, and inflammation12,17,18,19. Research for the development of drugs modulating blood vessels is, therefore high, and multiple molecular players and concepts have already been identified for therapeutic targeting. In this context, the protocol described is particularly suitable for building disease models and for drug testing as it recapitulates key features of in vivo sprouting angiogenesis, including endothelial tip and stalk cell selection, endothelial cell migration and proliferation, endothelial cell guidance, tube formation, and mural cell recruitment. It also shows similarities with recently described 3D vascular assays based on human iPSC technologies20.
Protocol
1. Media preparation and culture of mESC
- Prepare conditioned medium +/- (CM+/-) using the supplement 1x Glasgow MEM (G-MEM BHK-21) medium with 10% (vol/vol) heat-inactivated Fetal Bovine Serum (FBS), 0.05 mM β-mercaptoethanol, 1x non-essential amino acids (NEAA 1x), 2 mM L-glutamine, and 1 mM sodium pyruvate.
- Prepare conditioned medium +/+ (CM+/+) using the supplement CM+/- medium with Leukemia Inhibitory Factor (LIF) (1,500 U/mL) and 0.1 mM β-mercaptoethanol.
- Prepare conditioned medium +/+ in the presence of two inhibitors (CM+/+ 2i) using the supplement CM+/+ medium with 1 µM PD0325901 and 3 µM CHIR99021.
- Prepare 0.1% gelatin solution by mixing 25 mL of pre-warmed 2% gelatin solution in 500 mL of phosphate-buffered saline without calcium and magnesium (DPBS).
- Prepare 10x Trypsin Versene Phosphate (TVP 10x) buffer by mixing Trypsin (2.5%) with TVP 1x (9.5% 10x DPBS, 1 mM EDTA, 0.01% chicken serum, 0.01% Trypsin (2.5%) in H2O) (1:10 ratio, vol/vol).
NOTE: Filter all the solutions through a 0.22 µm pore filter. Store the culture medium at -20 °C for long term and keep other reagents at 4 °C for up to 3 weeks (see Table of Materials). - Coat two 12-well cell culture plates with 0.1% gelatin solution (500 µL per well) and incubate them for 30 min in a CO2 incubator (37 °C, 5% CO2, humid atmosphere).
- Wash the gelatin-coated plates with PBS and add 500 µL of CM+/- medium.
- Thaw two vials of 1 x 106 cryopreserved irradiated mouse embryonic fibroblasts (MEFs) at 37 °C and transfer the cell suspension to a conical tube with 5 mL of CM+/- medium.
- Centrifuge the cells at 200 x g for 5 min at room temperature (RT). Aspirate the medium and gently resuspend the cell pellet in 12 mL of CM+/- medium at a concentration of 1.67 x 105 cells per mL.
- Seed 500 µL of MEF suspension in a 12-well cell culture plate (2.4 x 105 cells per cm2) and incubate the plate overnight (o/n) in a CO2 incubator.
- Thaw one vial of cryopreserved mESCs (1 x 106) in CM+/+ 2i medium.
- Seed the mESC suspension on a pre-washed 12 well plate with MEFs in 1 mL of CM+/+ 2i medium and transfer the plate to a CO2 incubator. Refresh the medium daily.
- At 70% confluency, wash the mESC colonies with DPBS. Add 150 µL of warm 10x TVP buffer and incubate at RT for 30 s to initiate enzymatic dissociation.
- Carefully remove the TVP buffer, resuspend the cells in 1 mL of CM+/+ 2i medium and dissociate the colonies into single cells by gentle pipetting.
- Passage the cells by transferring them to a new 12-well cell culture plate with MEFs (splitting ratio: 1:3-1:5). Mix gently to distribute the cells and incubate in a CO2 incubator.
- Refresh the culture medium (2-3 mL) and observe the cell growth/morphology daily. Repeat serial passaging at 70% confluency every 2 days. Switch to CM+/+ medium for two passages before initiating cell differentiation.
2. EB formation in hanging drop
- Prepare fresh mesoderm differentiation medium by supplementing the CM+/- medium with basic Fibroblast Growth Factor (bFGF) (50 ng·mL-1) and with Bone Morphogenetic Protein 4 (BMP-4) (5 ng·mL-1) and keep it at 4 °C until use.
- Coat one well of the 6-well cell culture plate with 500 µL of 0.1% gelatin solution and place it in a CO2 incubator for 30 min.
- Wash the gelatin-coated plates with PBS and add 500 µL of CM+/- medium.
- To obtain a pure population of mESCs, trypsinize the cell culture plate with 10x TVP buffer for 30 s at RT, resuspend the cells in 1 mL mesoderm differentiation medium and then transfer them to the gelatin-coated 6-well plate for 30 min allowing the MEFs to attach while the mESCs stay in suspension.
- Collect the cell suspension in a 50 mL conical tube and count the cells using a Neubauer hemocytometer and Trypan blue dye for a live/dead cell exclusion.
- Centrifuge the cells at 200 x g for 5 min at RT. Remove the supernatant and resuspend the cell pellet in mesoderm differentiation medium to reach 4.55 x 104 cells per mL.
- Fill the bottom of 94 mm low attachment polystyrene dishes with 15 mL of sterile water.
NOTE: Four dishes containing hanging drops (1.6 x 105 cells in 3.52 mL of medium) will be required for testing one particular condition using the 3D sprouting angiogenesis assay. - Transfer the cell suspension into a sterile plastic reservoir and load four positions of a multichannel pipette with 22 µL of cell suspension per channel (1 x 103 cells per 22 µL drop).
- Lift and invert the lid of the 94 mm dish and place it on the clean surface of the flow cabinet with the inner side facing upwards.
- Deposit 40 drops of the cell suspension on the inner surface of each lid. Carefully invert the lid without disturbance and place it back on the dish, so that the drops face the water.
- Incubate the dishes in a CO2 incubator. Consider this as differentiation day 0. Maintain the plates for 4 days to form EBs.
3. Competition assay for the tip cell position
- Culture one fluorescent and one non-fluorescent mESC line as previously described13. As an example, 7ACS/EYFP mESCs labeled in yellow and R1 mESCs are used.
- Prepare mosaic EBs by mixing equal amounts of the two mESC lines (1:1 ratio) and incubate the hanging drop dishes in a CO2 incubator as described in step 2.
4. Floating EBs culture for vascular differentiation
- Before collecting EBs from the hanging drops, prepare the following.
- Prepare 5% agar solution in H2O and sterilize it by autoclaving (20 min at 120 °C).
- Use the warm 5% agar solution to prepare G-MEM BHK-21 medium containing 1% agar and quickly pour 3 mL in one of the 60 mm polystyrene dishes. Allow the agar to solidify for 1 h at RT. Store the dishes at 4 °C until use.
- Prepare fresh 2x vascular differentiation medium by supplementing the CM+/- medium with bFGF (100 ng·mL-1) and VEGF-A (50 ng·mL-1). Store the medium at 4 °C until use.
- Collect the hanging drops in a 15 mL conical tube using a P1000 pipette and remove the supernatant after a few minutes of EB sedimentation.
- Resuspend the EBs in 3 mL of 2x vascular differentiation medium, transfer the EB suspension to one agar-coated dishand distribute the EBs homogenously to avoid aggregation.
- Incubate the dishes in CO2 incubator and refresh the medium every 2 days until day 9 using 1x vascular differentiation medium in the presence of bFGF (50 ng·mL-1) and VEGF-A (25 ng·mL-1).
- Alternatively, add Platelet Derived Growth Factor-BB (PDGF-BB) (10 ng·mL-1 on day 4 and 5 ng·mL-1 on day 6 and day 8) to the vascular differentiation medium to promote mural cell differentiation.
5. Flow cytometry analysis
- Collect the 9-day-old EBs in a 15 mL conical tube using a P1000 pipette and wash them once with warm PBS.
- Add 1mL of G-MEM BHK-21 medium containing 0.2 mg·mL-1 of collagenase A and incubate the cells in a CO2 incubator for 5 min.
- Dissociate the EBs by gently pipetting up and down with a P1000 pipette.
- Stop the collagenase activity by adding 1 mL of cold G-MEM BHK-21 medium with 10% FBS.
- Centrifuge the cells at 200 x g for 5 min at RT.
- Resuspend the cells in 500 µL of PBS with 2% FBS.
- Count the cells using a Neubauer hemocytometer.
- Resuspend 400,000 cells in 100 µL of PBS with 2% FBS per staining condition.
- Incubate the cells for 45 min at 4 °C with the following antibodies: APC conjugated rat anti-mouse PECAM-1 antibody (clone MEC13.3) and FITC conjugated rat anti-mouse CD45 (clone 30-F11) or isotype control antibodies.
- Wash the cells twice with 1 mL of PBS containing 2% FBS.
- Resuspend the cells to reach a final concentration of 5 x 106 cells per mL.
- Filter the cells using a round bottom polystyrene test tube, with a cell strainer snap cap.
- Analyze 20,000 PECAM-1 (+) events by flow cytometry.
6. 3D sprouting angiogenesis assay and immunofluorescence staining
- At day 9, prepare a sprouting medium by adding 10% FBS (vol/vol), bFGF (50 ng·mL-1), VEGF-A (25 ng·mL-1), human recombinant Erythropoietin (hEPO) (20 ng·mL-1), human interleukin-6 (IL-6) (10 ng·mL-1), 0.05 mM β-mercaptoethanol, NEAA (1x), L-glutamine (1x), sodium pyruvate (1x), type I rat tail collagen (1.25 mg·mL-1), and NaOH (3.1 mM) to GMEM BHK-1 medium.
- To avoid collagen gelation, maintain the sprouting medium on ice until use.
- To evaluate the effect of pro/anti-angiogenic molecules, add the selected drug or vehicle to the sprouting medium at the selected concentration.
- Collect 9-day-old EBs from one 60 mm agar dish in a 15 mL conical tube (equivalent to one condition) and remove the supernatant after a few minutes of sedimentation.
- Cover the bottom of a 35 mm culture dish with 1 mL of the sprouting medium and incubate at 37 °C for 5 min to induce gelation.
- Resuspend the EBs in 2 mL of cold sprouting medium.
- Transfer the suspension to the 35 mm culture dish coated with the first layer of sprouting medium.
- Distribute the EBs all over the plate and ensure they are at an equal distance from each other. Incubate the dish in a CO2 incubator. The first sprout formation happens within 24-48 h.
- At day 12, the collagen gel containing EBs is carefully transferred to a glass slide (75 x 26 mm) using a spatula.
- Remove the excess liquid using a pipette (P1000) and dehydrate the gel by placing a gauze sheet of nylon linen and absorbent filter cards (gel blotting paper) on top of the gel. Apply pressure by placing a weight (250 g) for 2 min. Carefully remove the nylon/filter papers and allow the slides to air dry for 30 min at RT.
- Wash the slides three times with PBS for 5 min at RT.
- Fix the EBs using zinc solution (see Table of Materials) o/n at 4 °C. Alternatively, fix the mosaic fluorescent EBs with Paraformaldehyde (PFA) (4%) o/n at 4 °C in the dark.
- Remove the fixative. Wash the slides five times with PBS for 5 min at RT.
- Permeabilize the EBs in PBS containing 0.1% Triton-X100 for 15 min at RT.
- Remove the permeabilization solution. Wash the slides five times with PBS for 5 min.
- Incubate the EBs in the blocking buffer (PBS with 2% Bovine Serum Albumin, BSA) for 1 h at RT.
- To stain the endothelial sprouts, use a rat anti-mouse anti-PECAM-1 primary antibody (1:100 dilution) in blocking buffer o/n at 4 °C.
- Wash the slides five times with PBS for 5 min.
- Incubate the slides with goat anti-rat Alexa 555 secondary antibody in blocking buffer (1:250 dilution) and when required, with FITC-conjugated anti-α-SMA antibody in blocking buffer (1:250 dilution) to stain mural cells for 2 h at RT in the dark.
- Wash the slides three times with PBS for 5 min and one time with H20 before mounting them.
7. Confocal imaging, morphometric, and quantitative analysis of EB endothelial sprouts
- Acquire high-resolution images of the immunostained EBs by focal plane merging (z-stacking) using a confocal microscope. Use 10x magnification objective to image whole EBs.
- Analyze the images acquired using ImageJ to evaluate the morphology and quantify the characteristics of PECAM-1 positive endothelial sprouts according to established quantification methods13,14,21.
- Calculate the mean number of endothelial sprouts per EBs by manually counting the total sprout number per individual EBs.
- Measure the individual sprout lengths using the ImageJ drawing tool. Define the base of the endothelial sprout starting at the EB core area and manually draw a line until the sprout tip end.
- Calculate the mean number of tip cells per sprout by manually counting the number of tip cells per individual sprout and then calculate the mean per EB.
- Calculate the filopodia orientation by manually determining the axis of the parent sprout and measure the acute angle between them using the ImageJ software angle tool. Calculate the number of sprouts with an orientation >50° and divide it by the total number of sprouts of the EB of interest.
NOTE: The angles always ranged from 0° to 90°.
- Acquire high-resolution images of the immunostained EBs using a confocal microscope. Use 40x magnification objective to realize high-resolution images of single endothelial sprouts.
- Quantify the vessel coverage of PECAM-1 positive EC sprouts surrounded by α-SMA positive MCs using the ImageJ software.
- Split the merged images into separate red and green channels.
- Convert the images into its binary form.
- Measure the total cellular area of the sprout occupied separately by PECAM-1 (endothelial cells labeled in red) and by α-SMA (mural cells labeled in green) positive cells.
- Generate the merged image using the image calculator function and the AND operator. Measure the total cellular area of the image. To calculate the coverage, divide the area of the colocalized image by the area of the PECAM-1 binary image.
- To analyze the cell competition for endothelial tip/stalk cell position of sprouts developed by mosaic EBs, manually score the number of tip cells and mark their genotypic origin based on the fluorescence signal. Calculate the mean values per EB.
Results
The protocol overview of the blood vessel sprouting assay is shown in Figure 1. Nine-day-old EBs derived from three independent 129/Ola mESC lines (Z/Red, R1, and E14) were enzymatically dissociated into single cells using collagenase A. Cells were stained for PECAM-1 and analyzed by Fluorescence-activated cell sorting (FACS) as described. All the cell lines exhibited robust endothelial differentiation, and no differences in their ability to differentiate into endothelial cells were observed. All the cell lines produced about 10.5% ± 1.3% of endothelial cells (Figure 2A). The relative expression levels of PAN-endotelial cell markers in the PECAM-1 (+) cell populations were also quantified. The mRNA expression levels of all the analyzed markers (Flk1, Flt1, Flt4, Eng, Tie2, and Cdh5) were comparable between the cell lines and the experiments, confirming the robustness of the differentiation protocol (Figure 2B). PECAM-1 (+) cell populations only expressed very low mRNA levels of arterial (Notch1 and Efnb2) or venous (Couptf2 and Ephb4) markers supporting the relatively immature state of the endothelial cells that were generated by the protocol (Figure 2C).
The ability of the vascular sprouting EB model to screen for drugs modulating angiogenesis was then demonstrated (Figure 3). DC101 and DAPT (N-[N-(3,5-difluorophenacetyl)-Lalanyl]-S-phenylglycine t-butylester) were tested. These compounds are widely used in mice to respectively block angiogenesis by inhibiting VEGFR2 activity or to promote endothelial tip cell differentiation and the formation of a dense vascular plexus by targeting Notch signaling (Figure 3A). Both VEGF and Notch signaling pathways are key regulators of sprouting angiogenesis in vivo. The effects of various concentrations of DC101 and DAPT in EBs plated in collagen I were evaluated. High doses of DC101 ranging from 6-30 mg·L-1 inhibited both the number and the length of the vessel sprouts while DAPT had opposite effects at the dose of 1 μmol·L-1 (Figure 3B-C). High magnification pictures of the vessel sprouts stained for PECAM-1 of EBs cultured in the presence of DAPT is also provided (Figure 3D). DAPT even at low doses ranging from 0.5-1 μmol·L-1 strongly increased the number of endothelial tip cells with vessel sprouts that had a misguidance phenotype (Figure 3D-F). High dose of DAPT also led to vessel coalescence and the formation of large and flat endothelial cell areas without organization (Figure 3A). The results confirmed the ability of the model to test drugs that either promote or inhibit angiogenesis.
To confirm that this model is suitable for mimicking vascular diseases, the confocal images of EBs derived from Acvrl1+/- mESCs are provided. Acvrl1 gene encodes for ALK1 (Activin Receptor-like Kinase 1), a receptor specifically expressed in endothelial cells that if mutated is responsible for the development of a vascular rare disease with angiodysplasia named Hereditary Hemorrhagic Telangiectasia (HHT). A high magnification picture of the Acvrl1+/- endothelial sprouts revealed that they had more endothelial tip cells and more branches per sprout that were at random angles relative to the parent vessels. These confirmed in vitro a misguidance phenotype as observed in HHT mice (Figure 4).
By forming chimeric EBs that contain differentiated mESC fluorescently-labeled and mESC of a particular genotype, an alternative protocol to study the process of endothelial tip selection is included (Figure 5A-C). A confocal image of PECAM-1 labeled vessel sprouts identified the genotypic origin of the leading endothelial tip cells (Figure 5B). Mixtures of a wild-type YFP (yellow fluorescent protein) mESC line at 1:1 ratio with one unlabeled mESC wild-type line consistently led to the equal contribution of each population to the leading endothelial tip cells (Figure 5C).
This protocol is also suitable for quantifying mural cell coverage of the vessel sprout. EBs that undergo angiogenesis were fixed and stained for PECAM-1 (endothelial cells, red) and for α-Smooth Muscle Actin (α-SMA) (mural cells, green) (Figure 5D). A high magnification image revealed how one individual vessel sprout was surrounded by mural cells (Figure 5E, left). Binary transformation was performed after color channel separation (Figure F-G) to quantify the ratio of PECAM-1 (+) vessel covered by α-SMA (+) mural cells using the ImageJ software (Figure 5H).

Figure 1: Timeline of the protocol procedures. Please click here to view a larger version of this figure.
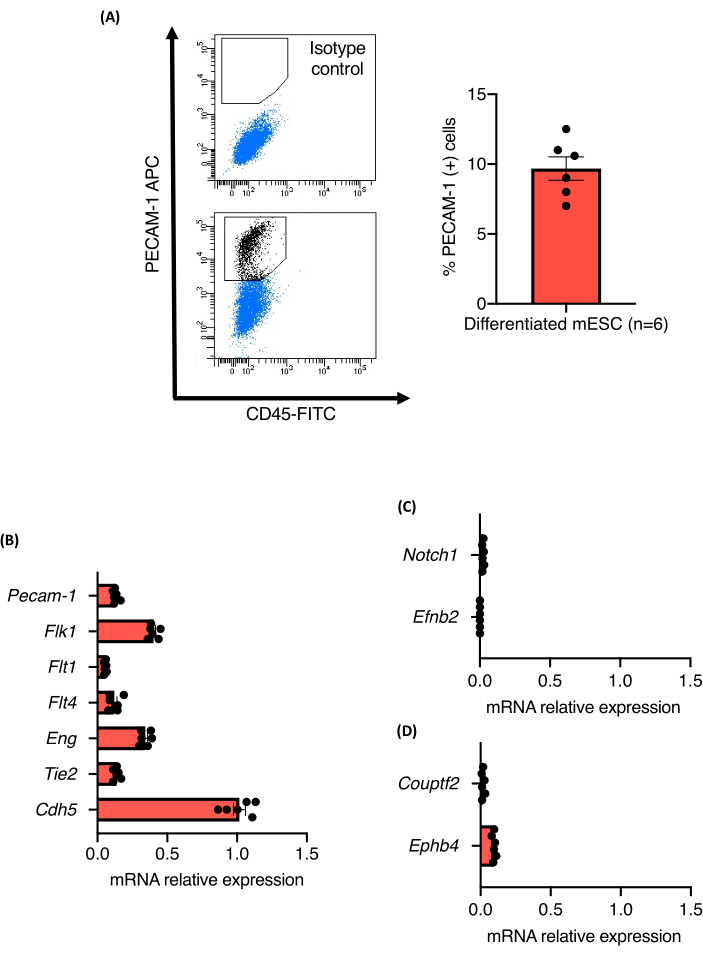
Figure 2: Characterization of ECs derived from mESC vascular differentiation inside EBs. (A) Flow cytometric analysis of CD31 expression from 9-day-old EBs and quantification of the percent of Pecam-1 (+) cells. (B-D) mRNA expression levels of Pecam-1, Flk1, Flt1, Flt4, Eng, Tie2, Cdh5, Notch1, EfnB2, Couptf2, and EphB4 in sorted endothelial cells from 9-day-old EBs. Error bars represent the mean ± standard error of the mean (SEM). Please click here to view a larger version of this figure.
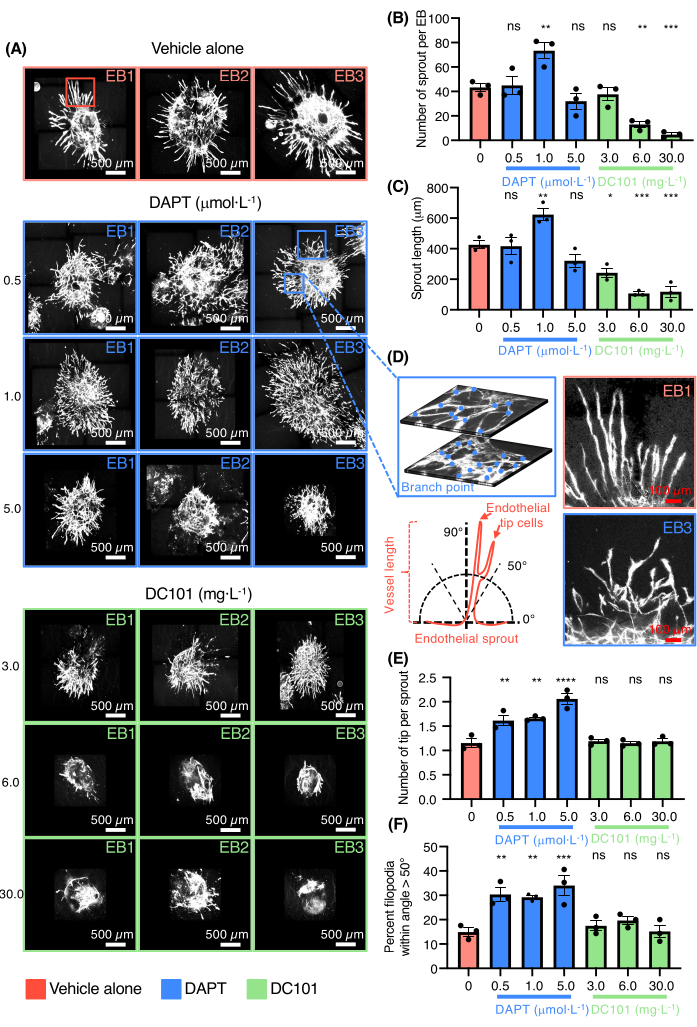
Figure 3: 3D sprouting angiogenesis assay for drug testing. (A) Confocal images of three representative EBs stained for Pecam-1 (white, endothelial cells) treated with vehicle alone, DAPT (0.5 µmol·L-1, 1.0 µmol·L-1, 5.0 µmol·L-1) or DC101 (3 mg·L-1, 6 mg·L-1, 30 mg·L-1). (B) Quantification of the number of sprouts per EB. (C) Quantification of the sprout length. (D) On top-left panel, high magnification of endothelial sprout showing the network complexity and the branch point counting on two different layers from the same EB, on the bottom-left panel a schematic illustration representing the measurement method of endothelial sprout orientation. On the top-right panel, high magnification of endothelial sprouts from the vehicle alone and on the bottom-right panel, high magnification of endothelial sprouts from DAPT (0.5 µmol·L-1) condition. (E) Quantification of the number of tip cells per sprout. (F) Quantification of the percent of filopodia within angle >50°. All bars represent mean ± SEM and p values from unpaired, one-way ANOVA test. ns = non-significant, *p < 0.05, **p < 0.01, ***p < 0.001, and ****p < 0.0001. Please click here to view a larger version of this figure.

Figure 4: Defective vessel sprouting in Hereditary Hemorrhagic Telangiectasia EBs. On the top panel, confocal images of representative 12-day-old EBs from Acvrl1+/+ and Acvrl1+/- genotypes stained for Pecam-1 (white, endothelial cells). On the bottom panel, high magnification of Acvrl1+/- endothelial sprouts (white box from top panel) showing numerous tip cells (red arrows), endothelial branch points (blue dots) and sprouting misguidance phenotype (green arrows). Please click here to view a larger version of this figure.

Figure 5: Studying tip/stalk cell position and vessel maturation using the 3D sprouting assay. (A) Confocal image of a representative 12-day-old chimeric EB made from R1 wild-type cell line mixed 1:1 with 7ACS/EYFP wild-type cell line stained for Pecam-1 (red, endothelial cells). Red arrows indicate tip cells from the R1 cell line and green arrows indicate tip cells from 7ACS/EYFP cell line. (B) High magnification of an endothelial sprout showing the distribution of R1 and 7ACS/EYFP endothelial cell along the sprout. (C) Quantification of the relative genotyping tip cells from representative wild-type EBs. (D) Confocal image of a representative 12-day-old EB stained for Pecam-1 (red, endothelial cells) and α-SMA (green, mural cells). (E) High magnification of an endothelial sprout (white rectangular dotted box from D) showing mural/endothelial cell interaction. (F-G) Images of the colored endothelial sprout and their associated binary transformed images. (H) Binary image of co-localized Pecam-1 and α-SMA staining. Ratio of endothelial cell sprouts covered by mural cells. All bars represent mean ± SEM. Please click here to view a larger version of this figure.
Discussion
This protocol describes an unbiased, robust, and reproducible 3D EB-based vascular sprouting assay that is amenable to screening for drugs and genes modulating angiogenesis. This method offers advantages over many widely used two dimensional (2D) assays using endothelial cell cultures such as Human Umbilical Vein Endothelial Cells (HUVECs) to monitor migration (lateral scratch assay or the Boyden chamber assay)22,23 or proliferation (counting cell number, detection of DNA synthesis, detection of proliferation markers, or metabolic assays)24 in that it uniquely allows the study of both endothelial and mural cell differentiation and their organization into a vascular network mimicking key steps of sprouting angiogenesis. These steps include the endothelial tip cell selection, proliferation of the stalk cells, spatial orientation and migration of the vessel sprout, and the recruitment of mural cells to the nascent blood vessel25. It also offers advantages to many 3D angiogenesis models. The fibrin bead26,27 or collagen gel assay28,29,30 mimicking the tubulogenesis commonly use HUVECs or Endothelial Colony Forming Cells derived Endothelial Cells (ECFC-EC) as they have a high proliferative rate but are not suitable for mouse primary endothelial cells that are difficult to maintain in culture. The ex vivo retina explant31 or vascularized micro-organ assay32 can recapitulate well all the blood vessel formation steps but they have complex experimental procedures and are not suitable for high throughput drug or genetic screening. This is also true for the ex vivo aortic ring assay33,34 and for many in vivo assays such as tumor implanted in mice or loss of function studies in mice that have often high cost and difficulties in obtaining large amount of data35. This protocol also nicely complements similar in vitro angiogenesis assays using human iPSCs allowing comparisons between mouse and human data. Although it is important to note that human iPSC-derived endothelial cells show less ability to sprout than the mouse cells36,37.
The method developed here has also some limitations. It cannot evaluate the effects of fluid flow on blood vessel maturation, vessel permeability and does not produce nascent blood vessel in a specific tissue environment in comparison to recent microfabricated devices that are under development. Indeed, organ-on-chip technologies that combine microfluidics with tissue engineering can provide cultured endothelial cells with a microenvironment similar to that in vivo38,39. Microfluidic systems contain the correct extracellular matrix composition and are designed to produce mechanical signals such as shear stress. Some are designed to incorporate mural cells and other supportive cells of a given tissue or can generate chemical gradients. They contain networks of micron-scale fluid filled channels that are similar in size and in structure to the blood capillaries. The organ-on-chip technologies also enable the quantification of specific vascular functions, including the permeability and the trans endothelial electrical resistance. Although organ-on-chip technologies offer promise, they are as far beyond the research expertise of most biology laboratories, still need proper standardization, and require specialized fabrication techniques. Commercialization of organ-on-chip technology manufacture is only beginning, and these systems are considered cost-and-time prohibitive for pharmaceutical companies at present40,41.
There are several critical steps that should be taken into account. Use high-quality cells which robustly expresses well-accepted markers (Nanog, Oct4, Sox2, and SSEA-1) of the pluripotent state. It is essential to carefully monitor their growth, mES cell shape, and the size and morphology of mES colonies. As karyotypic stability is stochastic in cultured cells, reassessing it after extensive passaging is essential.It is recommended to use products already tested for mESC culture and test MEF feeders, Fetal calf serum, and all chemical compounds for several passages to detect whether mESCs maintain their cellular properties or whether they differentiate or acquire an epiblastic phenotype. The medium should be refreshed daily and mESC colonies should not be allowed to become too large and dense. Finally, mESCs need to be cultured at least for two passages without 2i before differentiation to ensure the best yield of endothelial cells.
The endothelial differentiation includes two important steps: the formation of EBs using the hanging drop method and their culture under floating conditions in the presence of vascular differentiation medium13,14. Movement of the hanging drop dishes should be minimized to achieve uniform cell aggregation. The number of mESCs used to form an aggregate in most cases, ranges between 800-1,000 cells, but it may need to be optimized if mESCs have a different genetic background than 129/ola to ensure an optimal differentiation into vascularized EBs. When cultured under floating conditions, EBs need to be carefully distributed and avoid movement that favors EB clumping.
EBs are finally cultured in collagen I gel to form vessel sprouts. Angiogenic medium should be freshly prepared and once mixed with collagen I must be maintained on ice to avoid spontaneous gelation. In case of drug testing, drugs are added in the cold mixture at the right concentration during this step. Adjusting the pH with NaOH before resuspending the EBs is crucial, otherwise collagen acidity will cause cell toxicity. Finally, EBs should be spread at equidistance from each other to ensure reproducible results.
In conclusion, this method introduces a 3D vascular sprouting assay based on mESC that has the required robustness and scalability to be used for genetic screening as recently described by Elling U. et al. that generated a large haplobank of hemi/homozygous mutant mESC16 and for phenotypic drug discovery program.
Disclosures
The authors have nothing to disclose.
Acknowledgements
This work was supported by grants from the Nederlandse organisatie voor gezondheidsonderzoek en zorginnovatie (ZonMW 446002501), Health Holland (LSHM19057-H040), Leading Fellows Programme Marie Skłodowska-Curie COFUND, and by the Association Maladie de Rendu-Osler (AMRO).
Materials
| Name | Company | Catalog Number | Comments |
| 2-mercaptoethanol | Milipore, Merck | 805740 | Biohazard: adequate safety instructions should be taken when handling |
| Agar Noble | Difco, BD Pharmigen | 214220 | |
| Alexa Fluo 555 goat anti rat IgG | Life technologies | A21434 | |
| APC conjugated rat anti-mouse PECAM-1 antibody (clone MEC13.3) | BD Biosciences | 551262 | |
| APC Rat IgG2a κ Isotype Control (Clone R35-95) | BD Biosciences | 553932 | |
| Axiovert 25 inverted phase contrast tissue culture microscope | ZEISS | ||
| Basic Fibroblast Growth Factor-2 (bFGF) | Peprotech | 450-33 | |
| Benchtop Centrifuge, Allegra X-15R | Beckman Coulter | 392932 | |
| Biosafety cabinet BioVanguard (Green Line) | Telstar | 133H401001 | |
| Bovine Serum Albumin (BSA) | Sigma-Aldrich | A9418 | |
| Cell counting chamber, Buerker, 0.100mm | Marienfeld | 640211 | |
| Cell culture dishes 60 x 15mm | Corning | 353802 | |
| Cell culture dishes, 35 x 10 mm | Corning | 353801 | |
| Cell culture plates 12-well | Corning | 3512 | |
| CFX96 Touch Real-Time PCR Detection System | Biorad | 1855196 | |
| Chicken serum | Sigma-Aldrich | C5405 | |
| CHIR-99021 (CT99021) HCl | Selleckchem | S2924 | |
| Collagen I, High Concentration, Rat Tail, 100mg | Corning | 354249 | |
| Collagenase A | Roche | 10103586001 | |
| Confocal Laser Scanning Microscope, TCS SP5 | Leica | ||
| Cover glasses, 24 × 50 mm | Vwr | 631-0146 | |
| DAPT γ?secretase inhibitor | Sigma Aldrich | D5942 | |
| DC101 anti mouse VEGFR-2 Clone | BioXcell | BP0060 | |
| DC101 isotype rat IgG1 | BioXcell | BP0290 | |
| Dimethyl sulfoxide (DMSO) | Sigma-Aldrich | D2438-5X | Biohazard: adequate safety instructions should be taken when handling |
| DPBS (10x), no calcium, no magnesium | Gibco, Thermofisher scientific | 14200067 | |
| EDTA 40 mM | Gibco, Thermofisher scientific | 15575-038 | |
| Embryonic stem-cell Fetal Bovine Serum | Gibco, Thermofisher scientific | 16141-079 | Should be lot-tested for maximum ES cell viability and growth. Heat inactivate at 60°C and store at −20 °C for up to 1 year |
| Eppendorf Microcentrifuge 5415R | Eppendorf AG | Z605212 | |
| Erythropoietin, human (hEPO), 250 U (2.5 µg) (1 mL) | Roche | 11120166001 | |
| ESGRO Recombinant Mouse LIF Protein (10? units 1 mL) | Milipore, Merck | ESG1107 | |
| Falcon tubes 15 mL | Greiner Bio-One | 188271 | |
| Falcon tubes 50 mL | Greiner Bio-0ne | 227270 | |
| Filter tip ,clear ,sterile F.Gilson, P-200 | Greiner Bio-One | 739288 | |
| Filter tip ,clear ,sterile F.Gilson, P10 | Greiner Bio-One | 771288 | |
| Filter tip ,clear ,sterile F.Gilson, P1000 | Greiner Bio-One | 740288 | |
| FITC conjugated anti-α Smooth Muscle Actin (SMA) (clone 1A4) | Sigma Aldrich | F3777 | |
| FITC conjugated rat anti-mouse CD45 (clone 30-F11) | Biolegend | 103107 | |
| FITC Rat IgG2b, κ Isotype Ctrl Antibody (clone RTK4530) | Biolegend | 400605 | |
| Fluorscent mounting media | DAKO | S3023 | |
| Gascompress | Cutisoft | 45846 | |
| Gauze Cutisoft 10 x 10 cm | Bsn Medical | 45844_00 | |
| Gel blotting paper, Grade GB003 | Whatman | WHA10547922 | |
| Gelatin solution, type B | Sigma-Aldrich | G1393-100 ml | |
| Glasgow's MEM (GMEM) | Gibco, Thermofisher scientific | 21710082 | |
| IHC Zinc Fixative | BD Pharmigen | 550523 | |
| IncuSafe CO2 Incubator | PHCBi | MCO-170AICUV-PE | |
| Interleukin-6, human (hIL-6) | Roche | 11138600001 | |
| L-Glutamine 200 mM | Gibco, Thermofisher scientific | 25030-024 | |
| MEM Non-Essential Amino Acids Solution (100x) | Gibco, Thermofisher scientific | 11140035 | |
| Microscope slide box | Kartell Labware | 278 | |
| Microscope slide, Starfrost | Knittel glass | VS113711FKB.0 | |
| Mm_Cdh5_1_SG QuantiTect Primer Assay | Qiagen | QT00110467 | |
| Mm_Eng_1_SG QuantiTect Primer Assay | Qiagen | QT00148981 | |
| Mm_Epha4_1_SG QuantiTect Primer Assay | Qiagen | QT00093576 | |
| Mm_Ephb2_1_SG QuantiTect Primer Assay | Qiagen | QT00154014 | |
| Mm_Flt1_1_SG QuantiTect Primer Assay | Qiagen | QT00096292 | |
| Mm_Flt4_1_SG QuantiTect Primer Assay | Qiagen | QT00099064 | |
| Mm_Gapdh_3_SG QuantiTect Primer Assay | Qiagen | QT01658692 | |
| Mm_Kdr_1_SG QuantiTect Primer Assay | Qiagen | QT00097020 | |
| Mm_Notch1_1_SG QuantiTect Primer | Qiagen | QT00156982 | |
| Mm_Nr2f2_1_SG QuantiTect Primer Assay | Qiagen | QT00153104 | |
| Mm_Pecam1_1_SG QuantiTect Primer | Qiagen | QT01052044 | |
| Mm_Tek_1_SG QuantiTect Primer Assay | Qiagen | QT00114576 | |
| Mouse (ICR) Inactivated Embryonic Fibroblasts (2 M) | Gibco, Thermofisher scientific | A24903 | Store vials in liquid nitrogen (195.79 °C) indefinitely |
| Mouse embryonic stem cell line 7AC5/EYFP (ATCC SCRC-1033) | ATCC | SCRC-1033 | Generated by Dr A Nagy, Samuel Lunenfeld Research Institute, Mount Sinai Hospital, 600 University Ave, Toronto, Ontario, M5G 1X5, Canada. [Hadjantonakis, A. K., et al. Mechanisms of Development. 76 (1–2), 79–90 (1998)]. |
| Mouse embryonic stem cell lines Acvrl1 +/- and Acvrl1 +/+ | Generated at Leiden University Medical Centre [Thalgott, J.H. et al. Circulation. 138 (23), 2698–2712 (2018)]. | ||
| Mouse embryonic stem cells line E14 | Provided by M Letarte laboratory and generated according to Cho, S. K., et al. Blood. 98 (13), 3635–3642 (2001). | ||
| Mouse embryonic stem cells line R1 (ATCC SCRC-1011) | ATCC | SCRC-1011 | Generated by Dr A Nagy, Samuel Lunenfeld Research Institute, Mount Sinai Hospital, 600 University Ave, Toronto, Ontario, M5G 1X5, Canada. [Nagy, A., et al. Procedings of the National Academy of Sciences of the United States of America. 90 (18), 8424–8428 (1993)]. |
| Mouse embryonic stem cells line Z/Red (strain 129/Ola) | Generated by Dr A Nagy, Samuel Lunenfeld Research Institute, Mount Sinai Hospital, 600 University Ave, Toronto, Ontario, M5G 1X5, Canada [Vintersten, K., et al. Genesis. 40 (4), 241–246 (2004)]. | ||
| NanoDrop 1000 UV/VIS Spectrophotometer | Thermo Fischer Scientific | ND-1000 | |
| PD0325901 | Selleckchem | S1036 | |
| PDGF-BB, Recombinant Human | Peprotech | 100-14B | |
| Pecam-1 antibody, Rat Anti-Mouse | BD Biosciences | 550274 | |
| Penicillin-streptomycin (10,000 U/mL) | Gibco, Thermofisher scientific | 15140122 | |
| Petri dish, PS, 94/16 mm, standard ,with vents, sterile | Greiner Bio-One | 633181 | |
| Pipetboy acu 2 | Integra-Biosciences | 155 019 | |
| Pipetman G Multichannel P8 x 200G | Gilson | F144072 | |
| Pipetman G Starter Kit, 4 Pipette Kit, P2G, P20G, P200G, P1000G | Gilson | F167360 | |
| Recombinant Human BMP-4 Protein | R&D Systems | 314-BP | |
| RNeasy Plus mini Kit | QIAGEN | 74134 | |
| Serological pipettes, 10 mL | Greiner Bio-One | 607 180 | |
| Serological pipettes, 25 mL | Greiner Bio-One | 760 180 | |
| Serological pipettes, 5 mL | Greiner Bio-One | 606 180 | |
| Sodium hydroxide (NaOH) | Merck | 106498 | |
| Sodium pyruvate 100 mM | Gibco, Thermofisher scientific | 11360039 | |
| Test tubes 5ml round-bottom with cell-strainer cap | Corning | 352235 | |
| Thermal cycler, T100 | Biorad | 1861096 | |
| Triton X-100 (BioXtra) | Sigma Aldrich | T9284 | |
| Trypan Blue Solution, 0.4% | Gibco, Thermofisher scientific | 15250061 | |
| Trypsin (2.5%) | Gibco, Thermofisher scientific | 15090046 | |
| Vacuum Filter/Storage Bottle System, 500 mL | Corning | 430758 | |
| VEGFA165 , recombinant murine | Peprotech | 450-32 | |
| Water, Sterile | Fresenius-Kabi | B230531 | |
| Waterbath, Lab-Line Digital | Thermo Fischer Scientific | 18052A |
References
- Moffat, J. G., Vincent, F., Lee, J. A., Eder, J., Prunotto, M. Opportunities and challenges in phenotypic drug discovery: an industry perspective. Nature Reviews Drug Discovery. 16 (8), 531-543 (2017).
- Horvath, P., et al. Screening out irrelevant cell-based models of disease. Nature Reviews. Drug Discovery. 15 (11), 751-769 (2016).
- Low, L. A., Mummery, C., Berridge, B. R., Austin, C. P., Tagle, D. A. Organs-on-chips: into the next decade. Nature Reviews. Drug Discovery. , (2020).
- Ma, C., Peng, Y., Li, H., Chen, W. Organ-on-a-Chip: A new paradigm for drug development. Trends in Pharmacological Sciences. 42 (2), 119-133 (2021).
- Swinney, D. C., Anthony, J. How were new medicines discovered. Nature Reviews Drug Discovery. 10 (7), 507-519 (2011).
- Hussain, S., et al. High-content image generation for drug discovery using generative adversarial networks. Neural Networks: The Official Journal of the INternational Neural Network Society. 132, 353-363 (2020).
- Scheeder, C., Heigwer, F., Boutros, M. Machine learning and image-based profiling in drug discovery. Current Opinion in Systems Biology. 10, 43-52 (2018).
- Wagner, B. K., Schreiber, S. L. The power of sophisticated phenotypic screening and modern mechanism-of-action methods. Cell Chemical Biology. 23 (1), 3-9 (2016).
- Scannell, J. W., Bosley, J. When quality beats quantity: Decision theory, drug discovery, and the reproducibility crisis. PLoS One. 11 (2), 0147215 (2016).
- Webster, J. D., Santagostino, S. F., Foreman, O. Applications and considerations for the use of genetically engineered mouse models in drug development. Cell and Tissue Research. 380 (2), 325-340 (2020).
- Howland, D. S., Munoz-Sanjuan, I. Mind the gap: models in multiple species needed for therapeutic development in Huntington's disease. Movement Disorders: Official Journal of the Movement Disorder Scoiety. 29 (11), 1397-1403 (2014).
- Galaris, G., Thalgott, J. H., Lebrin, F. P. G. Pericytes in hereditary hemorrhagic telangiectasia. Advances in Experimental Medicine and Biology. 1147, 215-246 (2019).
- Thalgott, J. H., et al. Decreased expression of vascular endothelial growth factor receptor 1 contributes to the pathogenesis of hereditary hemorrhagic telangiectasia type 2. Circulation. 138 (23), 2698-2712 (2018).
- Lebrin, F., et al. Thalidomide stimulates vessel maturation and reduces epistaxis in individuals with hereditary hemorrhagic telangiectasia. Nature Medicine. 16 (4), 420-428 (2010).
- Czechanski, A., et al. Derivation and characterization of mouse embryonic stem cells from permissive and nonpermissive strains. Nature Protocols. 9 (3), 559-574 (2014).
- Elling, U., et al. A reversible haploid mouse embryonic stem cell biobank resource for functional genomics. Nature. 550 (7674), 114-118 (2017).
- Cheng, J., et al. Targeting pericytes for therapeutic approaches to neurological disorders. Acta Neuropathologica. 136 (4), 507-523 (2018).
- Chade, A. R. Small vessels, big role: Renal microcirculation and progression of renal injury. Hypertension. 69 (4), 551-563 (2017).
- Jourde-Chiche, N., et al. Endothelium structure and function in kidney health and disease. Nature Reviews. Nephrology. 15 (2), 87-108 (2019).
- van Duinen, V., et al. Standardized and scalable assay to study perfused 3D angiogenic sprouting of iPSC-derived endothelial cells in vitro. Journal of Visualized Experiment: JoVE. (153), e59678 (2019).
- Chappell, J. C., Taylor, S. M., Ferrara, N., Bautch, V. L. Local guidance of emerging vessel sprouts requires soluble Flt-1. Developmental Cell. 17 (3), 377-386 (2009).
- Sato, Y., Rifkin, D. B. Inhibition of endothelial cell movement by pericytes and smooth muscle cells: activation of a latent transforming growth factor-beta 1-like molecule by plasmin during co-culture. Journal of Cell Biology. 109 (1), 309-315 (1989).
- Tchaikovski, V., Olieslagers, S., Bohmer, F. D., Waltenberger, J. Diabetes mellitus activates signal transduction pathways resulting in vascular endothelial growth factor resistance of human monocytes. Circulation. 120 (2), 150-159 (2009).
- Staton, C. A., Reed, M. W., Brown, N. J. A critical analysis of current in vitro and in vivo angiogenesis assays. International Journal of Experimental Pathology. 90 (3), 195-221 (2009).
- Herbert, S. P., Stainier, D. Y. Molecular control of endothelial cell behaviour during blood vessel morphogenesis. Nature Reviews Molecular Cell Biology. 12 (9), 551-564 (2011).
- Nakatsu, M. N., Hughes, C. C. An optimized three-dimensional in vitro model for the analysis of angiogenesis. Methods in Enzymology. 443, 65-82 (2008).
- Nakatsu, M. N., Davis, J., Hughes, C. C. Optimized fibrin gel bead assay for the study of angiogenesis. Journal of Visualized Experiments: JoVE. (186), (2007).
- Gau, D., et al. Pharmacological intervention of MKL/SRF signaling by CCG-1423 impedes endothelial cell migration and angiogenesis. Angiogenesis. 20 (4), 663-672 (2017).
- Torres-Estay, V., et al. Androgens modulate male-derived endothelial cell homeostasis using androgen receptor-dependent and receptor-independent mechanisms. Angiogenesis. 20 (1), 25-38 (2017).
- Merjaneh, M., et al. Pro-angiogenic capacities of microvesicles produced by skin wound myofibroblasts. Angiogenesis. 20 (3), 385-398 (2017).
- Rezzola, S., et al. In vitro and ex vivo retina angiogenesis assays. Angiogenesis. 17 (3), 429-442 (2014).
- Wang, X., Phan, D. T. T., George, S. C., Hughes, C. C. W., Lee, A. P. 3D Anastomosed microvascular network model with living capillary networks and endothelial cell-lined microfluidic channels. Methods in Molecular Biology. 1612, 325-344 (2017).
- Nicosia, R. F., Ottinetti, A. Growth of microvessels in serum-free matrix culture of rat aorta. A quantitative assay of angiogenesis in vitro. Laboratory Investigation; A Journal of Technical Methods and Pathology. 63 (1), 115-122 (1990).
- Nicosia, R. F. The aortic ring model of angiogenesis: a quarter century of search and discovery. Journal of Cellular and Molecular Medicine. 13 (10), 4113-4136 (2009).
- Nowak-Sliwinska, P., et al. Consensus guidelines for the use and interpretation of angiogenesis assays. Angiogenesis. 21 (3), 425 (2018).
- Belair, D. G., Schwartz, M. P., Knudsen, T., Murphy, W. L. Human iPSC-derived endothelial cell sprouting assay in synthetic hydrogel arrays. Acta Biomaterialia. 39, 12-24 (2016).
- Bezenah, J. R., Kong, Y. P., Putnam, A. J. Evaluating the potential of endothelial cells derived from human induced pluripotent stem cells to form microvascular networks in 3D cultures. Scientific Reports. 8 (1), 2671 (2018).
- Henderson, A. R., Choi, H., Lee, E. Blood and lymphatic vasculatures on-chip platforms and their applications for organ-specific in vitro modeling. Micromachines (Basel). 11 (2), 147 (2020).
- Lin, D. S. Y., Guo, F., Zhang, B. Modeling organ-specific vasculature with organ-on-a-chip devices. Nanotechnology. 30 (2), 024002 (2019).
- Pollet, A., den Toonder, J. M. J. Recapitulating the vasculature using organ-on-chip technology. Bioengineering. 7 (1), 17 (2020).
- Cochrane, A., et al. Advanced in vitro models of vascular biology: Human induced pluripotent stem cells and organ-on-chip technology. Advanced Drug Delivery Reviews. 140, 68-77 (2019).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved