Method Article
Organotypic Slice Cultures as Preclinical Models of Tumor Microenvironment in Primary Pancreatic Cancer and Metastasis
In This Article
Summary
This protocol describes the preparation of organotypic slice cultures (OTSCs). This technique facilitates the ex vivo cultivation of intact multicellular tissue. OTSCs can be used immediately to test for their respective response to drugs in a multicellular environment.
Abstract
Realistic preclinical models of primary pancreatic cancer and metastasis are urgently needed to test the therapy response ex vivo and facilitate personalized patient treatment. However, the absence of tumor-specific microenvironment in currently used models, e.g., patient-derived cell lines and xenografts, only allows limited predictive insights. Organotypic slice cultures (OTSCs) comprise intact multicellular tissue, which can be rapidly used for the spatially resolved drug response testing.
This protocol describes the generation and cultivation of viable tumor slices of pancreatic cancer and its metastasis. Briefly, tissue is casted in low melt agarose and stored in cold isotonic buffer. Next, tissue slices of 300 µm thickness are generated with a vibratome. After preparation, slices are cultured at an air-liquid interface using cell culture inserts and an appropriate cultivation medium. During cultivation, changes in cell differentiation and viability can be monitored. Additionally, this technique enables the application of treatment to viable human tumor tissue ex vivo and subsequent downstream analyses, such as transcriptome and proteome profiling.
OTSCs provide a unique opportunity to test the individual treatment response ex vivo and identify individual transcriptomic and proteomic profiles associated with the respective response of distinct slices of a tumor. OTSCs can be further explored to identify therapeutic strategies to personalize treatment of primary pancreatic cancer and metastasis.
Introduction
Existing preclinical models of pancreatic ductal adenocarcinoma (PDAC) and respective metastases are poor predictors of response to treatment in patients which is a major drawback in drug development and the identification of predictive biomarkers1. Although models such as patient-derived organoids and patient-derived xenografts are promising, their use remains limited2. Major limitations of these in vitro models are the lack of the tumor microenvironment and xenografting in non-human immunocompromised species. Especially in PDAC and its metastases, the tumor microenvironment has considerably gained interest over the last years because of its crucial functions in tumor biology. It comprises cellular and acellular components, such as (myo-)fibroblasts, pancreatic stellate cells, immune cells, blood vessels, extracellular matrix, cytokines, and growth factors3. This microenvironment is not a non-functional tumor component, but induces tumor progression and metastasis and seems to contribute substantially to radio- and chemotherapy resistance4. The PDAC microenvironment not only mechanically compromises drug delivery, but also possesses immune and drug-scavenging activity5,6,7. Thus, preclinical models which reflect the complex interaction of tumor cells and the tumor microenvironment are urgently needed to adequately test patients' treatment response ex vivo and guide individualized clinical treatment.
Ex vivo cultures of fresh tumor samples represent a close approximation of the tumor in situ. Organotypic slice cultures (OTSCs) have been recently developed and studied for several tumors, such as head, neck, breast, prostate, lung, colon, and pancreatic cancers8,9,10,11,12. It has been shown that OTSCs maintain their baseline morphology, proliferative activity, and microenvironment during the cultivation for a defined, tissue-dependent period11,12,13. OTSCs of PDACs maintained their viability, morphology, and most components of their tumor microenvironment for 4-9 days in several in vitro studies5,12,14. Perspectively, this technique enables an immediate application of the treatment to viable human tumor tissue ex vivo and subsequent downstream analyses, such as profiling of the transcriptome and proteome.
The establishment of OTSCs provides a unique opportunity to test the treatment response ex vivo promptly after surgery. Thus, OTSCs will prospectively allow to identify therapeutic strategies to personalize treatment of metastatic disease. This protocol describes the generation and cultivation of viable OTSCs of pancreatic cancer.
Protocol
Tissue specimens were collected and processed after approval by the local ethics committee of the University of Lübeck (# 16-281).
1. Fresh tissue collection and handling
NOTE: Every unfixed human tissue specimen should be handled with caution to prevent the risk of infection from blood-borne pathogens. All patients should be tested to be negative for HIV, HBV, and HCV prior to tissue processing. Wear a protective coat and handle human tissue specimens with gloves.
- Collect fresh, unfixed, and unfrozen PDAC tissue specimen with a minimum size of 0.4 x 0.4 cm immediately after surgery and transport the specimen to the laboratory in a tissue storage solution.
- When possible, process the fresh tissue immediately.
- Alternatively, store tissues in tissue storage solution on wet ice at 4 °C overnight. However, tissue storage might result in impaired viability and should generally be avoided.
2. Preparation
- Low melting agarose preparation
- Prepare 100 mL of low-melting agarose (8%) by dissolving 8 g of agarose in 100 mL of prewarmed Ringer's solution and store it at 4 °C until needed.
- Upon the announcement of tumor resection, melt the agarose in a microwave.
- Place the agarose in a pre-heated water bath (37 °C) allowing it to cool down to physiological temperatures prior to the preparation.
- Vibratome setup
- Place a razor blade into the holder of the vibratome and perform an automated angle adjustment according to the manufacturer's instructions, if applicable.
- Cool down the jacket of the cutting chamber using a cooling unit or wet ice.
- Fill the cutting chamber with approximately 100 mL of a physiological cutting solution (e.g., Ringer's solution).
- Place the mounted razor blade into the pre-chilled cutting solution, allowing the razor blade to cool down.
3. Tissue embedding in low-melting agarose
- Wash the tissue specimen with cooled (4 °C) PBS and place the tissue in PBS onto a large (~14 cm) Petri dish on ice.
- Remove macroscopically visible excess connective tissue on ice using a scalpel since it might impede the cutting efficiency.
- Place the tissue into a small Petri dish (~3 cm).
- Adjust the tissue orientation so that remaining macroscopically visible connective tissue has the same orientation as the plane of the bottom of the Petri dish. The bottom of the Petri dish has the same orientation as the cutting plane.
- Pour the prepared low-melting agarose into the small Petri dish.
- Readjust the orientation of the tissue, if needed, using forceps.
- Place the Petri dish on wet ice for faster hardening of the agarose.
- Carefully cut the tissue using a scalpel, leaving at least 5 mm surrounding agarose on each side of the tissue.
- Carefully transfer the embedded tissue and glue it on the sample holder using super glue.
- After a few seconds, place the sample holder into the cutting chamber.
- Adjust the orientation of the tissue toward the razor blade, if needed.
4. Slicing of the agarose-embedded tissue using a vibratome
- Define the outer limits of the cutting range (y axis) according to the size of the tissue specimen.
- Adjust the blade toward the top of the tissue block.
- Set cutting speed to 0.04 mm/s, cutting amplitude to 1 mm and slice thickness to 300 µm.
- Carefully cut the first slices and transfer the slices to a separate container with prechilled (4 °C) cutting solution on wet ice.
5. Culture of organotypic slice cultures
- Prepare a 6-well plate with 1 mL of the appropriate cultivation medium per well.
- Medium A: Advanced DMEM/F12, 10% FBS, 1% Penicillin/Streptomycin.
- Medium B: RPMI 1640, 10% FBS, 1% Penicillin/Streptomycin, 4 µg/mL Insulin, 8 ng/mL EGF, 0.3 µg/mL hydrocortisone.
NOTE: The medium for optimal culture conditions might vary depending on the tissue and the patient. Two distinct tissue culture media were compared, one based on DMEM/F12 (medium A), the second based on RPMI (medium B). No substantial differences were detected between these media. For all experiments shown in this protocol, medium A was used.
- Place the 6-well plate with the medium into an incubator, allowing temperature and pH to adjust prior to cultivation.
- Place slices onto cell culture inserts (e.g., hydrophilic PTFE inserts with 0.4 µm pore size) using a gaze filter.
- Remove any excess cutting solution by placing the loaded filter onto a sterile cloth.
- Place the loaded filter into the prepared 6-well plate. Do not add any additional medium to the insert.
- Place the 6-well plate in an incubator (37 °C, 5% CO2).
- Change the medium every 2 days by repeating steps 5.1, 5.2, and 5.5 with a new 6-well plate.
NOTE: Organotypic slice cultures can be cultured for various periods depending on the individual research question.
6. Resazurin viability assay
NOTE: The resazurin viability assay measures general metabolic activity of the organotypic slice cultures based on the reduction of non-fluorescent blue resazurin to red fluorescent resorufin in living cells15. The assay has no toxic effects on cells and can be applied to the cultures repeatedly depending on the individual research question. Viability was measured using the resazurin assay every 2-3 days.
- Preparation of resazurin stock solution
- Turn off the light of the sterile hood, since resazurin stock solution is light sensitive.
- Prepare a stock solution with 10 mg/mL of resazurin sodium salt in 1x PBS.
- Store the stock solution in light protected aliquots at 4 °C in the fridge until use.
- Assessment of overall slice viability
- Turn off the light of the sterile hood, since resazurin stock solution is light sensitive.
- Dilute the resazurin stock solution 1:250 with an appropriate medium.
NOTE: The medium used for dilution should be the same as used for cultivation (medium A or medium B). - Prepare 1 mL of the final resazurin solution per slice and add an additional 1 mL for the blank control, e.g., for 6 slices dilute 28 µL of resazurin stock solution in 7 mL of medium.
- Dispense the resazurin solution in 6-well plates, with 1 mL of the diluted resazurin solution per well.
- Transfer the cultivation filters with the slices into the wells with the resazurin solution. One well with the resazurin solution is kept empty as blank control.
NOTE: To simplify the experimental procedure, this step can be combined with changing the medium (step 5.7). However, in case additional viability measurements are needed, the assay can be done anytime during the cultivation of the slice cultures. - Place the tissue slices in an incubator for 1 h at 37 °C and 5% CO2.
- Prepare new 6-well plates with the culture medium if slice culture is continued (see steps 5.1 and 5.2).
- Remove the cultivation filters with the slices from the resazurin solution and remove excess solution by placing the loaded filter onto a sterile cloth.
- Transfer the cultivation filters with the slices onto the previously prepared culture plate.
- From each 6-well, take 100 µL of resazurin solution and transfer it onto a 96-well plate. From blank control, place three samples (3 x 100 µL) in separate wells of the 96-well plate.
- Quantify the extinction with a plate photometer according to the manufacturer's instructions. Excitation wavelength is set to 545 nm and emission wavelength is set to 600 nm.
7. Formalin fixation and paraffin embedding of OTSCs
- Cautiously transfer the cultivated tissue slices to a plastic embedding cassette. To do so, follow the steps below.
- Place the cultivation filter with the mounted slice on a Petri dish.
- With a scalpel, carefully cut out the filter membrane with the mounted tissue slice.
- Carefully transfer the filter membrane with the mounted slice into a biopsy nylon bag and place it in an embedding cassette.
- Subsequently transfer plastic embedding cassettes in a container with pre-chilled (4 °C) 4.5% formalin. Slices can be kept in formalin solution at 4 °C until further use but should be incubated for at least 24 h.
NOTE: OTSCs need to be transferred with great caution as they tear apart easily.
- Cautiously rinse the formalin-fixed slice culture with running tap water for 1.5 h.
- Dehydrate the formalin-fixed tissue slice by incubation in 70% ethanol (2x for 3 h), 95% ethanol (1x for overnight, 1x for 3 h), followed by absolute ethanol (1x for 3 h, 1x for overnight).
- Clear the formalin-fixed tissue slice by 3 h incubation in xylene twice.
- Immerse the tissue with paraffin at 60 °C (1x for overnight, 1x for 2 h). Embed the tissue in a paraffin block in a tissue embedding mold.
- Section the paraffin-embedded tissue block at 4 µm thickness with a microtome and float in a 40 °C water bath containing distilled water. Transfer the sections onto glass slides.
- Incubate paraffin sections for 1 h at 60 °C to bond the tissue to the glass. Incubate the slides overnight at 37 °C.
8. Hematoxylin and Eosin (H&E) staining
- Deparaffinize sections from step 7.7 by incubation in xylene (3x for 5 min).
- Re-hydrate by incubation in absolute alcohol (2x for 5 min), 95% alcohol (2x for 5 min), and 70% alcohol (1x for 5 min). Rinse briefly with distilled water.
- Stain in Mayer hematoxylin solution for 5 min. Rinse with running tap water for 10 min.
- Counterstain in 0.5% Eosin solution for 40 s. Rinse with distilled water.
- Dehydrate by incubation in 70% alcohol (maximal 1 min), 95% ethanol (2x for 3 min), and absolute alcohol (2x for 3 min), respectively.
- Clear in three changes of xylene (few seconds each). Place a drop of mounting medium and cover slides with a coverslip.
9. Immunohistochemistry of OTSCs
- Deparaffinize sections by incubation in xylene 2x for 10 min, followed by 1:1 ethanol/xylol for 10 min.
- Transfer slides to 100% ethanol (2x for 3 min), 96% ethanol (2x for 3 min), 70% ethanol (1x for 3 min), and then to 50% ethanol (1x for 3 min).
- Perform antigen retrieval to unmask the antigenic epitope. Heat the slides in a microwave in citrate buffer for 5 min at 900 W, followed by 2x for 8 min at 600 W. Allow the slides to cool to room temperature for 20 min.
- Wash in PBS 3x for 3 min on a shaker.
- Perform permeabilization of cell membranes by incubation in 0.1% Triton X-100 in PBS (200 µL per slide) in a humidified chamber at room temperature for 10 min.
- Wash in PBS for three changes, 3 min each on the shaker.
- Incubate sections with 3% H2O2 solution in methanol (200 µL per slide) in a humidified chamber at room temperature for 10 min to block endogenous peroxidase activity.
- Wash in PBS 3x for 3 min on a shaker.
- Add 200 µL of blocking buffer (1:50 horse serum in PBS) and incubate in a humidified chamber at room temperature for 25 min.
- Drain off the blocking buffer from the slides by tilting the slide on a paper tissue.
- Apply 200 µL of appropriately diluted primary antibody in antibody diluent on the slides and incubate in a humidified chamber at 4 °C overnight. As a negative control, use appropriate isoform of mouse immunoglobulins at the same dilution as the primary antibody.
- Wash 3x for 3 min in PBS on a shaker.
- Apply 200 µL of biotinylated secondary antibody (1:50 solution in PBS) on the slides and incubate in a humidified chamber at room temperature for 30 min.
- Wash 3x for 3 min in PBS on a shaker.
- Prepare the avidin/biotin-based peroxidase complex according to the manufacturer's instructions prior to application. Apply 200 µL of avidin-biotin-peroxidase complex on the slides and incubate in a humidified chamber at room temperature for 30 min.
- Wash 3x for 3 min in PBS on a shaker.
- Apply 200 µL of DAB substrate solution (freshly made directly before use: 1 drop of DAB in 1 mL of substrate), 200 µL per slide. Allow the color development 1-3 min until the desired color intensity is reached.
- Rinse with running tap water for 10 min.
- Counterstain the slides by immersing sides in Hematoxylin for 5 min.
- Rinse with running tap water for 10 min.
- Cover the slides using aqueous mounting medium and coverslips. The mounted slides can be stored at room temperature permanently.
Results
Figure 1 provides an overview of the workflow to culture OTSCs from fresh, unfrozen tumor tissue. Specimens of primary PDACs and metastases were collected directly after surgical resection and stored overnight on wet ice at 4 °C in the tissue storage solution. The specimens were processed, and slices were cultured as described in the protocol. The macroscopic morphology of each OTSC did not change grossly during cultivation. However, the size of the surface area of the OTSCs decreased over time as exemplarily shown in Figure 2A during cultivation for 6 days. The overall viability of OTSC was assessed by resazurin viability assay on days 0, 2/3, and 6 (Figure 2B). The resazurin viability assay measures general metabolic activity of the OTSC based on the reduction of non-fluorescent blue resazurin to red fluorescent resorufin in living cells15. Figure 2B shows the comparison of viability measured by resazurin reduction after the cultivation of OTSCs from two representative primary tumors in two different media. OTSCs were cultivated either in medium A (advanced DMEM/F12, 10% FBS, 1% Penicillin/Streptomycin) or medium B (RPMI 1640, 10% FBS, 1% Penicillin/Streptomycin, 4 µg/mL Insulin, 8 ng/mL EGF, 0.3 µg/mL hydrocortisone), which resulted in similar overall viability independently of the culture medium. Of note, a decrease in viability after day 0 can be observed frequently and is expected due to the slicing procedure and adjustment to culture conditions. However, we also observed increased viability as shown exemplarily in the right panel (Figure 2B).
After defined periods of cultivation in medium A, OTSCs were fixed in formalin for further immunohistological characterization (IHC). After formalin fixation, OTSCs were paraffin embedded and sectioned. H&E stained sections showed that the overall structure of the tissue was preserved over the entire time of cultivation ex vivo (Figure 3). Tumor and stroma cells were discriminated by IHC for cytokeratin 7 and vimentin, respectively. No substantial changes in the tumor to stroma ratios were detected during cultivation.
Microscopic histopathologic evaluation of H&E sections did not reveal a substantial increase in necrosis of all cultivated tissues during cultivation (Figure 3, Figure 4, Figure 5). Additionally, Ki-67 and cleaved caspase 3 were stained for evaluation of proliferation and apoptosis, respectively. Again, we did not detect gross changes of proliferation and apoptosis during the culture period of 6 days (Figure 3). However, the proportion of apoptotic cells increases over time during extended cultivation periods as measured by cleaved caspase 3 staining. Figure 4 shows the increase in cleaved caspase 3 positive cells after cultivating a primary PDAC for 15 days. Figure 5 shows the histopathology of a peritoneal metastasis of a PDAC. This experiment demonstrates a high intratumor heterogeneity between individual slices regarding the tumor/stroma content as well as naturally occurring apoptosis measured by cleaved caspase 3 staining. Hence, histopathological evaluation of cultivated OTSCs is important for the evaluation of the tumor/stroma architecture and viability of each cultivated tumor or its metastasis.
Figure 6 shows the histopathology of a PDAC metastasis of the abdominal wall. H&E staining as well as IHC for cytokeratin 7 showed that the derived OTSCs did not contain any tumor cells, but only consisted of connective tissue, which was partially necrotic. A drawback of the OTSC technology is that fresh, unfixed, and unfrozen tissue cannot be evaluated for its tumor cell content prior to cultivation.
Overall, these data demonstrate that the multicellular architecture of a tumor and its respective metastasis, comprising distinct cell types, are reflected in OTSCs. Particularly tumor-stroma interactions are preserved.
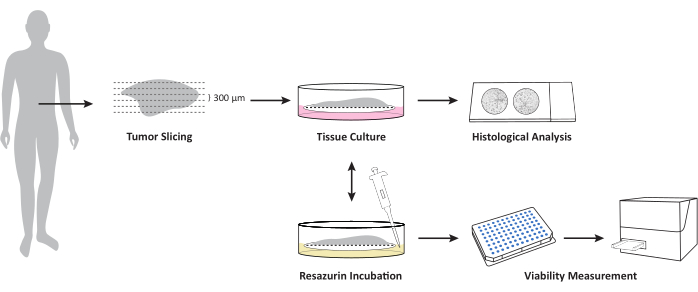
Figure 1: Overview over the workflow for cultivation of OTSCs from fresh, unfrozen tissue tumor/metastasis specimens. The fresh, unfrozen tissue specimen is sectioned using a vibratome and cultivated at air-liquid interface on PTFE cell culture inserts. Overall viability can be measured at defined time points by resazurin viability assay. This assay allows further cultivation after viability measurement. Slices are formalin-fixed at defined time points for further histopathological examination. Please click here to view a larger version of this figure.
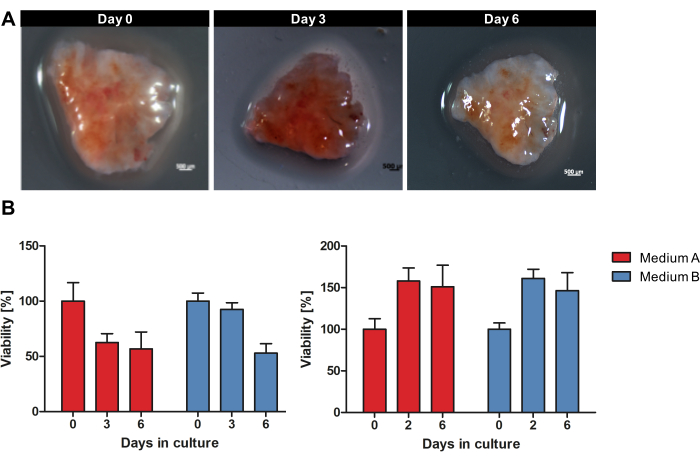
Figure 2: Macroscopic morphology and viability of OTSCs during cultivation. A PDAC specimen was cultivated as OTSCs for 6 days. (A) The macroscopic morphology of each OTSC did not change grossly during cultivation. However, the size of the surface area of the OTSCs decreased over time. (B) Overall viability was quantified by resazurin viability assay in medium A and B. A decrease in viability can frequently be observed after day 0 due to the sectioning procedure and adjustment to culture conditions. However, we also observed increases of viability as shown exemplarily in the right panel. Each panel represents an individual tumor specimen with different yields of derived slice cultures (left panel n = 10, right panel n = 10). Please click here to view a larger version of this figure.

Figure 3: Histopathological evaluation of OTSCs after a total cultivation time of 6 days. OTSCs (n = 6) were cultured in medium A. Tissue morphology was assessed by H&E staining. Tumor and stroma cells were discriminated by IHC for cytokeratin 7 and vimentin, respectively. Ki-67 and cleaved caspase 3 were stained for evaluation of proliferation and apoptosis, respectively. No substantial increase in apoptosis was observed over a total cultivation period of 6 days. Each scale bar represents 100 µm. Please click here to view a larger version of this figure.

Figure 4: Histopathological evaluation of OTSCs after a total cultivation time of 15 days. OTSCs (n = 8) were cultured in medium A. Tissue morphology was assessed by H&E staining. Tumor and stroma cells were discriminated by IHC for cytokeratin 7 and vimentin, respectively. Ki-67 and cleaved caspase 3 were stained for evaluation of proliferation and apoptosis, respectively. Apoptosis substantially increased after 15 days of cultivation as exemplified by cleaved caspase-3 staining. Each scale bar represents 100 µm. Please click here to view a larger version of this figure.

Figure 5: Histopathological evaluation of OTSCs of a peritoneal metastasis of a PDAC. OTSCs (n = 8) were cultured in medium A. Tissue morphology was assessed by H&E staining. Tumor and stroma cells were discriminated by IHC for cytokeratin 7 and vimentin, respectively. Ki-67 and cleaved caspase 3 were stained for evaluation of proliferation and apoptosis, respectively. Histopathological evaluation revealed a high degree of intratumor heterogeneity between individual slices regarding the tumor/stroma content as well as naturally occurring apoptosis measured by cleaved caspase 3 staining. Each scale bar represents 100 µm. Please click here to view a larger version of this figure.

Figure 6: Histopathological evaluation of OTSCs of a PDAC metastasis of the abdominal wall. OTSCs (n = 5) were cultured in medium A. Tissue morphology was assessed by H&E staining. Tumor and stroma cells were discriminated by IHC for cytokeratin 7 and vimentin, respectively. Ki-67 and cleaved caspase 3 were stained for evaluation of proliferation and apoptosis, respectively. Histopathological evaluation revealed the lack of tumor cells in the cultivated metastasis and partial necrosis, which could not be determined prior to cultivation. Each scale bar represents 100 µm. Please click here to view a larger version of this figure.
Discussion
OTSCs of fresh tumor samples are a close approximation of the tumor in situ. They maintain their baseline morphology, proliferative activity, and microenvironment during the cultivation for a defined, tissue-dependent period11,12,13. This technique enables the immediate application of treatment to viable human tumor tissue ex vivo and subsequent downstream analyses, such as profiling of the transcriptome and proteome. A specific advantage of OTSCs is that spatially resolved downstream analyses such as MALDI-imaging can be applied to intact multicellular tumor tissue.
Here we describe a method for the generation, cultivation, and histopathological evaluation of OTSCs of PDAC and its metastases. The key issue for this technique is to cut fresh tissue without any freezing procedures and cultivation at an air-liquid interface. A limitation of this method is that fresh, unfixed, and unfrozen tissue cannot be evaluated for its tumor cell content prior to cultivation. As exemplified in our results, OTSCs may reveal to consist of tissue components other than expected from rapid sections of other specimens from the same tumor. Histopathological evaluation of the OTSCs after cultivation is imperative for data evaluation of individual slice cultures. The described viability assay only allows a general assessment of overall viability of each OTSC. In general, the described method can be implemented in any laboratory equipped with a vibratome and tissue culture unit. Due to the simplicity of this technique, it can be easily modified toward various tumors and research questions.
Multiple methodologies have been developed to culture human tumor tissue ex vivo, e.g., primary cell lines, patient-derived organoids, and xenografts. However, their use for drug development and the identification of predictive biomarkers remains limited mostly due to the loss of the tissue context, i.e., interaction of stroma and tumor cells2.
Although the presented method of organotypic slice culture establishment preserves the multicellular tissue architecture, several limitations need to be considered. First, a considerable degree of (intra)tumor heterogeneity needs to be taken into account. Distinct OTSCs derived from a single tumor biopsy might show considerable variations in their proportions of tumor and stroma cells. Besides overall tissue quality, this might be one reason for different slice viability during culture. Secondly, the medium for optimal culture conditions might vary depending on the tissue and patient. We compared two distinct tissue culture media: the first based on DMEM/F12 (medium A) and the second based on RPMI (medium B). Each specimen of primary tumors or its metastases might require modifications. Thirdly, tumor necrosis might occur already in situ16 and is not necessarily due to insufficient culture conditions of the OTSCs. Comparison with the histopathology of the primary tumor should be considered.
The establishment of OTSCs as described in this protocol provides an opportunity to test treatment response promptly after surgery in an ex vivo model system that preserves the tumor microenvironment. Prospectively, OTSCs will facilitate the development of individual therapeutic strategies to personalize treatment of metastatic disease.
Disclosures
The authors disclosed no potential conflicts of interest.
Acknowledgements
R. Braun was supported by the Clinician Scientist School Lübeck (DFG #413535489) and the Junior Funding Program of the University of Lübeck.
Materials
| Name | Company | Catalog Number | Comments |
| Advanced DMEM/F-12 Medium | Gibco | 12634028 | |
| Agarose Low Melt | Roth | 6351.2 | 8% in Ringer solution |
| Antibody Diluent, Background Reducing | Dako | S3022 | |
| AquaTex | Merck | 108562 | |
| Bioethanol (99%, denatured) | CHEMSolute | 2,21,19,010 | |
| Citric Acid monohydrate | Sigma Aldrich | C7129 | |
| Cleaved Caspase-3 (Asp175) (5A1E) Rabbit mAb | Cell Signalling Technology | 9664 | 1:400 dilution |
| Derby Extra Double Edge Safety Razor Blades | Derby Tokai | ||
| Embedding cassettes | Roth | H579.1 | |
| Eosin Y-solution 0,5% aqueous | Merck | 10,98,44,100 | |
| Eukitt Quick hardening mounting medium | Sigma-Aldrich | 3989 | |
| Fetal bovine serum | Gibco | 10270106 | |
| Formaldehyde solution 4,5%, buffered | Büfa Chemikalien | B211101000 | |
| Hem alum solution acid acc. to Mayer | Roth | T865 | |
| Human EGF | Milteniy Biotec | 130-097-794 | |
| Hydrocortisone | Sigma Aldrich (Merck) | H0888 | |
| Hydrogen peroxide 30% | Merck | 1,08,59,71,000 | |
| Insulin human | Sigma Aldrich (Merck) | 12643 | |
| Liquid DAB+ Substrate Chromogen System | Dako | K3468 | |
| MACS Tissue Storage Solution | Milteniy Biotech | 130-100-008 | |
| Methanol | Merck | 10.600.092.500 | |
| Microscope Slides Superfrost Plus | Thermo Scientific | J1800AMNZ | |
| Millicell Cell Culture Insert, 30 mm, hydrophilic PTFE, 0.4 µm | Millipore (Merck) | PICM0RG50 | |
| Monoclonal mouse anti-human Cytokeratin 7 (Clone OV-TL 12/30) | Dako | M7018 | 1:200 dilution |
| Monoclonal mouse anti-human Ki67 Clone MIB-1 | Dako | M7240 | 1:200 dilution |
| Monoclonal mouse Anti-vimentin (Clone V9) | Dako | M0725 | 1:200 dilution |
| Negative control Mouse IgG2a | Dako | X0943 | 1:200 dilution |
| Negative control Mouse IgG1 | Dako | X093101-2 | 1:200 dilution |
| Paraffin (melting temperature 56°- 58°) | Merck | 10,73,37,100 | |
| Penicillin-Streptomycin (10.000 U/ml) | Gibco | 15140122 | |
| PBS pH 7,4 (1x) Flow Cytometry Grade | Gibco | A12860301 | |
| Resazurin sodium salt; 10 mg/ml in PBS | Sigma Aldrich | R7017 | 1:250 dilution |
| Ringer's solution | Fresenius Kabi | 2610813 | |
| RPMI-1640 Medium | Sigma Aldrich (Merck) | R8758 | |
| Tissue culture testplate 6 | TPP | 92006 | |
| Triton X-100 | Sigma Aldrich | 9002-93-1 | |
| VECTASTAIN Elite ABC-Peroxidase Kit | Vector Laboratories | PK-6200 | |
| Xylene (extra pure) | J.T.Baker | 8,11,85,000 | |
| Equipment | |||
| ClarioStar Microplate Reader | BMG Labtech | ||
| Paraffin Embedding Center E61110 | Leica | ||
| Rotary Microtome Microm HM355S | Thermo Scientific | ||
| Section Transfer System Microm STS | Thermo Scientific | ||
| VT 1200S Vibratom | Leica |
References
- Nevala-Plagemann, C., Hidalgo, M., Garrido-Laguna, I. From state-of-the-art treatments to novel therapies for advanced-stage pancreatic cancer. Nature Reviews. Clinical Oncology. 17 (2), 108-123 (2020).
- Tiriac, H., et al. Organoid profiling identifies common responders to chemotherapy in pancreatic cancer. Cancer Discovery. 8 (9), 1112-1129 (2018).
- Waghray, M., Yalamanchili, M., di Magliano, M. P., Simeone, D. M. Deciphering the role of stroma in pancreatic cancer. Current Opinion in Gastroenterology. 29 (5), 537-543 (2013).
- Hwang, R. F., et al. Cancer-associated stromal fibroblasts promote pancreatic tumor progression. Cancer Research. 68 (3), 918-926 (2008).
- Jiang, X., et al. Long-lived pancreatic ductal adenocarcinoma slice cultures enable precise study of the immune microenvironment. Oncoimmunology. 6 (7), 1333210 (2017).
- Hessmann, E., et al. Fibroblast drug scavenging increases intratumoural gemcitabine accumulation in murine pancreas cancer. Gut. 67 (3), 497-507 (2018).
- Feig, C., et al. The pancreas cancer microenvironment. Clinical Cancer Research. 18 (16), 4266-4276 (2012).
- Koerfer, J., et al. Organotypic slice cultures of human gastric and esophagogastric junction cancer. Cancer Medicine. 5 (7), 1444-1453 (2016).
- Gerlach, M. M., et al. Slice cultures from head and neck squamous cell carcinoma: a novel test system for drug susceptibility and mechanisms of resistance. British Journal of Cancer. 110 (2), 479-488 (2014).
- Holliday, D. L., et al. The practicalities of using tissue slices as preclinical organotypic breast cancer models. Journal of Clinical Pathology. 66 (3), 253-255 (2013).
- Vaira, V., et al. Preclinical model of organotypic culture for pharmacodynamic profiling of human tumors. Proceedings of the National Academy of Sciences of the United States of America. 107 (18), 8352-8356 (2010).
- Lim, C. Y., et al. Organotypic slice cultures of pancreatic ductal adenocarcinoma preserve the tumor microenvironment and provide a platform for drug response. Pancreatology. 18 (8), 913-927 (2018).
- Brandenburger, M., et al. Organotypic slice culture from human adult ventricular myocardium. Cardiovascular Research. 93 (1), 50-59 (2012).
- Misra, S., et al. Ex vivo organotypic culture system of precision-cut slices of human pancreatic ductal adenocarcinoma. Science Reports. 9 (1), 2133 (2019).
- O'Brien, J., Wilson, I., Orton, T., Pognan, F. Investigation of the Alamar Blue (resazurin) fluorescent dye for the assessment of mammalian cell cytotoxicity. European Journal of Biochemistry. 267 (17), 5421-5426 (2000).
- Beutler, B., Cerami, A. Tumor necrosis, cachexia, shock, and inflammation: a common mediator. Annual Review of Biochemisty. 57, 505-518 (1988).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionExplore More Articles
This article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved