Method Article
Development of a Surgical Technique for Subretinal Implants in Rats
In This Article
Summary
The present protocol describes the scleral approach for subretinal device implantation, a feasible surgical technique for implementation in animal models of retinal diseases in research.
Abstract
Retinal degeneration, such as age-related macular degeneration (AMD), is a leading cause of blindness worldwide. A myriad of approaches have been undertaken to develop regenerative medicine-based therapies for AMD, including stem cell-based therapies. Rodents as animal models for retinal degeneration are a foundation for translational research, due to the broad spectrum of strains that develop retinal degeneration diseases at different stages. However, mimicking human therapeutic delivery of subretinal implants in rodents is challenging, due to anatomical differences such as lens size and vitreous volume. This surgical protocol aims to provide a guided method for transplanting implants into the subretinal space in rats. A user-friendly comprehensive description of the critical steps has been included. This protocol has been developed as a cost-efficient surgical procedure for reproducibility across different preclinical studies in rats. Proper miniaturization of a human-sized implant is required prior to conducting the surgical experiment, which includes adjustments to the dimensions of the implant. An external approach is used instead of an intravitreal procedure to deliver the implant to the subretinal space. Using a small sharp needle, a scleral incision is performed in the temporal superior quadrant, followed by paracentesis to reduce intraocular pressure, thereby minimizing resistance during the surgical implantation. Next, a balanced salt solution (BSS) injection through the incision is carried out to achieve focal retinal detachment (RD). Lastly, insertion and visualization of the implant into the subretinal space are conducted. Post-operative assessment of the subretinal placement of the implant includes imaging by spectral domain optical coherence tomography (SD-OCT). Imaging follow-ups ascertain the subretinal stability of the implant, before the eyes are harvested and fixated for histological analysis.
Introduction
Age-related macular degeneration (AMD) is a leading cause of blindness worldwide. The number of people affected with AMD in 2020 was estimated at 196 million, and this is projected to increase to about 288 million by 20401. Over the past decade, several therapeutics have been developed to mitigate the visual changes associated with the late stages of AMD, mainly to treat the development and progression of the choroidal neovascularization observed in wet AMD. Conversely, the treatment of dry AMD, where dysfunction and loss of retinal pigment epithelium (RPE) cell progress to RPE and retinal atrophy, has been estimated to account for 85% to 90% of AMD, with a prevalence of 0.44% worldwide1,2. AMD has been described as a multifactorial disease with, age, genetic, and environmental factors contributing to the onset and progression of the disease; several therapies are in development to address the different pathophysiological pathways associated with this disease3.
Stem cell-based therapy has been developed as a novel therapeutic option to replace the failing RPE in dry AMD4. Although the usage of pluripotent stem cells is still in early clinical trials, safety has been demonstrated in several clinical trials5,6,7. To date, there are two main routes to deploy stem cells into the subretinal space: suspension or inserting a monolayer patch seeded on a biocompatible implant8,9,10,11,12. New strategies using stem cell-based therapies in preclinical studies require animal models where the stem cell-based therapeutics can be delivered to the same targeted site as intended in humans. The difference in anatomy might mandate minor changes to the procedures, surgical equipment, and approach compared to those used with the final human product13,14. Modifying the ocular surgical techniques is one of the required changes that has been widely described as a successful approach for use across different animal models15,16,17.
Although previous publications have mentioned surgical techniques for subretinal implants in rats, there are no comprehensive descriptions of such techniques to overcome the technical difficulties researchers may encounter. Therefore, there is a need to properly describe the surgical techniques in detail, provide best practices and lessons learned to avoid, and, if needed, address problems during critical steps throughout the procedure. The purpose of this manuscript is to provide a comprehensive guideline for surgical implantation of the implant into the subretinal space in rats.
Protocol
All experiments were approved by the University of Southern California Institutional Animal Care and Use Committee (IACUC) and were performed following the National Institutes of Health (NIH) Guide for the Care and Use of Laboratory Animals and The Association for Research in Vision and Ophthalmology (ARVO) Statement for the Use of Animals in Ophthalmic and Vision Research. A total of 12 Royal College of Surgeon (RCS) male rats were used in the present study. Animals were bred in the animal facility and included in the study once they reached the age of 28 ± 1 days postnatal. A complete eye exam was performed to verify the lack of eye abnormalities. The subretinal implants, ultrathin membranes made from Parylene C and coated with vitronectin, were designed by a specific commercial organization (see Table of Materials). These membranes replicate human-size membranes in terms of their thickness and permeability (6.0 µm thickness mesh frame with 20 µm circular pores in the ultrathin areas). Miniaturizing the length and width (1.0 mm × 0.4 mm) from human-size membranes was achieved to accommodate the subretinal implants inside the rodent eyes18.
1. Animal care and surgical preparation
- Weigh the animal to calculate the dosage of anesthesia and anesthetize following step 1.2.
- Anesthetize the animal through intraperitoneal injection of a mixture of ketamine and xylazine (35-50 mg/kg and 5-10 mg/kg, respectively; see Table of Materials). Inject with a 1.0 mL syringe and 30 G needle.
- Load another 1.0 mL syringe with half the amount of the mixture of the anesthesia (ketamine and xylazine) to maintain the proper level of anesthesia during the procedure.
- Confirm adequate anesthesia by the absence of a pedal reflex (firm toe pinch).
- To properly visualize the vitreous cavity and retina, dilate the pupil by the instillation of 1% tropicamide and 2.5% phenylephrine eye drops (see Table of Materials) in the treated eye.
- Apply a second dose of dilation eye drops after 5 min.
- Apply either artificial tears or ocular lubricant gel in the non-surgical eye every 5-10 min while the animal is anesthetized to moisturize the cornea.
- Place a sterile drape over the heating pad (see Table of Materials). Place a sterile drape covering the surgical tray.
- Prepare the sterile surgical area by placing the following sterile instruments on the surgical tray: surgical gloves, subretinal implant, and surgical tools: mosquito forceps (3), microsurgical fine scissors (1), needle holder (1), fine forceps with teeth (1), fine straight forceps without teeth (2), microliter syringe (1), 32 G blunt needle (1), 27 G or 30 G needle (2), 4-0 silk suture (3), cotton swabs, balanced salt solution (BSS), and a cover slip (see Table of Materials).
- Under sterile conditions and using sterile gloves, load the microliter syringe with the BSS and attach the 32 G blunt needle.
- Insert the cotton swap into the 27 G or 30 G needle hub to create a handle for more sensitive manipulation of the needle.
- To maintain sterile conditions throughout the procedure, change the sterile gloves if non-sterile instruments or areas are manipulated, such as the surgical microscope.
2. Scleral approach for subretinal implantation: surgical technique
- Expose the surgical area following the steps below.
- Once the animal is under anesthesia and the pupil is dilated (step 1.5), place the animal belly-side down with the head toward the researcher.
NOTE: Keep the rat on the heating pad covered with a sterile drape during the entire surgery and until the animal is fully recovered. - Apply 5% povidone drops (see Table of Materials) onto the eye and clean the eye surface and eyelids with cotton swabs.
- Place and adjust the surgical microscope (see Table of Materials) over the surgical eye.
NOTE: It is recommended to use a surgical ophthalmic microscope throughout the entire surgical procedure to get a sharper and larger visualization of the ocular structures. - Ensure proper exposure of the surgical area by lifting the superior eyelid and protruding the eyeball using 4-0 non-absorbable sutures.
- Elevate the superior eyelid using a 4-0 silk suture. Place the suture in the anterior side of the eyelids at the level of the meibomian glands. Secure the tractional suture by clamping mosquito forceps to the surgical surface.
NOTE: If the suture is placed higher than the level of the meibomian gland, eversion of the eyelid will occur instead of eyelid elevation. - Perform peritomy of the temporal superior quadrant using microsurgical fine scissors.
- Place two tractional sutures to allow protrusion and forward displacement of the eyeball. Perform isolation of the superior rectus muscle.
- Through gentle manipulation of the eye with fine teeth forceps (0.12 mm forceps) in the superior aspect of the limbus, roll the eye down to expose the sclera.
- Place a 4-0 silk suture behind the superior limbus up to 1 mm away, which is the location of the superior extraocular muscle. Clamp both tails of the suture by using mosquito forceps.
- Perform temporal rectus muscle isolation. Place a second suture (4-0 silk) up to 1 mm away from the limbus in the temporal quadrant (at the corresponding area of the temporal extraocular muscle). Clamp both tails of the suture by using mosquito forceps.
- Once both extraocular muscle sutures are properly placed and clamped, pull the sutures down and inward to expose the temporal superior quadrant of the sclera.
- Elevate the superior eyelid using a 4-0 silk suture. Place the suture in the anterior side of the eyelids at the level of the meibomian glands. Secure the tractional suture by clamping mosquito forceps to the surgical surface.
- Extend the peritomy toward the back of the eye with microsurgical fine scissors.
- Control conjunctival bleeding by using cotton swabs.
- Once the animal is under anesthesia and the pupil is dilated (step 1.5), place the animal belly-side down with the head toward the researcher.
- Perform scleral incision, retinal detachment (RD), and implant insertion following the steps below.
- Perform scleral incision by using a 27 G or 30 G needle. Ensure that the size of the incision is ~1.2 mm and is 1.5 mm posterior to the limbus.
NOTE: Development of a tunnel-like scleral incision configuration is recommended to stabilize intraocular structures while manipulating the eye and introducing the implant. This incision shape will prevent sudden fluctuations in intraocular pressure. - Sometimes, proper configuration requires practice. Therefore, if proper configuration is not achieved, release some tension from the extraocular muscle tractional sutures to facilitate keeping the ocular structures inside of the vitreous cavity.
- By using the same needle used for the scleral incision (27 G or 30 G), perform paracentesis in the peripheral cornea at the same quadrant.
- Insert the 32 G blunt needle mounted on a microliter syringe through the scleral incision.
- Inject 100 µL of BSS to create a focal RD.
- Release the extraocular muscle tractional sutures to get the eye back into the regular position.
- For direct visualization of the RD, rest and hold the cover slip, loaded with ophthalmic lubricant gel, onto the cornea.
- Adjust the microscope objective to focus on the retina.
- If the RD is not observed or is too small (smaller than the size of one quadrant), perform a second injection of BSS to achieve the desired RD size by rolling the eye down and inward and repeating steps 2.2.4 to 2.2.8.
- Once the RD is completed, verify the scleral length (as mentioned in step 2.2.1) with a caliper (see Table of Materials).
- Cut the choroidal tissue in both directions with fine microsurgical scissors.
- Run the blunt needle sideways along the scleral incision to verify all scleral and choroidal structures have been dissected.
- Insert the implant into the subretinal space.
- Using two fine surgical forceps, such as tying forceps (see Table of Materials), grab the implant from the back so as not to damage the active part of the implant.
- Place the implant parallel to the incision plane and gently slide in the implant.
- Once the implant is completely introduced in the subretinal space, release it, and push the implant further in by introducing the jaws of one of the forceps 1-1.5 mm into the incision.
- Release the extraocular muscle tractional sutures and verify the placement of the implant by using the microscope and the coverslip as described above (steps 2.2.7 and 2.2.8).
- Perform scleral incision by using a 27 G or 30 G needle. Ensure that the size of the incision is ~1.2 mm and is 1.5 mm posterior to the limbus.
- Release and remove all the sutures.
NOTE: After concluding the surgical procedure, SD-OCT is performed (step 3) to validate the clinical findings. The SD-OCT imaging session is conducted right after the surgery. If proper visualization of the implant location was not obtained, then conduct the SD-OCT again 7-10 days post-surgery.
3. SD-OCT imaging
- Click on the Heidelberg Eye Explorer icon (an image management software; see Table of Materials) on the desktop screen.
- Click on the New Patient icon at the top of the screen.
- Complete all information requested to generate the animal ID and click on Accept.
- Select the OCT imaging system (HRA + OCT; see Table of Materials) for the device type.
- Select the operator and press OK.
- For the correct cornea curvature option, press the OK button to accept default eye data.
- Ensure that the SD-OCT acquisition window is launched and ready for SD-OCT.
- Turn on the SD-OCT camera by pressing the yellow Start button located on the touchscreen display next to the camera.
- Place the animal on the SD-OCT animal stage that has been adapted on top of the SD-OCT headrest.
NOTE: The animal stage adaptation includes a piece of polystyrene foam (see Table of Materials) to fit within the SD-OCT head stage that is large enough to place the animal on, including the heating pad. - Adjust the table height and animal position to align the pupil with the center of the SD-OCT lens.
NOTE: Once the eye is aligned, an infrared (IR) live image will appear on the screen. - Press either the IR + OCT button on the touch screen or the OCT button at the bottom right of the monitor to start the image acquisition.
- Once the SD-OCT goes live, select a single scan mode with the highest number (100) of ART frames. The 100 ART scan mode is the default mode.
- Using the camera joystick, center the optic nerve on the IR image.
- Push the camera forward until the IR image is evenly filled with the retinal image.
NOTE: Dark corners indicate the camera is either too far or close to the eye. - Move the camera sideways until the implant is visualized in the temporal aspect of the retina. Use the IR image to guide the movements.
- To visualize the implant by the B-scan, press CTRL + ALT + SHIFT + O simultaneously to display the SD-OCT B-scan adjustment window.
- Adjust the "Reference Arm" (at the bottom of the window) until the retina/implant is visualized in the SD-OCT image.
NOTE: The SD-OCT image must be displayed within the blue reference corners. - Close the window.
- By using the joystick and the camera handle, slide and rotate the camera in all directions until a better, flatter, and sharper SD-OCT quality image is obtained.
- Drag the blue arrow on the IR image until it is placed along the implant.
- Once the saturation and placement of the B-scan are optimal, activate the "Automatic Real-time Tracking (ART)" by pressing the black Gain Control button below the touch screen.
- Once the ART reaches 100 frames, press Acquire on the touch screen.
- When all images have been acquired, click on Save Images on the top of the window and then click on Exit.
- Keep the cornea moist during the entire imaging session by applying lubricant eye drops frequently (every 5 min).
4. Animal recovery
- At the end of the imaging session, apply antibiotic ointment to the cornea to prevent ocular infection and moisturize the cornea.
- Keep the animal resting on its belly on the heating pad until fully recovered (~20-30 min) and ambulatory.
- Give systemic analgesics (1.0-1.2 mg/kg Buprenorphine SR once subcutaneous; see Table of Materials) at the end of the surgical procedure.
- Do not leave the animal unattended until it has regained sufficient consciousness.
- Do not return the animal to the company of other animals until fully recovered.
- Once fully recovered, place the animal back into the housing area with access to food and water ad libitum.
Results
Implantation of a subretinal implant in RCS rats (N = 12) demonstrated the feasibility and reproducibility of the surgical technique for subretinal delivery in rats. In this study, the right eye was the treated eye (N = 12) with the implant. In the clinical assessment conducted at the end of the procedure using the surgical microscope, nine of the 12 treated eyes demonstrated a subretinal localization of the implant (75.00%), two eyes (16.67%) were identified as an intraretinal placement of the implant, and in one eye (8.33%) direct visualization was not possible due to media opacity caused by a subretinal hemorrhage at the surgical area, with a limited view of both the implant and retinal structures (Table 1). SD-OCT scans performed immediately after the surgical procedure demonstrated the subretinal or intraretinal position of the implant (10 [83.33%] and one [8.33%], respectively) (Figure 1A). SD-OCT could not completely identify the implant's placement subretinally in the same animal (n = 1) with media opacity described above (direct visualization not possible), even after a 10 day follow-up. Figure 1B,C shows two different animals with an implant properly placed in the subretinal space. There were no other surgical complications associated with the surgical technique. By Hematoxylin and Eosin (H&E) staining, verification of the subretinal placement of the implant was observed (Figure 1D).
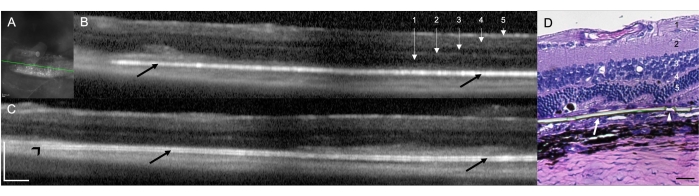
Figure 1: Spectral domain optical coherence tomography (SD-OCT) scans at 1 week post-surgical implantation. (A) Infrared image of the subretinal implant. The green line demarcates the cross-section shown in (B). Scale bar: 200 µm. (B,C) Two different animals with an implant properly placed in the subretinal space (black arrows). The implant's tip points toward the optic nerve (black arrowhead). 1 = Retinal Nerve Fiber/Ganglion Cell Layer, 2 = Inner Plexiform Layer, 3 = Inner Outer Layer, 4 = Outer Plexiform Layer, and 5 = Outer Nuclear Layer. Scale bar: 200 µm. (D) Histology section stained with H&E to demonstrate the subretinal implantation of the parylene membrane (white arrow). The arrowhead shows one of the micropores in the ultrathin areas. 1 = Retinal Nerve Fiber/Ganglion Cell Layer, 2 = Inner Plexiform Layer, 3 = Inner Outer Layer, 4 = Outer Plexiform Layer, and 5 = Outer Nuclear Layer. Scale bar: 20 µm. Magnification: 20x. Please click here to view a larger version of this figure.
| Clinical Assessment | SD-OCT | |||||
| Subject | SR | IR | Unknown | SR | IR | Unknown |
| 1 | X | X | ||||
| 2 | X | X | ||||
| 3 | X | X | ||||
| 4 | X | X | ||||
| 5 | X | X | ||||
| 6 | X | X | ||||
| 7 | X | X | ||||
| 8 | X | X | ||||
| 9 | X | X | ||||
| 10 | X | X | ||||
| 11 | X | X | ||||
| 12 | X | X | ||||
| 9 | 2 | 1 | 10 | 1 | 1 | |
| 75.00% | 16.67% | 8.33% | 83.33% | 8.33% | 8.33% | |
Table 1: Comparison of the ocular findings between clinical assessments and SD-OCT imaging among all the animals. Abbreviations: SR = subretinal, IR = intraretinal, and SD-OCT = spectral domain optical coherence tomography.
Discussion
Although the procedure has been previously described with slight variations, the scope of this manuscript is to provide a comprehensive description of a surgical procedure for subretinal implants in rats to be followed while learning the technique and to overcome the surgical challenges and potential complications that investigators may encounter. The surgical protocol outlined here includes the usage of the ultrathin parylene membrane that has been widely utilized in our lab for several years9,10,16,18. However, the reproducibility of the technique using different injectors and materials implanted in the subretinal space has been observed18,19.
A scleral approach for subretinal device implantation is not limited to stem cell-based therapies; retinal transplant procedures in small animal models have also been described20,21. In the retinal electrical stimulation field, this surgical procedure for subretinal implants in rats has been used for more than a decade22. More recently, Ho et al.23 implanted an array to stimulate the rat retina, and Thomas et al.24 used retina organoids as a source of stem cells. As mentioned previously, stem cell-based therapies have been well-published, including publications on the surgical implantation of biocompatible implants seeded with stem cells4. There are slight variations in the surgical approaches described by different authors, which will be discussed and compared with the surgical technique described in this manuscript.
Scleral closure and surgical instrumentation require additional discussion. There are two common approaches to managing scleral incision: (1) closure with a suture and (2) closure without a suture. Several authors use 10-0 nylon to close the scleral incision with a suture as part of their regular procedure23,25,26,27. However, other groups (including ours) have found that the 10-0 nylon suture is not required28. Those that support closure with the suture argue that the subretinal implant will slide out of the incision in the eye if there is no suture. As described in the results section, the current study did not find extrusion of the implant or intraocular tissue throughout the incision. This surgical approach without the suture has been used in our laboratory routinely and successfully9,10,12,13,16. The justification for a no-suture approach relies on two factors: First, a combination of the incision location and its configuration provides enough structure to generate a self-sealing incision. It should be kept in mind that proper configuration of the scleral tunnel is a step that investigators will achieve with practice. Second, the intraocular pressure increases once the traction is released, keeping the implant in place. The increased intraocular pressure results in the retina being pushed against the incision, bringing both scleral flaps close to each other and making a self-sealing incision. Therefore, a suture is not needed. Of note, the incision length is only up to 1.5 mm. In cases where the surgical incision requires a larger wound or if a proper scleral tunnel configuration is not achieved, a 10-0 nylon suture is a reasonable solution. The current technique is highly reliable if used with the recommended surgical instrumentation. Some authors have used customized injectors for their implants, which modifies the incision size and results in the need to use a scleral suture for proper closure25,29. However, in our experience, using different materials and injectors resulted in an increased incision length (~0.5 mm)18,19. We still did not observe instability or complications associated with a larger scleral incision, and no suture was needed. However, using instrumentation outside these guidelines during the procedure could be considered a limitation of this technique.
Another critical step that has been rarely referred to in previous publications is the paracentesis to reduce the intraocular pressure (IOP) prior to creating the focal RD and injecting the implant into the subretinal space4,10,13,15. Decreasing the IOP provides better control of the intraocular structures while detaching the retina and avoids extrusion of the intraocular content, which results in an unsuccessful procedure. Another advantage associated with a hypotonic eye is the reduction in resistance while injecting the implant through the scleral incision, which results in less damage to the implant itself. On the other hand, low IOP is prone to increased ocular bleeding at the surgical incision. Large amounts of blood at the scleral incision obscures the view and increases the risk of moving blood into the subretinal space during the subretinal implantation. We recommend controlling the bleeding using cotton swaps and BSS to clean the area and avoid surgical complications.
It is worth mentioning that the size of the RD is important for proper placement of the implant in the subretinal space. Unlike in other animal models and humans5,14,30, because this scleral approach does not provide direct visualization of the subretinal space, it is more difficult to generate a focal RD. To provide enough space for the implant to be deployed gently into the subretinal space without placing outside of this area, the recommendation is to inject 100 µLof BSS. This recommendation is based on the generation of an RD of at least one quadrant of the retina. If an RD smaller than at least one quadrant of the retina is created, the implant will be incorrectly injected into the intravitreal, intraretinal, or suprachoroidal space. As described throughout the protocol, if a small RD is observed, repeating steps 2.2.4 to 2.2.8 are recommended until the desired RD is achieved.
Most of the surgical complications and critical steps discussed in the manuscript may occur during the learning curve, which could compromise the success of the subretinal implantation. This learning curve also includes the amount of time the animals remain under anesthesia and the level of dehydration. A longer anesthesia time, anesthetics, and dehydration can lead to dry eye complications, such as corneal, lens, and scleral changes31. Additionally, anesthetics, such as ketamine and xylazine, have been associated with media opacity in the cornea and lens along with changes in the aqueous humor composition32. Using lubricant eye drops (BSS) on the surgical eye throughout the anesthetic time addresses these complications. In summary, the methodology described in this manuscript is meant to be used as a surgical recommendation in developing subretinal therapeutics in rat eyes.
Disclosures
M.S.H., D.R.H., and J.L. are co-founders and consultants to Regenerative Patch Technologies (RPT). The other authors certify that they have no affiliations with or involvement in any organization or entity with any financial or non-financial interest in the subject matter or materials discussed in this manuscript.
Acknowledgements
This study was supported by CIRM DT3 (MSH) and Research to Prevent Blindness (USC Roski Eye Institute). We want to thank Fernando Gallardo and Dr. Ying Liu for their technical assistance.
The sponsor had no role in the design or conduct of this research.
Materials
| Name | Company | Catalog Number | Comments |
| 1 cc syringe | VWR | BD309659 | |
| 27 G needle 1/2'' | VWR | BD305109 | |
| 30 G needle 1/2'' | VWR | BD305106 | |
| 32 G Blunt needle - Small hub RN | Hamilton | 7803-04 | |
| 4-0 Perma Hand silk black 1X18" PC-5 | Ethicon | 1984G | |
| 6'' sterile cotton tips | VWR | 10805-154 | |
| Betadine 5% sterile ophthalmic prep solution | Alcon | 8007-1 | |
| BSS irrigating solution 15 mL | Accutome | Ax17362 | |
| Buprenorphine ER | ZooPharm | N/A | |
| Castroviejo Caliper | Storz | E2405 | |
| Castroviejo suturing forceps 0.12 mm | Storz | E1796 | |
| Clayman-Vannas scissors straight | Storz | E3383S | |
| Cover glass, square | WVR | 48366-227 | |
| EPS Polystyrene block | Silverlake LLC | CFB8x12x2 | |
| Gonak 15 mL | Accutome | Ax10968 | Eye lubricant |
| Halstead straight hemostatic mosquito forceps non-magnetic | Storz | E6772 | |
| Hamilton syringe 700 series 100 µL | Hamilton | 7638-01 | |
| HEYEX Software | Heidelberg | N/A | an image management software |
| Kelman-McPherson tying forceps angled | Storz | E1815 AKUS | |
| Ketamine (100 mg/mL) | MWI | 501072 | |
| Needle holder 9mm curved fine locking | Storz | 3-302 | |
| Neomycin/Polymyxin B sulfactes/Bacitracin zinc ointment 3.5 g | Accutome | Ax0720 | |
| Ophthalmic surgical microscope | Zeiss | SN: 233922 | |
| Phenylephrine 2.5% 15 mL | Accutome | Ax0310 | |
| Spectralis SD-OCT | Heidelberg | SPEC-CAM-011210s3600 | |
| Sterile Drape | VWR | 100229-300 | |
| Sterile surgical gloves | VWR | 89233-804 | |
| T-Pump heating system | Gaymar | TP650 | |
| Tropicamide 1% 15 mL | Accutome | Ax0330 | |
| Ultrathin membranes made from Parylene C and coated with vitronectin | Mini Pumps LLC, CA | specifically designed for this study | used as subretinal implants |
| Xylazine (100 mg/mL) | MWI | 510650 |
References
- Wong, W. L., et al. Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: a systematic review and meta-analysis. Lancet Global Health. 2 (2), 106-116 (2014).
- Schultz, N. M., Bhardwaj, S., Barclay, C., Gaspar, L., Schwartz, J. Global burden of dry age-related macular degeneration: a targeted literature review. Clinical Therapeutics. 43 (10), 1792-1818 (2021).
- Deng, Y., et al. Age-related macular degeneration: Epidemiology, genetics, pathophysiology, diagnosis, and targeted therapy. Genes & Diseases. 9 (1), 62-79 (2021).
- Nazari, H., et al. Stem cell-based therapies for age-related macular degeneration: The promises and the challenges. Progress in Retinal and Eye Research. 48, 1-39 (2015).
- Kashani, A. H., et al. A bioengineered retinal pigment epithelial monolayer for advanced, dry age-related macular degeneration. Science Translational Medicine. 10 (435), (2018).
- Kashani, A. H., et al. Survival of an HLA-mismatched, bioengineered RPE implant in dry age-related macular degeneration. Stem Cell Reports. 17 (3), 448-458 (2022).
- da Cruz, L., et al. Phase 1 clinical study of an embryonic stem cell-derived retinal pigment epithelium patch in age-related macular degeneration. Nature Biotechnology. 36 (4), 328-337 (2018).
- Da Cruz, L., Chen, F. K., Ahmado, A., Greenwood, J., Coffey, P. RPE transplantation and its role in retinal disease. Progress in Retinal and Eye Research. 26 (6), 598-635 (2017).
- Hu, Y., et al. A novel approach for subretinal implantation of ultrathin substrates containing stem cell-derived retinal pigment epithelium monolayer. Ophthalmic Research. 48 (4), 186-191 (2012).
- Diniz, B., et al. Subretinal implantation of retinal pigment epithelial cells derived from human embryonic stem cells: improved survival when implanted as a monolayer. Investigative Ophthalmology & Visual Science. 54 (7), 5087-5096 (2013).
- Antognazza, M. R., et al. Characterization of a polymer-based, fully organic prosthesis for implantation into the subretinal space of the rat. Advanced Healthcare Materials. 5 (17), 2271-2282 (2016).
- Pennington, B. O., et al. Xeno-free cryopreservation of adherent retinal pigmented epithelium yields viable and functional cells in vitro and in vivo. Scientific Reports. 11 (1), 6286 (2021).
- Thomas, B. B., et al. A new immunodeficient retinal dystrophic rat model for transplantation studies using human-derived cells. Graefe's Archive for Clinical and Experimental Ophthalmology. 256 (11), 2113-2125 (2018).
- Koss, M. J., et al. Subretinal implantation of a monolayer of human embryonic stem cell-derived retinal pigment epithelium: a feasibility and safety study in Yucatán minipigs. Graefe's Archive for Clinical and Experimental Ophthalmology. 254 (8), 1553-1565 (2016).
- Yu, W., et al. Biocompatibility of subretinal parylene-based Ti/Pt microelectrode array in rabbit for further artificial vision studies. Journal of Ocular Biology, Diseases, and Informatics. 2 (1), 33-36 (2009).
- Thomas, B. B., et al. Survival and functionality of hESC-derived retinal pigment epithelium cells cultured as a monolayer on polymer substrates transplanted in RCS rats. Investigative Ophthalmology & Visual Science. 57 (6), 2877-2887 (2016).
- Adekunle, A. N., et al. Integration of perforated subretinal prostheses with retinal tissue. Translational Vision Science & Technology. 4 (4), 5 (2015).
- Lu, B., et al. Semipermeable parylene membrane as an artificial Bruch's membrane. 2011 16th International Solid-State Sensors, Actuators and Microsystems Conference. IEEE. , 950-953 (2011).
- Hu, Y., et al. Subretinal implantation of gelatin films with stem cells derived RPE in rats. Investigative Ophthalmology & Visual Science. 54 (15), 1763 (2013).
- Aramant, R. B., Seiler, M. J. Retinal transplantation-advantages of intact fetal sheets. Progress in Retinal and Eye Research. 21 (1), 57-73 (2002).
- Peng, Q., et al. Structure and function of embryonic rat retinal sheet transplants. Current Eye Research. 32 (9), 781-789 (2007).
- Pardue, M. T., et al. Neuroprotective effect of subretinal implants in the RCS rat. Investigative Ophthalmology & Visual Science. 46 (2), 674-682 (2005).
- Ho, E., et al. Characteristics of prosthetic vision in rats with subretinal flat and pillar electrode arrays. Journal of Neural Engineering. 16 (6), 066027 (2019).
- Thomas, B. B., et al. Co-grafts of human embryonic stem cell derived retina organoids and retinal pigment epithelium for retinal reconstruction in immunodeficient retinal degenerate Royal College of Surgeons rats. Frontiers in Neuroscience. 15, 752958 (2021).
- Seiler, M. J., et al. Vision recovery and connectivity by fetal retinal sheet transplantation in an immunodeficient retinal degenerate rat model. Investigative Ophthalmology & Visual Science. 58 (1), 614-630 (2017).
- McLelland, B. T., et al. Transplanted hESC-derived retina organoid sheets differentiate, integrate, and improve visual function in retinal degenerate rats. Investigative Ophthalmology & Visual Science. 59 (6), 2586-2603 (2018).
- Lin, B., McLelland, B. T., Mathur, A., Aramant, R. B., Seiler, M. J. Sheets of human retinal progenitor transplants improve vision in rats with severe retinal degeneration. Experimental Eye Research. 174, 13-28 (2018).
- Matsuo, T., Hosoya, O., Tsutsui, K. M., Uchida, T. Behavior tests and immunohistochemical retinal response analyses in RCS rats with subretinal implantation of Okayama-University-type retinal prosthesis. Journal of Artificial Organs. 16 (3), 343-351 (2013).
- Seiler, M. J., et al. A new immunodeficient pigmented retinal degenerate rat strain to study transplantation of human cells without immunosuppression. Graefe's Archive for Clinical and Experimental Ophthalmology. 252 (7), 1079-1092 (2014).
- Stanzel, B. V. Subretinal delivery of ultrathin rigid-elastic cell carriers using a metallic shooter instrument and biodegradable hydrogel encapsulation. Investigative Ophthalmology & Visual Science. 53 (1), 490-500 (2012).
- Fabian, R. J., Bond, J. M., Drobeck, H. P. Induced corneal opacities in the rat. The British Journal of Ophthalmology. 51 (2), 124-129 (1976).
- Calderone, L., Grimes, P., Shalev, M. Acute reversible cataract induced by xylazine and by ketamine-xylazine anesthesia in rats and mice. Experimental Eye Research. 42 (4), 331-337 (1986).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionExplore More Articles
This article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved