Method Article
Assessing Iron Deposition in the Brains of 5xFAD Mice by Perls'/DAB Staining
* Wspomniani autorzy wnieśli do projektu równy wkład.
W tym Artykule
Podsumowanie
This protocol presents methods for assessing the distribution and quantity of iron deposition in tissues, especially the brain. The protocol details the procedures for sample preparation, Perls/DAB staining, image capture, and data analysis.
Streszczenie
Alzheimer's disease (AD), a common neurodegenerative disease, is the leading cause of dementia in the elderly. Iron deposition is closely linked to the pathogenesis of AD. In the AD brain, elevated iron levels are strongly correlated with characteristic AD pathologies, including β-amyloid plaques, tau neurofibrillary tangles, and apoptosis. Increasing evidence suggests that oxidative stress, due to dysregulated iron metabolism, plays an important role in AD pathophysiology. Understanding the role of iron accumulation in the pathology of AD is, therefore, critical to treating AD. A comprehensive iron staining protocol is essential to detect iron expression and distribution in the brain. This protocol aims to describe a method to assess the quantity and distribution of iron deposition in the brain. We describe the processes involved in reagent preparation, mouse treatment, and preparation of brain sections. In addition, we present a detailed step-by-step protocol for histochemical iron detection in cerebral tissue sections, accompanied by a systematic analytical framework integrating both quantitative assessment and morphological evaluation to ensure a comprehensive interpretation of staining outcomes. This experimental protocol will help researchers understand iron depositions in the brain and support AD research.
Wprowadzenie
Alzheimer's disease (AD) is the most common neurodegenerative disease and has become a serious problem for society. The prevalence of AD increases dramatically with age1,2,3,4. The primary pathological hallmarks of Alzheimer's disease (AD) include the deposition of β-amyloid (Aβ) plaques and neurofibrillary tangles (NFTs) due to aberrant phosphorylation of tau proteins. Aβ is generated through the sequential cleavage of amyloid precursor protein (APP) by β-secretase and γ-secretase enzymes. Dysregulation in Aβ production or clearance leads to its accumulation in the brain, forming amyloid plaques, which are recognized as one of the earliest pathological markers of AD. These plaques not only cause direct neuronal damage but also initiate a cascade of detrimental effects, including neuroinflammation, oxidative stress, and synaptic dysfunction. Collectively, these processes contribute to neuronal death and the progressive cognitive decline observed in AD5. Despite decades of research predominantly targeting the pathological hallmarks of Alzheimer's disease (AD), including amyloid-β plaque deposition and hyperphosphorylated tau protein aggregation, clinically meaningful therapeutic breakthroughs remain elusive, with existing interventions demonstrating limited efficacy in modifying disease progression6.
Iron is an important metal for maintaining brain function and is involved in oxygen transport, DNA synthesis, mitochondrial respiration, myelin synthesis, neurotransmitter synthesis, and metabolism. However, iron becomes harmful when concentrations exceed the capacity of cellular sequestration6,7,8. Increased iron deposition, usually co-localized with Aβ plaques, has been observed in both cortical and hippocampal regions of the brain in patients with AD9,10. Through the Fenton reaction11, intracellular free iron can generate hydroxyl radicals (•OH) as well as reactive oxygen species.These harmful substances not only induce neuroinflammation by activating glial cells but also attack the cell's biomolecules through oxidative stress, disrupting normal cellular function and leading to synaptic dysfunction8,12. Furthermore, increased iron is directly involved in the development of pathological signs of AD: iron contributes to Aβ production12 and also increases tau protein dysfunction, leading to neurofibrillary tangles13.
Several techniques have been developed to assess the iron content in the brain, including magnetic resonance imaging (MRI), as well as chemical and histochemical methods14. In vivo quantification of intracerebral iron levels using MRI is clinically important for aging and neurodegeneration patients15. MRI has the advantage of being non-invasive and highly sensitive: it can detect iron deposition in the early stages of disease. In particular, the field-dependent R2 increase (FDRI) technique can distinguish between different forms of iron, such as ferritin iron and heme-iron16. However, MRI cannot detect iron accumulation at the cellular level, and it is expensive, so it is mainly used in clinical investigations17.
Colorimetric measurement is a chemical method that determines the concentration of a substance (such as iron) by measuring the intensity of the color produced by the reaction of the substance in solution with a chromogenic agent18. This method is easy to perform and uses low-cost reagents and instrumentation, making it suitable for laboratories with limited budgets18. However, the accuracy and sensitivity of this method are very limited, as the results are subjected to influence by environmental factors, color-development time, and the subjective judgment of the operator. Flame atomic absorption spectrometry (FAAS) is another method that can be used to assess iron levels19. In this method, a clarified sample solution is obtained by burning the tissue of interest in a high-temperature furnace. The sample is then dissolved using hydrochloric acid, and the concentration of iron in the solution is determined via atomic absorption spectrometry. This method is highly sensitive and suitable for all types of biological samples19. However, the process is time-consuming and requires expensive equipment, so its use is limited.
Perls'/DAB staining to detect non-heme ferric iron is the most commonly used iron-detection method because of its high specificity and ease of implementation compared with other histochemical methods14. Unlike colorimetric analysis, Perls' staining is a qualitative histochemical staining method that relies on the reaction between iron ions and potassium ferrocyanide to generate a blue precipitate. It is used to microscopically visualize the presence and distribution of iron in tissues. Moreover, this method allows us to distinguish iron deposition in different cell types and observe the subcellular distribution of iron deposition, providing a method for the study of AD20. The Perls' stain primarily detects nonheme ferric iron and depends on the formation of the Prussian blue complex:

(Prussian blue)
However, the traditional Perls' stain is ineffective for detecting iron localization at low iron levels. When diaminobenzidine (DAB) is added, it reacts with ferric hexacyanoferrate to form a brown compound, increasing the specificity of the assay21.
The goal of this protocol is to describe how to assess the quantity and distribution of iron deposition in the brains of an AD mouse model. In this protocol, 8-month-old 5×FAD (C57BL/6J) transgenic mice were used as the AD mouse model and were compared with wild-type mice of the same age. Details of the procedures for preparing the chemical reagent, sectioning the brain, performing the Perls'/DAB stain, and analyzing images taken after staining are presented here.
Protokół
All the procedures were performed in accordance with the guidelines of the Institutional Animal Care and Use Committee of Xuzhou Medical University and the Chinese governmental regulations for the Care and Use of Laboratory Animals.
1. Perfusion of animals
- Prepare the fixative: Dissolve 4% paraformaldehyde (PFA) in 1× phosphate-buffered saline(PBS) (see Table 1).
- Anesthetize the mouse by intraperitoneal injection with ketamine (100 mg/kg) and xylazine (15 mg/kg). Once the mouse is anesthetized, fix it to the perfusion surgery tray with four pins to secure the limbs.
CAUTION: To ensure proper anesthesia, check for the loss of responses to toe pinching, relaxation of limb muscles, and deep, slow, and steady breath. - Cut open the chest with scissors to expose the heart and cut the right atrium. Inject 20 mL of 1× PBS into the left ventricle with a syringe to flush the blood. Then, inject 20 mL of 4% PFA for fixation.
NOTE: Control the depth and direction of the needle to avoid puncturing the interventricular septum. Remember that an optimal perfusion features a pale liver and stiff limbs after the initial tremor. - Decapitate the perfused mouse, cut the bone around the skull with a pair of fine scissors, lift the skull carefully using blunt forceps, and extract the brain. Then, soak the brain in 4% PFA at 4 °C overnight.
CAUTION: Be careful with 4% PFA, which is a strong respiratory irritant. Wear an appropriate mask and perform this step in a fume hood.
NOTE: The method of euthanasia was approved by IACUC of the Xuzhou Medical University Animal Care and Use Program Euthanasia Guidelines: Mice / Rats.
2. Brain cryosectioning
- Dehydrate the brain with graded sucrose solutions (15% and 30%) at 4 °C for 24 h for each step. The brain should finally sink to the bottom of the sucrose solution.
- Hemisect the brain along the interhemispheric fissure with a scalpel. Put the left half into a 5 mL microcentrifuge tube filled with 1 mL of 30% sucrose solution and an equal volume of optimal cutting temperature (OCT) compound to enhance contact between the OCT and the brain.
- Trim extraneous tissue from the left hemisphere with a razor blade and move it to the OCT platform.
- Embed the tissue in OCT with the support of a circular wall made of aluminum foil.
- Freeze the tissue in a cryostat microtome at −22 °C for at least 1 h and set the cutting head temperature to −19 °C.
NOTE: Remember to precool the anti-roll glass and brushes in the cryostat along with the sample. - Mount the frozen tissue block. Trim the tissue block again to an appropriate position depending on the area of interest and then begin to section the brain into 20 µm thick sagittal sections.
NOTE: If the sections fold, use soft brushes to unfold the sections. - Collect each section on the gelatin-coated slides and smear a drop of 1× PBS onto it. Turn the brain section over using a fine brush and then quickly mount the section onto the slide.
- Dry the collected sections at room temperature overnight and store them at −20 °C.
NOTE: The protocol can be paused here for up to 2 weeks. Longer storage time may interfere with the tissue quality and thus influence the outcome of the experiment.
3. Perls'/DAB staining
- Prepare the following solutions: 2% potassium ferrocyanide (K4[Fe(CN)6]·3H2O), 2% hydrochloric acid (HCI), and 20× DAB solution (see Table 1).
- Put a slide containing sections into a staining jar filled with 1× PBS and place the jar on an orbital shaker for 5 min to wash the OCT out of the tissue.
NOTE: Adjust the speed of the orbital shaker carefully (recommended speed: 20 rpm) to avoid tissue falling off the slide at high speeds. - Mix 20 mL of 2% potassium ferrocyanide solution and an equal volume of 2% hydrochloric acid in a 50 mL centrifuge tube.
- Use plastic forceps to transfer the slide to the 50 mL tube and then heat the tube in a water bath at 60 °C for 30 min.
- Rinse the slide with 1× PBS and blot any excess solution off with tissue paper.
- Use a pipette to add 50 µL of DAB solution to each brain section and incubate for 10 min at room temperature.
- Aspirate the excess DAB solution with a pipette and then wash the slide 3 times in PBS.
- Dehydrate the sections with graded alcohol solutions (50%, 70%, 95%, 100%) and xylene(100%, 100%). Incubate the slides for 3 min each.
- Seal the sections with neutral gum22 and then place the coverslip.
- Dry the slides in a fume hood for 24 h before storing them in a microscope slide box.
4. Imaging
- Turn on the microscope, adjust the brightness of the light source, and focus on the brain section under a 4× magnification objective.
- Capture the image with camera control software and adjust parameter settings to prevent overexposure. The specific settings (10×, 40× objective) are shown in Figure 1.
NOTE: Avoid using automatic white balance to ensure the same exposure time for each image. - Switch to a 10× magnification objective, move the microscope stage to focus on the areas with high Perls'/DAB staining signals, especially the hippocampus and the cerebral cortex, and capture images (Figure 1A).
- Switch to a 40x magnification objective to capture images with clear staining signals.
- Save each image as an 8-bit TIFF and export the images for further analysis.
5. Image analysis
- In ImageJ/Fiji, select Plugins > stitching > Grid/Collection stitching in the menu, then select Unknown position to choose the images intended to be stitched (Figure 2A).
- Choose File > Save As > Tiff to save the finished image as a TIFF (Figure 2B).
- Use ImageJ/Fiji to open an image and ensure the image format is RGB Color (Figure 2C).
- Select Plugins > IHC Profiler Image in the menu, then choose Mode > Cytoplasmic Stained Image and the Color Deconvolution default H DAB (Figure 2D). Then convert the image format to 8-bit Gray-scale.
- Convert the gray values of the image to OD values by selecting Analyze > Calibrate > Uncalibrated OD (Figure 2E).
- Use the Threshold function to select the area of positive Perls'/DAB staining, then adjust the horizontal scroll bars until all the brown signal spots are covered with red (Figure 2F).
- Click Analyze > Set Measurements, then select the options Area, Mean gray value, Integrated density, and most importantly, Limit to threshold to exclude background noise (Figure 2G).
- Select Analyze > Measure to obtain the results table (Figure 2H).
- Group the results and use statistical software to perform statistical analysis. In our case, we compared iron accumulation in the hippocampus and cortex of 5xFAD transgenic mice with that in the negative control (wild-type mice). For three-group comparisons, one-way analysis of variance (ANOVA) was used for data from Gaussian distribution with the same standard deviation.
Wyniki
To investigate the distribution and accumulation of iron in a mouse model of AD, we performed Perls'/DAB staining in sagittal slices of 8-month-old 5xFAD mice. As shown in Figure 3, Perls'/DAB staining signals were observed in 8-month-old 5xFAD and wild-type mice and in 2-month-old wild-type mice. In 5xFAD mice, high Perls'/DAB staining signals were observed in the hippocampus and cortex, especially in the subiculum of the hippocampus. Wild-type mice served as validated negative controls; both 2- and 8-month-old cohorts exhibited significantly attenuated iron deposition signals (Figure 3A), with detectable yet non-significant age-progressive accumulation trends. Under 40× magnification, the signals in 5xFAD mice were observed in Aβ plaque-like structures, consistent with previous studies9,10(Figure 3B). The 4× magnification images were stitched to examine the distribution of iron staining in the brains of 5xFAD mice. Perls'/DAB staining signals were clearly stronger in the cortex, hippocampus, and cerebellum than in other regions. (Figure 3C). Three sections of the cerebral cortex and three hippocampus from each of the three mice in each condition and age group were selected for quantification. Iron deposition in 8-month-old 5xFAD mice was significantly higher than in wild-type controls of the same age. When comparing the density of the signal in 2-month-old and 8-month-old wild-type mice, we found no significant difference in iron accumulation between these two ages (Figure 3D). These results demonstrate the effectiveness of Perls'/DAB staining as a histochemical technique for iron detection. Figure 4 shows two instances of failed staining. Excessive background coloring will occur if the brain sections are left in the DAB solution for too long (Figure 4A). Inappropriate dehydration will result in folded sections (Figure 4B).
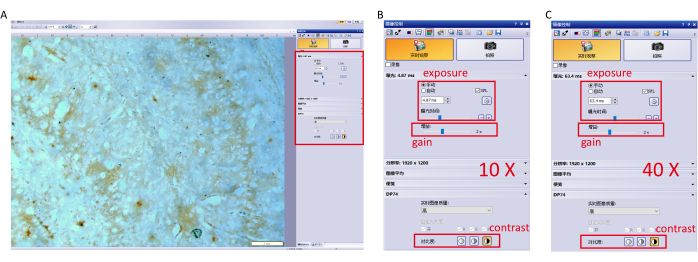
Figure 1: Parameter settings for the imaging software. (A) Adjust the parameter settings of the software (exposure, gain, and contrast.) at 10× objective and focus on the hippocampus. (B) Detailed parameter settings for a 10× objective. (C) Detailed parameter settings for a 40× objective. Please click here to view a larger version of this figure.

Figure 2: Data processing of the Perls'/DAB staining results. (A) In ImageJ/Fiji, select Plugins > stitching > Grid/Collection stitching in the menu, then select Unknown position to choose the images intended to be stitched. (B) Choose File > Save As> Tiff to save the finished image as a TIFF. (C) Use ImageJ/Fiji to open an image and ensure the image format is RGB Color. (D) Select Plugins > IHC Profiler Image, then choose Mode > Cytoplasmic Stained Image and the Color Deconvolution default H DAB. (E) Convert the gray values of the image to OD values by selecting Analyze > Calibrate > Uncalibrated OD. (F) Use the Threshold function to select the Perls'-positive area, then adjust the horizontal scroll bars until all the signal spots are covered. (G) Click Analyze > Set Measurements, select the options for Area, Mean gray value, Integrated density, and Limit to threshold to exclude background noise. (Integrated Density = Area × Mean). (H) Select Analyze > Measure to obtain the results table. Please click here to view a larger version of this figure.
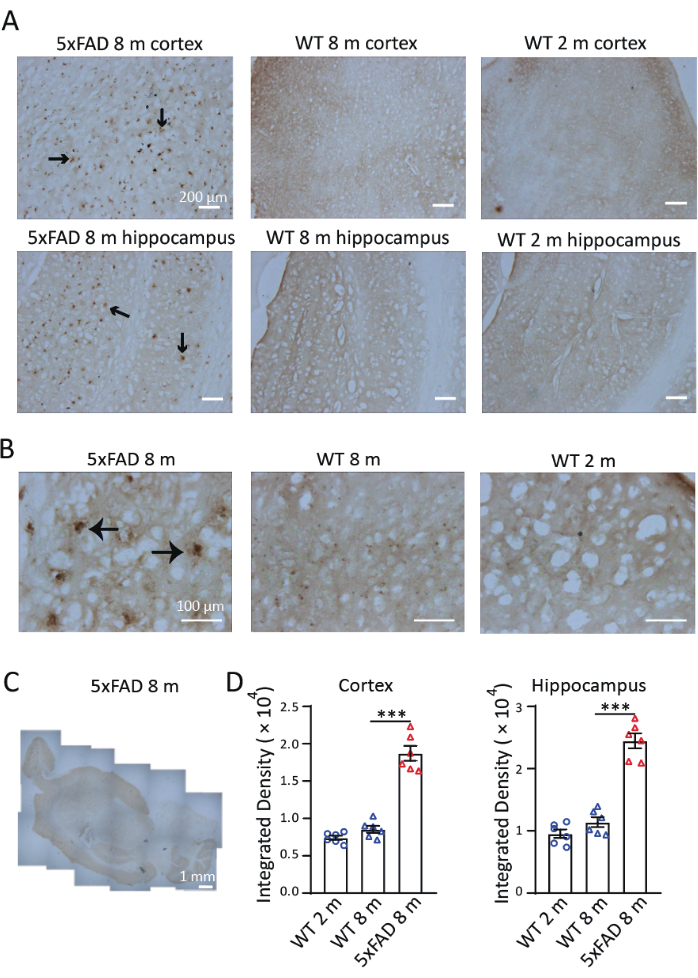
Figure 3: Representative Perls'/DAB staining in the brains of 5xFAD mice and wild-type (WT) mice. (A) Strong Perls'/DAB signals in longitudinal sections of hippocampus and cortex from 8-month-old 5xFAD mice, compared with staining in 2-month-old and 8-month-old wild-type mice. (B) Perls'/DAB signals were clustered around Aβ plaques, especially in the hippocampus. (C) A stitched longitudinal image showing the distribution of Perls'/DAB signals in a 5xFAD mouse brain. (D) Statistical analysis of the Perls'/DAB signal in the cortex or hippocampus of 2-month-old WT, 8-month-old WT, and 8-month-old 5xFAD mice. One-way ANOVA, ***, P < 0.001. Scale bars are displayed in the images. Please click here to view a larger version of this figure.

Figure 4: Two instances of unsuccessful Perls'/DAB staining. (A) An over-colored Perls'/DAB stain due to excessive DAB intensification. (B) The edge of the brain section is curled because of inappropriate dehydration. Please click here to view a larger version of this figure.
Dyskusje
Iron deposition is closely associated with Alzheimer's disease. On the one hand, it can lead to Aβ production through multiple mechanisms, including increased expression of amyloid precursor protein{NOTE:20}23, and also enhanced γ-secretase activity, which can lead to accelerated Aβ production24; on the other hand, lipid peroxidation induced by increased iron can promote tau polymerization, which can also lead to increased abnormal phosphorylation of tau protein in neurons25. Perls' Prussian blue staining is a well-established histochemical technique for selective visualization of ferric iron (Fe3+) deposits in biological specimens. The ferric iron reacts with ferrocyanide to produce blue ferric ferrocyanide (Prussian blue)14. Perls'/DAB staining is a modification of traditional Perls' staining. The reacting samples are transferred to a solution containing DAB (diamino benzenamine), whereupon the Prussian blue forms a brown-colored compound. Using this method, we observed the spatial distribution of iron accumulation in the brains of 5xFAD mice and quantitatively compared iron accumulation in AD model mice and wild-type mice.
In this protocol, we demonstrate that Perls'/DAB staining is feasible and effective for assessing pathological iron deposition. For ideal results, a few tips should be noted. First, iron-free reagents and tools, particularly iron-free hydrochloric acid, should be used to reduce background staining. The potassium ferrocyanide and hydrochloric acid should be freshly prepared. The extra solution left on the slide should be removed, and pure alcohol and xylene should be used to ensure thorough dehydration. During imaging, the use of automatic white balance will lead to inaccurate analyses. Remember to convert the gray values of the image to OD values, which correspond to the density of the histochemical signals.
The method described here enhances the specificity of the staining and is particularly useful in tissues with low levels of iron. The DAB stain produces a high contrast signal, which is important for microscopy and image analysis. In summary, Perls'/DAB staining provides a stronger signal and better background contrast than conventional Perls' staining, increasing the sensitivity and accuracy of iron detection. In addition, Perls' staining detects the ferric iron only in loosely bound protein complexes (as in hemosiderin)21. Iron that is strongly bound, as in hemoglobin, will not react. This greatly reduces the unwanted signals caused by iron in red blood cells and hemoglobin.
However, Perls'/DAB staining has some limitations. For example, DAB staining results in some non-specific background staining that can interfere with the observation of positive results. In addition, the staining process requires precise control of both time and concentration; otherwise, staining may be uneven or may have insufficient signal intensity. It has also been reported that the staining response is predominantly in oligodendrocytes rather than neurons26.
Perls'/DAB staining is appropriate for studies of pathology that require medium specificity and sensitivity. This method provides researchers with a less time-consuming and less costly way to visualize and quantify iron accumulation than MRI or FAAS. To further investigate the distribution of iron accumulation in different cell types and subcellular structures, co-staining with markers for neurons and glial cells is needed. Overall, Perls'/DAB staining will accelerate the exploration of AD pathology and facilitate research into therapeutics related to iron metabolism.
Ujawnienia
This research was supported by a general project of the Natural Science Foundation of China (grant number: 32271010).
Podziękowania
During the preparation of this work the authors used DeepSeek in order to improve language and readability. After using this tool/service, the authors reviewed and edited the content as needed and take full responsibility for the content of the publication.
Materiały
| Name | Company | Catalog Number | Comments |
| 0.1 M PBS | biosharp | BL601A | Add deionized water to make solution |
| 4% PFA | Beyotime | P0099 | Restore at 4 °C for use |
| 20X DAB solution | Bioss | C-0003 | Mix 10 μl solution A, 10 μl solution B and 180 μl PBS |
| Alcohol | Supelco | 107017 | |
| HCL | Sigma | 7647-01-0 | Add distilled water to 50 ml |
| ketamine | China National Medicines | H20193336 | 100 mg/kg for anesthetizing |
| Microscope | Olympus | CX43 | |
| Optimal cutting temperature compound (OCT) | Sakura Finetek | 4583 | |
| potassium ferrocynide solution | Sigma | 14038-43-8 | Add distilled water to 50 ml |
| Sucrose | Sigma | 57-50-1 | |
| xylazine | Y-S Biotechnology | YS-(BC)-2842 | 15 mg/kg for anesthetizing |
| Xylenes | Sigma | 1330-20-7 |
Odniesienia
- Karlawish, J., Jack, C. R., Rocca, W. A., Snyder, H. M., Carrillo, M. C. Alzheimer's disease: The next frontier-special report 2017. Alzheimer's Dement. 13 (4), 374-380 (2017).
- Praticò, D. Oxidative stress hypothesis in Alzheimer's disease: A reappraisal. Trends in Pharmacol Sci. 29 (12), 609-615 (2008).
- Sayre, L. M., et al. In situ oxidative catalysis by neurofibrillary tangles and senile plaques in alzheimer's disease. J. Neurochem. 74 (1), 270-279 (2001).
- Urrutia, P., et al. Inflammation alters the expression of dmt1, fpn1 and hepcidin, and it causes iron accumulation in central nervous system cells. J. Neurochem. 126 (4), 541-549 (2013).
- Hardy, J., Selkoe, D. J. The amyloid hypothesis of Alzheimer's disease: Progress and problems on the road to therapeutics. Science. 297 (5580), 353-356 (2002).
- Scheltens, P., et al. Alzheimer's disease. Lancet. 397 (10284), 1577-1590 (2021).
- Ficiarà, E., Munir, Z., Boschi, S., Caligiuri, M. E., Guiot, C. Alteration of iron concentration in alzheimer's disease as a possible diagnostic biomarker unveiling ferroptosis. Int J Mol Sci. 22 (9), 4479(2021).
- Yang, W. S., Stockwell, B. R. Ferroptosis: Death by lipid peroxidation. Trends in Cell Biol. 26 (3), 165-176 (2016).
- Lane, D. J. R., et al. Iron and Alzheimer's disease: An update on emerging mechanisms. J Alzheimers Dis. 64 (s1), S379-S395 (2018).
- Meadowcroft, M. D., Connor, J. R., Smith, M. B., Yang, Q. X. Mri and histological analysis of beta-amyloid plaques in both human Alzheimer's disease and app/ps1 transgenic mice. J Magn Reson Imaging. 29 (5), 997-1007 (2009).
- Winterbourn, C. C. Toxicity of iron and hydrogen peroxide: The Fenton reaction. Toxicol Lett. 82-83, 969-974 (1995).
- Silvestri, L., Camaschella, C. A potential pathogenetic role of iron in Alzheimer's disease. J Cell Mol Med. 12 (5a), 1548-1550 (2008).
- Gamblin, T. C., King, M. E., Kuret, J., Berry, R. W., Binder, L. I. Oxidative regulation of fatty acid-induced tau polymerization. Biochemistry. 39 (46), 14203-14210 (2000).
- Meguro, R., et al. Nonheme-iron histochemistry for light and electron microscopy: A historical, theoretical and technical review. Arch Histol Cytol. 70 (1), 1-19 (2007).
- Lehéricy, S., Roze, E., Goizet, C., Mochel, F. Mri of neurodegeneration with brain iron accumulation. Curr Opin Neurol. 33 (4), 462-473 (2020).
- Nabuurs, R. J. A., et al. High-field MRI of single histological slices using an inductively coupled, self-resonant microcoil: Application to ex vivo samples of patients with Alzheimer's disease. NMR Biomed. 24 (4), 351-357 (2010).
- Perl, D. P., Good, P. F. Comparative techniques for determining cellular iron distribution in brain tissues. Ann Neurol. 32 (S1), S76-S81 (1992).
- Riemer, J., Hoepken, H. H., Czerwinska, H., Robinson, S. R., Dringen, R. Colorimetric ferrozine-based assay for the quantitation of iron in cultured cells. Anal Biochem. 331 (2), 370-375 (2004).
- Luterotti, S., Kordić, T., Dodig, S. Simultaneous determination of iron and copper in children's sera by FAAS. Acta Pharm. 61 (1), 93-102 (2011).
- Hill, J. M., Switzer, R. C. The regional distribution and cellular localization of iron in the rat brain. Neuroscience. 11 (3), 595-603 (1984).
- Meguro, R., Asano, Y., Iwatsuki, H., Shoumura, K. Perfusion-perls and -turnbull methods supplemented by dab intensification for nonheme iron histochemistry: Demonstration of the superior sensitivity of the methods in the liver, spleen, and stomach of the rat. Histochem Cell Biol. 120 (1), 73-82 (2003).
- Liu, C., et al. S-nitrosylation of divalent metal transporter 1 enhances iron uptake to mediate loss of dopaminergic neurons and motoric deficit. J Neurosci. 38 (39), 8364-8377 (2018).
- Guillemot, J., Canuel, M., Essalmani, R., Prat, A., Seidah, N. G. Implication of the proprotein convertases in iron homeostasis: Proprotein convertase 7 sheds human transferrin receptor 1 and furin activates hepcidin. Hepatology. 57 (6), 2514-2524 (2013).
- Li, X., et al. Ferritin light chain interacts with pen-2 and affects γ-secretase activity. Neurosci Lett. 548, 90-94 (2013).
- Jin Jung, K., et al. Oxidative stress induces inactivation of protein phosphatase 2a, promoting proinflammatory nf-κb in aged rat kidney. Free Radic Biol Med. 61, 206-217 (2013).
- Connor, J. R., Menzies, S. L., Martin, S. M. S., Mufson, E. J. Cellular distribution of transferrin, ferritin, and iron in normal and aged human brains. J Neurosci Res. 27 (4), 595-611 (2004).
Przedruki i uprawnienia
Zapytaj o uprawnienia na użycie tekstu lub obrazów z tego artykułu JoVE
Zapytaj o uprawnieniaThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. Wszelkie prawa zastrzeżone