Method Article
Isolation Method for Long-Term and Short-Term Hematopoietic Stem Cells
W tym Artykule
Podsumowanie
We present a step-by-step protocol for the isolation of long-term hematopoietic stem cells (LT-HSCs) and short-term HSCs (ST-HSCs) using the Hoxb5 reporter system.
Streszczenie
Self-renewal capacity and multi-lineage differentiation potential are generally regarded as the defining characteristics of hematopoietic stem cells (HSCs). However, numerous studies have suggested that functional heterogeneity exists in the HSC compartment. Recent single-cell analyses have reported HSC clones with different cell fates within the HSC compartment, which are referred to as biased HSC clones. The mechanisms underlying heterogeneous or poorly reproducible results are little understood, especially regarding the length of self-renewal when purified HSC fractions are transplanted by conventional immunostaining. Therefore, establishing a reproducible isolation method for long-term HSCs (LT-HSCs) and short-term HSCs (ST-HSCs), defined by the length of their self-renewal, is crucial for overcoming this issue. Using unbiased multi-step screening, we identified a transcription factor, Hoxb5, which may be an exclusive marker of LT-HSCs in the mouse hematopoietic system. Based on this finding, we established a Hoxb5 reporter mouse line and successfully isolated LT-HSCs and ST-HSCs. Here we describe a detailed protocol for the isolation of LT-HSCs and ST-HSCs using the Hoxb5 reporter system. This isolation method will help researchers better understand the mechanisms of self-renewal and the biological basis for such heterogeneity in the HSC compartment.
Wprowadzenie
Hematopoietic stem cells (HSCs), which possess self-renewal capacity and multipotency, reside at the apex of the hematopoietic hierarchy1,2. In 1988, Weissman and colleagues demonstrated for the first time that the isolation of mouse HSCs could be achieved using flow cytometry3. Subsequently, a fraction defined by a combination of cell surface markers, Lineage−c-Kit+Sca-1+CD150+CD34−/loFlk2−, was reported to contain all HSCs in mice4,5,6,7,8.
Immunophenotypically defined (Lineage−c-Kit+Sca-1+CD150+CD34−/loFlk2−) HSCs (hereafter, pHSCs) were previously considered functionally homogeneous. However, recent single-cell analyses have revealed that pHSCs still exhibit heterogeneity with respect to their self-renewal capacity9,10 and multipotency11,12. Specifically, two populations seem to exist in the pHSC fraction with regard to their self-renewal capacity: long-term hematopoietic stem cells (LT-HSCs), which have continuous self-renewal capacity, and short-term hematopoietic stem cells (ST-HSCs), which have transient self-renewal capacity9,10.
To date, the molecular mechanisms of self-renewal capacity that distinguish LT-HSCs and ST-HSCs remain poorly understood. It is crucial to isolate both cell populations based on their self-renewal capacities and to discover underlying molecular mechanisms. Several reporter systems have also been introduced to purify LT-HSCs13,14,15; however, the LT-HSC purity defined by each reporter system is variable, and exclusive LT-HSC purification has not been achieved to date.
Therefore, developing an isolation system for LT-HSCs and ST-HSCs will accelerate research regarding self-renewal capacity in the pHSC fraction. In the isolation of LT-HSCs and ST-HSCs, a study using multi-step, unbiased screening identified a single gene, Hoxb5, that is heterogeneously expressed in the pHSC fraction16. Additionally, bone marrow analysis of the Hoxb5 reporter mice revealed that approximately 20%-25% of the pHSC fraction consists of Hoxb5pos cells. A competitive transplantation assay using Hoxb5pos pHSCs and Hoxb5neg pHSCs revealed that only Hoxb5pos pHSCs possess long-term self-renewal capacity, while Hoxb5neg pHSCs lose their self-renewal capacity within a short period, indicating that Hoxb5 identifies LT-HSCs in the pHSC fraction16.
Here, we demonstrate a step-by-step protocol to isolate LT-HSCs and ST-HSCs using the Hoxb5 reporter system. In addition, we present a competitive transplantation assay to assess the self-renewal capacity of Hoxb5pos/neg pHSCs (Figure 1). This Hoxb5 reporter system allows us to prospectively isolate LT-HSCs and ST-HSCs and contributes to the understanding of LT-HSC-specific characteristics.
Protokół
All the animal experiments described were approved by the RIKEN Center for Biosystems Dynamics Research.
1. Preconditioning of the recipient mice
- Prepare male C57BL/6 congenic mice aged 8-10 weeks old as recipient mice. The number of recipient mice depends on the experimental protocol. We typically prepare 10-20 mice for each condition.
- Feed the mice with sterilized water supplemented with enrofloxacin (170 mg/L). As irradiated recipient mice are highly susceptible to infection, keep the cages as clean as possible.
NOTE: Supplementation with antibiotics starts 24 h prior to the irradiation and continues for 3 weeks after transplantation to avoid infections.
- Feed the mice with sterilized water supplemented with enrofloxacin (170 mg/L). As irradiated recipient mice are highly susceptible to infection, keep the cages as clean as possible.
- Total body irradiation
- Total body irradiation destroys the recipient bone marrow cells to ensure engraftment of the donor cells. Transfer the recipient mice to an irradiation cage. Lethally irradiate the recipient mice with a single dose of 8.7 Gy at 12-16 h before transplantation.
NOTE: The radiation dose and time may vary depending on the equipment. The lethal radiation dose should be confirmed with the researcher's irradiator and mouse strain. - Return them to their cages after the total body irradiation.
- Total body irradiation destroys the recipient bone marrow cells to ensure engraftment of the donor cells. Transfer the recipient mice to an irradiation cage. Lethally irradiate the recipient mice with a single dose of 8.7 Gy at 12-16 h before transplantation.
2. Collection of the donor bone marrow cells
- Prepare a male Hoxb5-tri-mCherry mouse aged 12 weeks old, and euthanize the mouse by CO2 exposure followed by cervical dislocation or using methods approved by the local animal ethics committee.
NOTE: The reagent volume per mouse is described in the following steps. - Under sterile conditions, remove the skin, and expose the bones (femur, tibia, pelvis, humerus). Cut the major muscles, and take the bones (femur, tibia, pelvis, humerus) from the mouse. Place them in sterile cell culture dishes with Ca2+- and Mg2+-free, ice-cold PBS.
- Trim the muscles and fibrous tissues from the bones using tweezers, small scissors, and wipes to prevent contamination. Be careful not to break the bones during this step. Discard any broken bones in order to maintain sterility.
- Sterilize a mortar and pestle with 70% ethanol (EtOH), and let them dry completely. Equilibrate with cell-staining buffer (Ca2+- and Mg2+-free PBS supplemented with 2% heat-inactivated FBS, 2 mM EDTA, 100 U/mL penicillin, and 100 µg/mL streptomycin).
- Put the bones in the mortar, and add 3 mL of cell-staining buffer. Crush the bones open with the pestle. Disaggregate the cell clumps by gentle pipetting, and transfer the cell suspension through a 100 µm cell strainer into a 50 mL tube.
- Repeat step 2.5 until the solution becomes clear. Usually, three times is enough.
3. Separation of the c-kit+ cells by magnetic sorting
- Antibody staining for magnetic sorting
- Centrifuge the samples at 400 x g and 4 °C for 5 min. Aspirate the supernatant, and resuspend the pellet in 1 mL of cell staining buffer. Add 10 µL of rat-IgG (5 mg/mL) to reduce non-specific antibody binding, and gently pipet up and down using a P1,000 pipette. Incubate on ice for 15 min.
- Add the c-Kit antibody (clone 2B8) at a concentration of 4 µg/mL, and mix with a P1,000 pipette. Incubate on ice for 15 min.
- Add 5 mL of cell staining buffer, and mix well. Centrifuge the samples at 400 x g, 4 °C, 5 min. Aspirate the supernatant, and resuspend the pellet in 500 µL of cell staining buffer.
- Add 35 µL of anti-APC microbeads to enrich the c-Kit+ cells, and mix with a P1,000 pipette. Incubate on ice for 15 min.
- Add 4-5 mL of cell-staining buffer. Centrifuge the samples at 400 x g and 4 °C for 5 min. Aspirate the supernatant, and resuspend the pellet in 1 mL of cell staining buffer.
- Sorting the c-Kit+ cells magnetically
- Follow the manufacturer's instructions to sort the cells. In brief, prime a magnetic sorting column with 3 mL of cell staining buffer. Filter the sample (1 mL) through a 40 µm cell strainer and load the sample onto the magnetic sorting column.
- Wash by adding 3 mL of cell staining buffer three times. Add the cell staining buffer only when the column reservoir is empty.
- Put the magnetic sorting column on top of an ice-cold 15 mL tube and add 5 mL of cell staining buffer. Flush out the cells by firmly pushing the plunger into the column. Keep the flow-through on ice.
NOTE: In case of accidental sample loss, we keep the flow-through until the end of the experiment.
4. Hematopoietic stem cell staining
- Centrifuge the sample prepared in step 3.2.3 at 400 x g and 4 °C for 5 min. Aspirate the supernatant.
- Add the CD34 antibody (clone RAM34) at a concentration of 50 µg/mL to the pellet, and incubate on ice for 60 min.
NOTE: Preparation of the supporting cells during the first hour of incubation with CD34 is recommended to shorten the processing time. - Prepare the antibody master mix according to Table 1. Add 100 µL of the master mix to the sample, and incubate on ice for 30 min. The antibody for the CD34 antigen (clone; RAM34) requires 90 min for sufficient staining.
- Add 4-5 mL of cell staining buffer. Centrifuge the sample at 400 x g and 4 °C for 5 min. Aspirate the supernatant. Add streptavidin-BUV737 at a concentration of 3 µg/mL to the pellet, and incubate on ice for 30 min.
- Add 4-5 mL of cell staining buffer. Centrifuge the sample at 400 x g and 4 °C for 5 min. Aspirate the supernatant, and resuspend the pellet in 400 µL of cell staining buffer. Keep the sample on ice.
5. Supporting cell preparation
- Prepare a CD45.1+ CD45.2+ congenic mouse aged 12 weeks old; ideally, this should be the same age as the donor mouse. Euthanize the mouse by CO2 exposure followed by cervical dislocation or using methods approved by the local animal ethics committee.
NOTE: In the provided example, CD45.1+ CD45.2+ congenic mice were bred in-house by crossing B6.CD45.1 congenic mice with C57BL/6J mice16. - Under sterile conditions, take both femurs and tibiae, and place them in sterile cell culture dishes with Ca2+- and Mg2+-free, ice-cold PBS. Trim the muscles and fibrous tissues from the bones using tweezers and small scissors.
- Cut both ends of the bones with sharp, sterile scissors. Use a 23 G needle and a 5 mL syringe filled with ice-cold cell-suspension buffer (Ca2+- and Mg2+-free PBS supplemented with 2% heat-inactivated FBS, 100 U/mL penicillin, and 100 µg/mL streptomycin) to flush the bone marrow out into a sterile cell culture dish with cell suspension buffer. Disaggregate the cell clumps by gentle pipetting.
- Filter the cell suspension through a 40 µm cell strainer using a P1,000 pipette. Count the cell number of the cell suspension using a hemocytometer and prepare a bone marrow cell suspension containing 1 x 106 cells/mL.
- Transfer 200 µL of the bone marrow cell suspension (2 x 105 cells) to a 96-well, round-bottom plate. Keep on ice until use.
NOTE: Round-bottom plates are recommended for easy cell collection.
6. Hoxb5pos or Hoxb5neg pHSC sorting
- Gating setup
- Transfer 400 µL of the sample prepared in step 4.5 to a round-bottom polystyrene test tube with a 35 µm cell strainer snap cap. Prepare dead cell staining reagent, and add it to the sample before the analysis according to the manufacturer's instructions.
- Turn on a flowcytometer, and start the analysis software according to the manufacturer's instructions. Then, press Load, and acquire the data.
NOTE: A flow cytometer equipped with five lasers and a 70 µm nozzle is recommended to enhance the purity of the sorted cells. - After excluding doublets, dead cells, and lineage-positive cells, gate the Lineage−c-Kit+Sca-1+ fraction. Next, gate out the Flk2+ fraction. Then, gate Hoxb5pos or Hoxb5neg pHSCs in the CD150+CD34−/low fraction (Figure 2). Perform compensation for the spectral overlap in the first experiment.
NOTE: Hoxb5 positive cells are expected to represent 20%-25% of the Lineage−c-Kit+Sca-1+CD150+CD34−/lowFlk2− fraction.
- Hoxb5pos or Hoxb5neg pHSC sorting
- Prepare a 1.5 mL low protein binding tube with 600 µL of Ca2+- and Mg2+ -free PBS supplemented with 10% heat-inactivated FBS.
- Set the 1.5 mL low protein binding tube on a sort collection device, and sort the Hoxb5pos or Hoxb5neg pHSCs into the 1.5 mL tube using the gating strategy set in step 6.1.3. In the first sorting, use the yield from the sorting precision mode.
- Set the 96-well plate with the supporting cells prepared in step 5.5 on the automated cell deposition unit (ACDU) stage. Set the 1.5 mL low protein binding tube prepared in step 6.2.2 to the loading port of a flow cytometer.
- Sort the Hoxb5pos or Hoxb5neg pHSCs into a 96-well plate with the supporting cells using the gating strategy set in step 6.1.3. Sort 10 Hoxb5pos or Hoxb5neg pHSCs to test their self-renewal capacities.
NOTE: Double-sorting is recommended to enhance the purity. In the second sorting, use purity as the recommended sorting precision mode. - Typically, 500-1,000 Hoxb5pos pHSCs and 1,500-4,000 Hoxb5neg pHSCs are harvested after double-sorting. Proceed with the transplantation procedures as soon as possible after the HSC sorting to enhance the cell viability (ideally within 1-2 h).
7. Transplantation
- Place the HSC-sorted, 96-well plate prepared in step 6.2.4 onto ice. Handle the HSC-sorted, 96-well plate in sterile conditions, ideally in a cell hood.
- Anesthetize a recipient mouse with 2% isoflurane in a gas anesthesia induction chamber. Once the animal is fully anesthetized, remove it, and place it on its side. To ensure sufficient anesthesia, confirm no movement in response to a noxious stimulus.
- After the confirmation of proper anesthesia, perform a retro-orbital injection as soon as possible to prevent the mouse from regaining consciousness. The retro-orbital injection takes less than 30 s.
NOTE: Since the injection time is short, we finish the procedure without applying ophthalmic ointment in the eyes. However, if the procedure takes longer, we recommend the use of ophthalmic ointment. - Gently pipette the cells in the HSC-sorted 96-well plate to mix them. Collect the donor cells in the sorting plate using a 30 G insulin syringe, and inject them into the retro-orbital venous plexus of the recipient mice. The recommended injectable volume is ≤200 µL.
- Observe until the mice are conscious and moving about in a clean cage. Return them to their cages after confirming their recovery.
8. Peripheral blood analysis
- Collect 50 µL of peripheral blood from the tail vein, and resuspend it with 100 µL of Ca2+- and Mg2+-free PBS with 2 mM EDTA. Transfer all the samples to a 96-well plate. Centrifuge at 400 x g and 4 °C for 5 min, and discard the supernatant.
- Add 200 µL of red blood cell lysis buffer, and incubate on ice for 3 min. Centrifuge the samples at 400 x g and 4 °C for 5 min, and discard the supernatant. Repeat one more time.
- Add 200 µL of cell staining buffer. Centrifuge the samples at 400 x g and 4 °C for 5 min, and discard the supernatant.
- Prepare antibody master mix according to Table 2. Add 50 µL of antibody master mix, and incubate on ice for 30 min.
- Add 150 µL of cell staining buffer. Centrifuge the samples at 400 x g and 4 °C for 5 min, and discard the supernatant.
- Add 200 µL of cell staining buffer. Centrifuge the samples at 400 x g and 4 °C for 5 min, and discard the supernatant. Resuspend in 200 µL of cell staining buffer, and add dead cell staining reagent before the analysis according to the manufacturer's instructions.
- Analyze peripheral blood chimerism using a flow cytometer as described previously16. Collect blood at 4 weeks, 8 weeks, 12 weeks, and 16 weeks after transplantation to follow multi-lineage reconstitution. Representative flow cytometry plots are provided in Figure 3.
Wyniki
Previously, self-renewal capacity has been measured using competitive transplantation assays, in which donor HSCs are thought to retain their self-renewal capacity only if multi-lineage donor cells in the recipient peripheral blood are observed17. In addition, several reports define LT-HSCs as cells that continue to produce peripheral blood cells several months after the second bone marrow transplantation10,18. Therefore, in order to compare their self-renewal abilities, 10 Hoxb5pos or Hoxb5neg pHSCs isolated from Hoxb5 reporter mice were transplanted into lethally irradiated primary recipient mice with 2 x 105 whole bone marrow cells. Then, 16 weeks after the primary transplantation, 1 x 107 bone marrow cells isolated from the primary recipient mice were transplanted into lethally irradiated secondary recipient mice to assess the long-term self-renewal capacity (Figure 1). Figure 2 shows representative flow cytometry plots of the bone marrow analysis of the Hoxb5-tri-mCherry mice. Approximately 20%-25% of the cells in the pHSC fraction defined by Lineage−c-Kit+Sca-1+CD150+CD34−/loFlk2− were Hoxb5pos pHSCs, which account for only 0.001%-0.00125% of mouse bone marrow. Figure 3 displays representative flow cytometry plots of the peripheral blood analysis in the recipient mice. The CD45.2 donor mice (Hoxb5-tri-mCherry mice), CD45.1/CD45.2 supporting cells, and CD45.1 recipient mice were prepared, respectively, to separately analyze the donor, supporting, and recipient cells.
Figure 4 shows peripheral blood analyses in the recipient mice at 4 weeks, 8 weeks, 12 weeks, and 16 weeks after transplantation to confirm donor chimerism. These analyses revealed that although Hoxb5pos and Hoxb5neg pHSCs present similar donor chimerism 4 weeks after transplantation, continuous hematopoiesis was observed only in the Hoxb5pos pHSC recipients (Figure 4A,B). On the other hand, Hoxb5neg HSCs started losing the ability to produce hematopoietic cells 8 weeks after transplantation (Figure 4A,B). In the secondary transplantation analysis, only the Hoxb5pos pHSC recipients presented robust hematopoiesis (Figure 5A,B). In contrast, donor cells were hardly observed in the Hoxb5neg pHSC recipient mice, suggesting that Hoxb5neg pHSCs lose their self-renewal ability within 16 weeks after transplantation in primary recipient mice. These data demonstrate that Hoxb5 expression can be used as a specific marker for LT-HSCs.

Figure 1: Experimental schematic for long-term hematopoietic reconstitution assays. The recipient mice were lethally irradiated and competitively transplanted with 10 HSCs and 2 x 105 whole bone marrow cells (supporting cells). For secondary transplants, 1 x 107 whole bone marrow cells were transferred from the primary recipient mice. Abbreviations: PB = peripheral blood; WBM = whole bone marrow. This figure has been modified from Chen et al.16. Please click here to view a larger version of this figure.
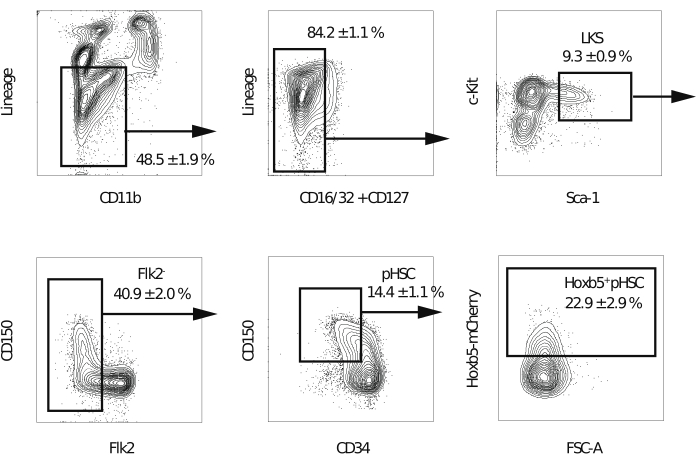
Figure 2: Gating strategy for sorting Hoxb5pos and Hoxb5neg pHSCs. Representative flow cytometry gating to isolate LKS, Flk2−, pHSC, Hoxb5pos, and Hoxb5neg pHSCs after the exclusion of doublets and dead cells. The values indicate the percentage of each fraction ± s.d. (n = 3). The lineages include B220, CD3ε, CD4, CD8a, Gr-1, and Ter-119. This figure has been modified from Chen et al.16. Please click here to view a larger version of this figure.
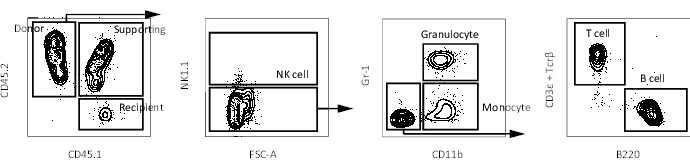
Figure 3: Representative FACS plots of peripheral blood in a recipient mouse. Gating scheme to identify peripheral blood cells (NK cell, granulocyte, monocyte, T cell, and B cell) in a recipient mouse after the exclusion of doublets and dead cells. Please click here to view a larger version of this figure.

Figure 4: Chimerism in recipient mice after primary transplantation. (A) Percentage chimerism at 4 weeks, 8 weeks, 12 weeks, and 16 weeks in primary recipients receiving 10 Hoxb5neg (n = 9), Hoxb5lo (n = 13), or Hoxb5hi (n = 18) pHSCs. Each column represents an individual mouse. The Hoxb5hi fraction was defined as the top 5% of Hoxb5 expression and others as the Hoxb5lo fraction. (B) The average donor lineage contribution in 10 cell primary transplants. The error bars denote the s.d. This figure has been modified from Chen et al.16. Please click here to view a larger version of this figure.

Figure 5: Chimerism in the recipient mice after the secondary transplantation. (A) Percentage chimerism at 4 weeks, 8 weeks, 12 weeks, and 16 weeks following the whole bone marrow secondary transplantation. (B) Individual donor chimerism by lineage in whole bone marrow secondary recipients. Each line represents an individual mouse. This figure has been modified from Chen et al.16. Please click here to view a larger version of this figure.
| Antibody | Clone | Concentration | Fluorochromes |
| Flk-2 | A2-F10 | 4 μg/mL | PerCP/eFlour710 |
| CD150 | TC15-12F12.2 | 4 μg/mL | BV421 |
| CD11b | M1/70 | 4 μg/mL | BV711 |
| Sca-1 | D7 | 4 μg/mL | BUV395 |
| CD16/32 | 93 | 4 μg/mL | A-700 |
| CD127 | A7R34 | 4 μg/mL | A-700 |
| CD3ε | 145-2C11 | 10 μg/mL | Biotin |
| CD4 | GK1.5 | 10 μg/mL | Biotin |
| CD8a | 53-6.7 | 10 μg/mL | Biotin |
| Gr-1 | RB6-8C5 | 10 μg/mL | Biotin |
| B220 | RA3-6B2 | 10 μg/mL | Biotin |
| Ter119 | TER119 | 10 μg/mL | Biotin |
Table 1: Antibody master mix for hematopoietic stem cell staining.
| Antibody | Clone | Concentration | Fluorochromes |
| CD45.1 | A20 | 1 μg/mL | FITC |
| CD45.2 | 104 | 1 μg/mL | PE |
| Gr-1 | RB6-8C5 | 2.5 μg/mL | A700 |
| NK1.1 | PK136 | 1 μg/mL | PerCP-Cyanine5.5 |
| CD11b | M1/70 | 1 μg/mL | BUV395 |
| CD3ε | 145-2C11 | 1 μg/mL | BV421 |
| TCRβ | H57-597 | 1 μg/mL | BV421 |
| B220 | RA3-6B2 | 1 μg/mL | BV786 |
Table 2: Antibody master mix for peripheral blood cell staining.
Dyskusje
Traditionally, cell surface marker-defined HSCs have been prepared to study the functions of HSCs, such as self-renewal capacity and multi-potency19,20,21. However, the immunophenotypically defined (Lineage−c-Kit+Sca-1+CD150+CD34−/loFlk2−) HSC fraction contains two discrete HSC populations: LT-HSCs and ST-HSCs9,10. Therefore, the specific analysis of bonafide HSCs, LT-HSCs, has not yet been achieved. Accordingly, an isolation method for LT-HSCs using the Hoxb5 reporter system will significantly benefit the search for the molecular mechanisms of self-renewal capacity.
Here, we will discuss critical steps in this protocol. First, step 1 to step 7 need to be completed without interruption. These steps usually take 9-12 h, and it is important to keep the samples at 4 °C throughout these procedures, as much as possible, in order to maintain sample viability. Next, approximately 1 x 108 bone marrow cells are harvested from a mouse. Thus, we need to use a sufficient volume of antibodies in order to reproduce the staining performance. In addition, the antibody for the CD34 antigen (clone; RAM34) requires 90 min for sufficient staining, while 30 min is enough for other antibodies. Second, irradiation usually causes pancytopenia in the recipient mice. If recipient-derived neutrophils persist in many recipient mice, this indicates that the radiation dose was insufficient. In such a case, optimization of the radiation dose is recommended. Third, if most of the mice die soon after the transplantation, there are two possible explanations: an inadequate number of supporting cells or unsuccessful retro-orbital injection.
For decades, it has been controversial whether the bonafide HSC fraction is homogeneous or heterogeneous22,23,24. In this study, the recipient mice that received the Hoxb5pos pHSC transplantation presented different donor chimeras and differentiation patterns (Figure 4A), indicating that this fraction could be heterogeneous. However, these fluctuations could be caused both by the use of unpurified bone marrow cells as the supporting cells and the different radio-sensitivities of individual mice25.
In summary, we have demonstrated a step-by-step protocol for the isolation of LT-HSCs and ST-HSCs using the Hoxb5 reporter system. To date, the detection of LT-HSCs has depended on the competitive transplantation assay, which requires more than 8 months. In contrast, the Hoxb5 reporter system enables us to identify both LT-HSCs and ST-HSCs prospectively and use them for various functional analyses. Figure 4 and Figure 5 also show that the Hoxb5 expression level seems to be correlated with the degree of donor chimerism in the second recipient mice. Additionally, taking advantage of the Hoxb5 reporter system, we previously revealed that LT-HSCs and ST-HSCs work in a complementary fashion for continuous hematopoietic reconstitution after hematopoietic stem cell transplantation26. Moreover, we demonstrated that exogenous Hoxb5 expression could partially reverse the cell fate of ST-HSCs to that of LT-HSCs, indicating that the presence or absence of Hoxb5 explains the heterogeneity of self-renewal ability in the cell surface marker-defined HSC fraction27.
In addition to these findings, the prospective isolation of LT-HSCs allows us to analyze LT-HSCs under various physiological conditions, such as aging, inflammation, and so on. These analyses will greatly facilitate the understanding of the functions of LT-HSCs.
Ujawnienia
The authors declare no conflicts of interest associated with this study.
Podziękowania
We gratefully acknowledge Hiroshi Kiyonari for the animal care and for providing recipient mice at RIKEN BDR, as well as Hitomi Oga, Kayoko Nagasaka, and Masaki Miyahashi for laboratory management at Kobe University. The authors also greatly appreciate the ongoing support for this work. Masanori Miyanishi was supported by the Japan Society for the Promotion of Science (JSPS) KAKENHI Grant Numbers JP17K07407 and JP20H03268, The Mochida Memorial Foundation for Medical and Pharmaceutical Research, The Life Science Foundation of Japan, The Takeda Science Foundation, The Astellas Foundation for Research on Metabolic Disorders, and AMED-PRIME, AMED under Grant Number JP18gm6110020. Taro Sakamaki is supported by JSPS KAKENHI Grant Numbers JP21K20669 and JP22K16334 and was supported by the JSPS Core-to-Core Program and RIKEN Junior Research Associate Program. Katsuyuki Nishi was supported by JSPS Grant Number KAKENHI JP18J13408.
Materiały
| Name | Company | Catalog Number | Comments |
| 0.2 mL Strip of 8 Tubes, Dome Cap | SSIbio | 3230-00 | |
| 0.5M EDTA pH 8.0 | Iinvtrogen | AM9260G | |
| 100 µm Cell Strainer | Falcon | 352360 | |
| 30G insulin syringe | BD | 326668 | |
| 40 µm Cell Strainer | Falcon | 352340 | |
| 5 mL Round Bottom Polystyrene Test Tube, with Cell Strainer Snap Cap | FALCON | 352235 | |
| 7-AAD Viability Staining Solution | BioLegend | 420404 | |
| 96 well U-Bottom | FALCON | 351177 | |
| Anti-APC-MicroBeads | Milteny biotec | 130-090-855 | |
| Aspirator with trap flask | Biosan | FTA-1 | |
| B220-Alexa Fluor 700 (RA3-6B2) | BioLegend | 103232 | |
| B220-Biotin (RA3-6B2) | BioLegend | 103204 | |
| B220-BV786 (RA3-6B2) | BD Biosciences | 563894 | |
| B6.CD45.1 congenic mice | Sankyo Labo Service | N/A | |
| Baytril 10% | BAYER | 341106546 | |
| BD FACS Aria II special order system | BD | N/A | |
| Brilliant stain buffer | BD | 566349 | |
| CD11b-Alexa Fluor 700 (M1/70) | BioLegend | 101222 | |
| CD11b-Biotin (M1/70) | BioLegend | 101204 | |
| CD11b-BUV395 (M1/70) | BD Biosciences | 563553 | |
| CD11b-BV711 (M1/70) | BD Biosciences | 563168 | |
| CD127-Alexa Fluor 700 (A7R34) | Invitrogen | 56-1271-82 | |
| CD150-BV421 (TC15-12F12.2) | BioLegend | 115943 | |
| CD16/CD32-Alexa Fluor 700 (93) | Invitrogen | 56-0161-82 | |
| CD34-Alexa Fluor 647 (RAM34) | BD Biosciences | 560230 | |
| CD34-FITC (RAM34) | Invitrogen | 11034185 | |
| CD3-Alexa Fluor 700 (17A2) | BioLegend | 100216 | |
| CD3ε -Biotin (145-2C11) | BioLegend | 100304 | |
| CD3ε -BV421 (145-2C11) | BioLegend | 100341 | |
| CD45.1/CD45.2 congenic mice | N/A | N/A | Bred in our Laboratory |
| CD45.1-FITC (A20) | BD Biosciences | 553775 | |
| CD45.2-PE (104) | BD Biosciences | 560695 | |
| CD4-Alexa Fluor 700 (GK1.5) | BioLegend | 100430 | |
| CD4-Biotin (GK1.5) | BioLegend | 100404 | |
| CD8a-Alexa Fluor 700 (53-6.7) | BioLegend | 100730 | |
| CD8a-Biotin (53-6.7) | BioLegend | 100704 | |
| Centrifuge Tube 15ml | NICHIRYO | 00-ETS-CT-15 | |
| Centrifuge Tube 50ml | NICHIRYO | 00-ETS-CT-50 | |
| c-Kit-APC-eFluor780 (2B8) | Invitrogen | 47117182 | |
| D-PBS (-) without Ca and Mg, liquid | Nacalai | 14249-24 | |
| Fetal Bovine Serum | Thermo Fisher | 10270106 | |
| Flk2-PerCP-eFluor710 (A2F10) | eBioscience | 46135182 | |
| FlowJo version 10 | BD Biosciences | https://www.flowjo.com/solutions/flowjo | |
| Gmmacell 40 Exactor | Best theratronics | N/A | |
| Gr-1-Alexa Fluor 700 (RB6-8C5) | BioLegend | 108422 | |
| Gr-1-Biotin (RB6-8C5) | BioLegend | 108404 | |
| Hoxb5-tri-mCherry mice (C57BL/6J background) | N/A | N/A | Bred in our Laboratory |
| IgG from rat serum, technical grade, >=80% (SDS-PAGE), buffered aqueous solution | Sigma-Aldrich | I8015-100MG | |
| isoflurane | Pfizer | 4987-114-13340-3 | |
| Kimwipes S200 | NIPPON PAPER CRECIA | 6-6689-01 | |
| LS Columns | Milteny biotec | 130-042-401 | |
| Lysis buffer | BD | 555899 | |
| MACS MultiStand | Milteny biotec | 130-042-303 | |
| Microplate for Tissue Culture (For Adhesion Cell) 6Well | IWAKI | 3810-006 | |
| MidiMACS Separator | Milteny biotec | 130-042-302 | |
| Mouse Pie Cages | Natsume Seisakusho | KN-331 | |
| Multipurpose refrigerated Centrifuge | TOMY | EX-125 | |
| NARCOBIT-E (II) | Natsume Seisakusho | KN-1071-I | |
| NK-1.1-PerCP-Cy5.5 (PK136) | BioLegend | 108728 | |
| Penicillin-Streptomycin Mixed Solution | nacalai | 26253-84 | |
| Porcelain Mortar φ120mm with Pestle | Asone | 6-549-03 | |
| Protein LoBind Tube 1.5 mL | Eppendorf | 22431081 | |
| Sca-I-BUV395 (D7) | BD Biosciences | 563990 | |
| Stainless steel scalpel blade | FastGene | FG-B2010 | |
| Streptavidin-BUV737 | BD Biosciences | 612775 | |
| SYTOX-red | Invitrogen | S34859 | |
| Tailveiner Restrainer for Mice standard | Braintree | TV-150 STD | |
| TCRb-BV421 (H57-597) | BioLegend | 109230 | |
| Ter-119-Alexa Fluor 700 (TER-119) | BioLegend | 116220 | |
| Ter-119-Biotin (TER-119) | BioLegend | 116204 | |
| Terumo 5ml Concentric Luer-Slip Syringe | TERUMO | SS-05LZ | |
| Terumo Hypodermic Needle 23G x 1 | TERUMO | NN-2325-R |
Odniesienia
- Weissman, I. L., Shizuru, J. A. The origins of the identification and isolation of hematopoietic stem cells, and their capability to induce donor-specific transplantation tolerance and treat autoimmune diseases. Blood. 112 (9), 3543-3553 (2008).
- Majeti, R., Park, C. Y., Weissman, I. L. Identification of a hierarchy of multipotent hematopoietic progenitors in human cord blood. Cell Stem Cell. 1 (6), 635-645 (2007).
- Spangrude, G. J., Heimfeld, S., Weissman, I. L. Purification and characterization of mouse hematopoietic stem cells. Science. 241 (4861), 58-62 (1988).
- Ogawa, M., et al. Expression and function of c-kit in hemopoietic progenitor cells. Journal of Experimental Medicine. 174 (1), 63-71 (1991).
- Ikuta, K., Weissman, I. L. Evidence that hematopoietic stem cells express mouse c-kit but do not depend on steel factor for their generation. Proceedings of the National Academy of Sciences of the United States of America. 89 (4), 1502-1506 (1992).
- Osawa, M., Hanada, K., Hamada, H., Nakauchi, H. Long-term lymphohematopoietic reconstitution by a single CD34-low/negative hematopoietic stem cell. Science. 273 (5272), 242-245 (1996).
- Christensen, J. L., Weissman, I. L. Flk-2 is a marker in hematopoietic stem cell differentiation: A simple method to isolate long-term stem cells. Proceedings of the National Academy of Sciences of the United States of America. 98 (25), 14541-14546 (2001).
- Kiel, M. J., et al. SLAM family receptors distinguish hematopoietic stem and progenitor cells and reveal endothelial niches for stem cells. Cell. 121 (7), 1109-1121 (2005).
- Morrison, S. J., Weissman, I. L. The long-term repopulating subset of hematopoietic stem cells is deterministic and isolatable by phenotype. Immunity. 1 (8), 661-673 (1994).
- Spangrude, G. J., Brooks, D. M., Tumas, D. B. Long-term repopulation of irradiated mice with limiting numbers of purified hematopoietic stem cells: In vivo expansion of stem cell phenotype but not function. Blood. 85 (4), 1006-1016 (1995).
- Dykstra, B., Olthof, S., Schreuder, J., Ritsema, M., de Haan, G. Clonal analysis reveals multiple functional defects of aged murine hematopoietic stem cells. Journal of Experimental Medicine. 208 (13), 2691-2703 (2011).
- Grover, A., et al. Single-cell RNA sequencing reveals molecular and functional platelet bias of aged haematopoietic stem cells. Nature Communications. 7, 11075 (2016).
- Kataoka, K., et al. Evi1 is essential for hematopoietic stem cell self-renewal, and its expression marks hematopoietic cells with long-term multilineage repopulating activity. Journal of Experimental Medicine. 208 (12), 2403-2416 (2011).
- Gazit, R., et al. Fgd5 identifies hematopoietic stem cells in the murine bone marrow. Journal of Experimental Medicine. 211 (7), 1315-1331 (2014).
- Acar, M., et al. Deep imaging of bone marrow shows non-dividing stem cells are mainly perisinusoidal. Nature. 526 (7571), 126-130 (2015).
- Chen, J. Y., et al. Hoxb5 marks long-term haematopoietic stem cells and reveals a homogenous perivascular niche. Nature. 530 (7589), 223-227 (2016).
- Ema, H., et al. Quantification of self-renewal capacity in single hematopoietic stem cells from normal and Lnk-deficient mice. Developmental Cell. 8 (6), 907-914 (2005).
- Morita, Y., Ema, H., Nakauchi, H. Heterogeneity and hierarchy within the most primitive hematopoietic stem cell compartment. Journal of Experimental Medicine. 207 (6), 1173-1182 (2010).
- Yamamoto, R., et al. Clonal analysis unveils self-renewing lineage-restricted progenitors generated directly from hematopoietic stem cells. Cell. 154 (5), 1112-1126 (2013).
- Fathman, J. W., et al. Upregulation of CD11A on hematopoietic stem cells denotes the loss of long-term reconstitution potential. Stem Cell Reports. 3 (5), 707-715 (2014).
- Oguro, H., Ding, L., Morrison, S. J. SLAM family markers resolve functionally distinct subpopulations of hematopoietic stem cells and multipotent progenitors. Cell Stem Cell. 13 (1), 102-116 (2013).
- Haas, S., Trumpp, A., Milsom, M. D. Causes and consequences of hematopoietic stem cell heterogeneity. Cell Stem Cell. 22 (5), 627-638 (2018).
- Schroeder, T. Hematopoietic stem cell heterogeneity: Subtypes, not unpredictable behavior. Cell Stem Cell. 6 (3), 203-207 (2010).
- Muller-Sieburg, C. E., Sieburg, H. B., Bernitz, J. M., Cattarossi, G. Stem cell heterogeneity: Implications for aging and regenerative medicine. Blood. 119 (17), 3900-3907 (2012).
- Duran-Struuck, R., Dysko, R. C. Principles of bone marrow transplantation (BMT): Providing optimal veterinary and husbandry care to irradiated mice in BMT studies. Journal of the American Association for Laboratory Animal Science. 48 (1), 11-22 (2009).
- Nishi, K., et al. Identification of the minimum requirements for successful haematopoietic stem cell transplantation. British Journal of Haematology. 196 (3), 711-723 (2022).
- Sakamaki, T., et al. Hoxb5 defines the heterogeneity of self-renewal capacity in the hematopoietic stem cell compartment. Biochemical and Biophysical Research Communications. 539, 34-41 (2021).
Przedruki i uprawnienia
Zapytaj o uprawnienia na użycie tekstu lub obrazów z tego artykułu JoVE
Zapytaj o uprawnieniaThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. Wszelkie prawa zastrzeżone