Method Article
Isolation, Purification, and Differentiation of Osteoclast Precursors from Rat Bone Marrow
W tym Artykule
Podsumowanie
Osteoclasts are tissue-specific macrophage polykaryons derived from the monocyte-macrophage lineage of hematopoietic stem cells. This protocol describes how to isolate bone marrow cells so that large quantities of osteoclasts are obtained while reducing the risk of accidents found in traditional methods.
Streszczenie
Osteoclasts are large, multinucleated, and bone-resorbing cells of the monocyte-macrophage lineage that are formed by the fusion of monocytes or macrophage precursors. Excessive bone resorption is one the most significant cellular mechanisms leading to osteolytic diseases, including osteoporosis, periodontitis, and periprosthetic osteolysis. The main physiological function of osteoclasts is to absorb both the hydroxyapatite mineral component and the organic matrix of bone, generating the characteristic resorption appearance on the surface of bones. There are relatively few osteoclasts compared to other cells in the body, especially in adult bones. Recent studies have focused on how to obtain more mature osteoclasts in less time, which has always been a problem. Several improvements in the isolation and culture techniques have developed in laboratories in order to obtain more mature osteoclasts. Here, we introduce a method that isolates bone marrow in less time and with less effort compared to the traditional procedure, using a special and simple device. With the use of density gradient centrifugation, we obtain large amounts of fully differentiated osteoclasts from rat bone marrow, which are identified by classical methods.
Wprowadzenie
Bone homeostasis is a complex physiological process that is regulated by bone-resorbing osteoclasts and bone-forming osteoblasts1. A balance between osteoblastic and osteoclastic activity mediated through osteoblasts and osteoclasts, respectively, is highly essential for maintaining bone health and homeostasis, because perturbations in bone homeostasis might lead to bone diseases, such as abnormal bone growth or loss of bone density. As unique bone-resorption cells, osteoclasts are important in diseases related to abnormal bone destruction, including osteoporosis, periodontitis, and periprosthetic osteolysis2,3.
The development of an osteoclast culture is mainly divided into two stages. The methods established by Boyde et al.4 and Chambers et al.5 composed the first stage in the 1980s. They obtained relatively abundant osteoclasts from the bones of newborn animals, which is the rapid remodeling period. Osteoclasts are released by fragmenting and stirring the bones in a special medium. However, the cells obtained by this method are low in quantity and purity. The second stage was the development of long-range cultures of osteoclast formation, using hematopoietic lineage cells derived from bone marrow6. Cytokines, such as 1α,25-dihydroxyvitamin D3, prostaglandin E2 (PGE-2), and parathyroid hormone (PTH), which are added into the culture medium, act through the system of osteoblasts/stromal cells to stimulate osteoclast formation7,8. However, the purity and quantity of osteoclasts obtained by this method cannot meet the needs of modern molecular biology research. Then, the discovery of macrophage colony-stimulating factor (M-CSF) and receptor activator for nuclear factor-κB ligand (RANKL) make osteoclastogenesis easier9,10,11, and the method of using M-CSF and RANKL to directly stimulate osteoclast formation is widely used around the world. However, there are still some details in the methodology that need to be improved.
Currently, the most commonly used osteoclast culture method, as described by Marino et al.12 and Pei et al.13, often requires the removal of the surrounding tissue around the bone and uses a sterilized needle to flush the marrow cavity with completed media. There are some drawbacks to this process, including the fact that (1) the removal of the surrounding tissue around the bone requires much time and great surgical technique, (2) the bones are fragile and can lead to bone marrow outflow, (3) the bone marrow cavity might be too tiny to flush, and (4) there is a risk of needle stick injury. To avoid these problems, we centrifuge the tubes containing the bones for bone marrow instead of needle-flushing the bone marrow. Here, we introduce a stable and safe method which isolates bone marrow in less time and with less effort compared to the traditional procedure. Together with the use of density gradient centrifugation, we obtain large amounts of fully differentiated osteoclasts in vitro.
Protokół
All the methods involving the animals described here are approved by the Institutional Animal Care and Use Committee (IACUC) of Nanjing University of Chinese Medicine.
1. Setup
- Prepare several longitudinally cut 1 mL pipette tips (1 cm) and several 1.5 mL microcentrifuge tubes. Put the pipette tips and microcentrifuge tubes at 103 kPa and 121 °C for 20 min and ensure that they are sterile.
- Prepare a box of ice and several sterile dishes to preserve the isolated tissues during the isolation procedure.
2. Preparation of Culture Medium
- Prepare the complete culture medium. Use minimum essential medium alpha (α-MEM) that contains a final solution of 1% of penicillin/streptomycin and 10% fetal bovine serum (FBS).
- Filter the media from step 2.1 using a 0.22 μm filter.
- Prepare the bone marrow induction medium. For 50 mL of the solution, add 125 µL of M-CSF at a concentration of 10,000 ng/mL (Table of Materials) to 49.875 mL of complete culture medium (from step 2.2) to make a final solution of 25 ng/mL M-CSF.
- Prepare the osteoclast induction medium. For 50 mL of the solution, add 500 µL of RANKL at a concentration of 10,000 ng/mL (Table of Materials) to 49.5 mL of bone marrow induction medium (from step 2.3) to make a final solution of 25 ng/mL M-CSF and 100 ng/mL RANKL.
3. Isolation of Bone mMarrow-derived Cells
- Euthanasia
- Euthanize four Sprague Dawley rats by CO2 inhalation followed by cervical dislocation, and immerse them in 75% ethanol for 1 min.
NOTE: Ensure that animals are of the same sex and of a similar age. Animals that are 2 to 3 weeks old are recommended. Two rats are prepared for the centrifugal method, while the other two are for the traditional method.
- Euthanize four Sprague Dawley rats by CO2 inhalation followed by cervical dislocation, and immerse them in 75% ethanol for 1 min.
- Traditional method
- Place the animals on a disinfectant board in a supine position. Make a small incision (approximately 1 cm) at the proximal femur to peel the skin, using sterile scissors.
- Dissect out the femurs and tibias. Cut the bilateral connection parts around the hip, knee, and ankle joints to isolate the tibias and femurs carefully and gently. Cut off part of the tissues around the bone; be thorough. Do not fracture the tibias and femurs during the whole process.
- Place the cleaned bones into a dish with 5 mL of the complete culture medium. Cut off the long bone with sterile scissors and use a 1 mL syringe needle to flush the marrow cavity carefully with 10 mL of complete media until the marrow cavity turns white.
- Transfer the cell suspension from step 3.2.3 to a 50 mL tube, and then, filter it with a 70 μm strainer to remove the remaining tissue.
- Add 5 mL of red blood cell lysis buffer (Table of Materials) to the cell suspension. Incubate the cells for 8 min on ice. Then, centrifuge the cells at 250 x g for 5 min to yield the cell pellet.
- Aspirate off the medium and resuspend the cells in 10 mL of complete media. Count the cells using a hemocytometer and calculate the time spent on the steps from this section (section 3.2).
- Improved method
- Place the animals on a disinfectant board in a supine position. Make a small incision (approximately 1 cm) at the proximal femur with sterile scissors to peel the skin.
- Dissect out the femurs and tibias. Properly cut off the part of the tissues around the bone (no need to remove completely). Do not fracture the tibias and femurs during the whole process.
- Rinse the tibias and femurs with 12 mL of phosphate-buffered saline (PBS) and cut them in half. Place the snipped tibias and femurs into a 1 mL pipette tip (from step 1.1), which is then put into a microcentrifuge tube and is centrifuged 3x at 1,000 x g for 45 s at 4 °C.
- After centrifugation, remove the pipette tip containing the bone, and leave the bone marrow in the tube. Add 200 μL of complete media into the microcentrifuge tube and repeat pipetting to disintegrate the marrow thoroughly.
- Transfer the cell suspension from step 3.3.4 to a 50 mL tube, and then, filter it with a 70 μm strainer to remove the remaining tissue.
- Count the cells using a hemocytometer, and calculate the time spent on the steps from this section (section 3.3).
4. Purification by Density Gradient Centrifugation
- Adjust the cell suspension from step 3.2.6 or step 3.3.5 to 2 mL with α-MEM.
- Prepare one sterile silicified centrifugal tube and add 8 mL of the cell separation solution (Table of Materials) to the tube.
- Add the cell suspension from step 4.1 to the cell separation solution. Adhere the pipette tip to the inner surface of the tube and keep a 45° to the inner surface. After adding the suspension, a clear limit will appear between the layer of the cells and the separation solution.
NOTE: The operation requires great care and needs to be performed slowly. - Centrifuge the layered solution in a horizontal centrifuge at 500 x g for 30 min. After centrifugation, aspirate off the cloudy second layer, which contains the target cells, from top to bottom.
NOTE: Cancel the acceleration and deceleration of the centrifuge before centrifugation. There are six layers after centrifugation; the first layer is the diluent layer, the second layer is the monocytes layer, the third layer is the layer of mononuclear cells, the fourth layer is the transparent separation liquid one layer, the fifth is the granular cell layer, and the sixth layer is the red cell layer. - Transfer the target cells to a new tube. Add 5 mL of PBS to wash the cells 3x. After each wash, centrifuge the target cells at 250 x g for 5 min to yield the cell pellet.
- Resuspend the cell pellet with bone marrow induction medium and count the cells using a hemocytometer. Add 5–8 mL of bone marrow induction medium from step 2.3 to obtain a final cell solution of 300,000 cells/mL. Add 1 mL to each well of a 24-well plate.
5. Culture and Differentiation
- After incubating the cells at 37 °C for 24 h, gently aspirate off the medium and add 1 mL of the osteoclast induction medium to each well. Agitate the plate gently and put it in an incubator at 37 °C.
- Change the osteoclast induction medium every 48 h. While doing so, change 0.8 mL of the medium in the well and agitate the plate gently.
NOTE: Large, motile, and multinucleated osteoclasts should be observed in the well under an inverted microscope, typically around days 4–6.
6. Tartrate-resistant Acid Phosphatase Staining
NOTE: Multinucleate osteoclasts will be present after 4–6 days if the induction (section 5) is successful.
- Prepare the fixative solution by combining 4 mL of 37% formaldehyde, 32.5 mL of acetone, and 12.5 mL of a citrate solution. Store the fixative solution at 4 °C.
- Prepare the tartrate-resistant acid phosphatase (TRAP) stain solution (Table of Materials) by adding 50 µL of sodium nitrite, 50 µL of Fast Garnet GBC base solution, 50 µL of naphthol AS-BI phosphate solution, 200 µL of an acetate solution, and 100 µL of a tartrate solution into 4.55 mL of deionized water that is prewarmed to 37 °C. Then, mix gently by inversion for 1 min, and let it stand for 2 min.
- After the successful induction of osteoclasts, aspirate off the medium, and gently wash the wells 3x with PBS.
- Bring the fixative solution to room temperature (RT). Add 2 mL of fixative solution to the wells for 30 s, and do not allow the cells to dry.
- Aspirate the fixative solution, gently wash it 3x with deionized water that is prewarmed to 37 °C, and then, aspirate the water.
- Add 2 mL of TRAP stain solution for 1 h at 37 °C and keep the sample in the dark.
- After 1 h, aspirate the stain, and gently wash the sample with deionized water that is prewarmed to 37 °C, and then, aspirate the water.
- Counterstain the cells for 1 min in a hematoxylin solution. After staining, aspirate the hematoxylin solution and gently wash the cells 3x with deionized water.
- Image osteoclasts using brightfield microscopy, and TRAP+ cells with three or more nuclei will appear purple.
7. Bone Resorption Assay Using Toluidine Blue Staining
- Pretreatment of the bone slices
- Cut the fresh bovine femoral bone cortex into 2 cm thick slices along the longitudinal axis by microelectric saw. Then, cut and grind the slices with hard tissue grinders into 80 µm.
- Wash the slices in a beaker with deionized water and 100 Hz ultrasound for 1 h, and repeat 3x.
- Immerse the slices into 75% alcohol for 2 h. Then, aspirate off the alcohol and expose each side of the slices to ultraviolet light for 1 h on a clean platform.
- Before planting the cells, immerse the slices in the culture medium for at least 2 h.
- Toluidine blue staining
- Place the pretreated bone slices into the 24-well plate. Plant the cells as mentioned in step 4.6 and induce the cells as mentioned in section 5.
- After the appearance of osteoclasts (after 4–6 days), wash the slices with 1 mL of 0.25 M ammonium hydroxide, and sonicate them 3x for 5 min each to remove the living cells to allow the analysis of the resorption pits on the bone slices. Then, remove the ammonium hydroxide and stain the slices with 1 mL of 1% (wt/vol) toluidine blue solution per slice for 2 min.
- Wash the slices with PBS. Randomly select five views and perform a semiquantitative analysis of the resorption area using an image analysis software.
8. Scanning Electron Microscopy
- Prefix the bone slices from step 7.2.1 with 1 mL of 2.5% glutaraldehyde per slice for 2 h at RT, and then, sonicate the slices 3x for 3 min each with 1 M ammonium hydroxide to remove the cells and remove the ammonium hydroxide.
- Wash the bone slices 3x for 12 min each with PBS.
- Fix the bone slices with 1% osmic acid for 2 h at RT.
- Perform ethanol gradient dehydration, with 50%, 70%, 80%, 90%, and 95% ethanol, for 15 min for every gradient, and then, replace the ethanol with isoamyl acetate for 15 min.
- Coat the slices with gold palladium, and then, analyze by scanning electron microscopy.
9. Immunofluorescence Staining of Calcitonin Receptor
NOTE: Multinucleate osteoclasts will be present after 4–6 days if the induction is successful (section 5).
- Aspirate off the medium and gently wash the well 3x with PBS.
- Fix the cells for 10 min with 4% paraformaldehyde, which is precooled to 4 °C.
- Add 1 mL of PBS with 0.3% nonionic surfactant per well for 30 min on ice. Then, aspirate off the PBS with 0.3% nonionic surfactant and add 1 mL of PBS with 5% FBS per well for 30 min on ice.
- Aspirate off the PBS and incubate the membranes with anti-calcitonin receptor (anti-CTR) at a 1:100 dilution in PBS at 4 °C overnight.
- Aspirate off the solution, and incubate the membranes with Alexa-488-conjugated anti-rabbit IgG antibodies at a 1:1,000 dilution in PBS for 1 h at RT.
- Aspirate off the solution and gently wash the cells 3x with PBS. Counterstain the nuclei with Hoechst 33342 stain for 3 min at RT.
- Aspirate off the solution, and gently wash the cells 3x with PBS. Observe the intensity of CTR by fluorescence microscopy.
Wyniki
The purpose of the protocol was to isolate and purify large numbers of osteoclast precursors conveniently and induce osteoclasts successfully. By supplementing with M-CSF and RANKL, giant osteoclasts were seen on days 5–6. The formation of osteoclasts was successfully identified by TRAP staining (Figure 1A). Large and purple cells were regarded as TRAP-positive cells with multiple nuclei (typically ≥ three nuclei). Through this method, it was typical to obtain 800 osteoclasts, containing as many as 30 nuclei per osteoclast, in a 24-well plate (Figure 1B). Compared to the traditional method, the improved method saved approximately 20 min in the whole isolation progress (Figure 1C).
Using bone slices to assess the activity of bone resorption is typical in vitro method. Through toluidine blue staining, the resorption area was visualized as light green (Figure 2A) and was calculated. The structure and characteristics of the bone pits were clearly observed by scanning electron microscopy (Figure 2B).
The CTR, one of the osteoclast-specific cell markers, is critical to identify osteoclasts and study the formation of osteoclasts in bone. The positive expression of CTR clearly identifies osteoclasts and distinguishes them from macrophage polykaryons. CTR was detected by the immunofluorescence assays. The green color indicated the expression of CTR and the blue indicated the nuclei (Figure 3).
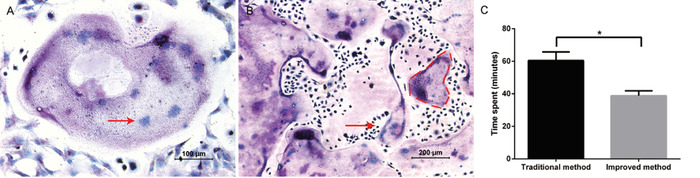
Figure 1: Osteoclastogenesis from bone marrow-derived cells. (A) Representative image of TRAP staining. The brightfield micrograph at 10x magnification demonstrates multiple giant, multinucleated osteoclasts that are TRAP-positive and were observed at 20x magnification. An example of a nucleus within a multinucleated osteoclast is shown by the red arrow. (B) The brightfield micrograph at 10x magnification proves that a large number of TRAP-positive osteoclasts were present. An example of a large, multinucleated osteoclast is outlined by the red dashed line. (C) Comparison of the time spent on the two isolation methods. All the operations were performed by the same group of experimenters. The data represent the means ± standard deviation. *P < 0.05, n = 3. Please click here to view a larger version of this figure.
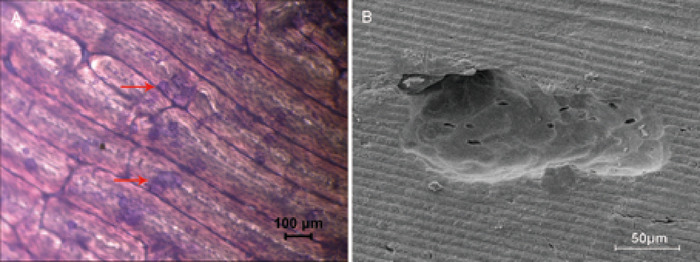
Figure 2: Bone resorption assays by bone slices. (A) The brightfield micrograph at 4x magnification demonstrates the bone resorption area, is stained light green by the toluidine blue stain, and is round-, oval-, or sausage-shaped. An example of the bone resorption area is shown by the red arrow. (B) Resorption pits observed with scanning electron microscopy at 500x magnification. Please click here to view a larger version of this figure.

Figure 3: Characterization of the CTR expression in the osteoclasts using immunofluorescence staining. The panel shows a clear CTR signal around the cells. (A) CTR-positive cells. (B) Nuclei. (C) Merged. Please click here to view a larger version of this figure.
Dyskusje
The ability to obtain and study osteoclasts in vitro is a critical and fundamental skill for any researcher wishing to study bone metabolism, which may help understand the mechanisms of bone-absorbing diseases and develop novel therapeutic agents. The present study described a protocol with some modifications based on previous methods.
By using pipette tips and microcentrifuge tubes to obtain bone marrow, it largely reduced the operation time of obtaining bone marrow and the workload of laboratory personnel compared to the traditional methods. Meantime, the method avoids the risk of bone marrow loss or needle stick injury. In a previous study, bone marrow-derived cells were plated immediately after isolation12,13. Density gradient centrifugation was used to select the bone marrow monocytes according to the differences in the settlement coefficients. In the process of density gradient centrifugation, the addition of the cell separation media must be performed gently and carefully along the wall, in order to make the boundaries clear. However, the technique is limited by the function of the centrifuge and in whether it is set up with the parameters of acceleration and deceleration. The seeding density is the key condition for the cultivation of osteoclasts. Several times, the protocol failed due to an improper seeding density when plating the cells. Thus, approximately 300,000 cells per 24-well plate well is recommended in this protocol. This protocol is also appropriate for obtaining various kinds of bone marrow-derived cells in rats or other animals (e.g., mouse, rabbit, and chicken).
The most classic method to evaluate the activity of osteoclasts is the resorption pit assay. Bovine bone cortex is commonly used for the resorption pit assay because its sources are widely available. With the help of a modern sectioning system, we can obtain thin bone slices more easily. The resorption pit assay has three factors. To generate successfully a resorption pit in the slices, the slices must be treated strictly as described for defatting in the protocol. In addition, rat osteoclasts are activated to form resorption pits in a slightly acidic environment, and the resorption function will essentially "shut down" when the pH goes up above 7.214,15. Thus, it is noteworthy that opening the incubator's door frequently during the progress of the experiments may lead to perturbations of the pH and pCO2 values, even influencing the resorption function of the osteoclasts16. Finally, the bone slices should remain at the bottom of the wells and should not be moved or float up when changing the medium, in order to avoid irritation.
The CTR is one member of the class II subfamily of the 7-transmembrane G-protein-coupled receptors that also contain the parathyroid hormone and secretin receptors17. As well as TRAP staining and the resorption pit assay, osteoclasts are identified by morphology and function, and CTR-positive identifies osteoclasts in immunology. Now, we can also use fluorescent staining of the actin ring and measure pyridinoline crosslinks in the supernatant to identify osteoclasts.
Although different researchers have different familiarities with experimental techniques and the total amount of cells in bone marrow is certain, we obtained bone marrow cells in a shorter time, relatively, and in the end, obtained large amounts of fully differentiated osteoclasts from the rat bone marrow.
Ujawnienia
The authors have nothing to disclose.
Podziękowania
This work was supported by the National Natural Science Foundation of China (81473692) to Yong Ma. The authors thank all the staff of the Medical Research Center of the First College of Clinical Medicine in Nanjing University of Chinese Medicine.
Materiały
| Name | Company | Catalog Number | Comments |
| Acid Phosphatase, Lekocyte (TRAP) kit | Sigma-Aldrich | 387A | |
| Automatic Hard Tissue Slicer | Lecia | RM2265 | |
| Bovine femoral bone | Purchased by ourselves | ||
| Cell Incubator | Heraus | BB16/BB5060 | |
| Fetal Bovine Serum | Sarana | s-fbs-au-015 | |
| Goat Anti-Rabbit IgG H&L (Alexa Fluor 488) | Abcam | ab150077 | |
| Hard Tissue Grinders | Lecia | SP-2600 | |
| Histopaque Kit | TianJing Haoyang | TBD2013DR | Silicified centrifugal tube amd cell separation solution |
| Hochest33342 | Sigma-Aldrich | B2261 | |
| Inverted Phase Contrast Microscope | Olympus | CKX31 | |
| Inverted Fluorescence Microscope | Lecia | DMI-3000 | |
| MEM, no Glutamine | Gibco | 11090-081 | |
| Penicillin-Streptomycin, Liquid | Gibco | 15140122 | |
| Rabbit Anti-Calcitonin receptor | Bioss | bs-0124R | |
| Recombinant Rat M-CSF | PeproTech | 400-28 | |
| Recombinant Rat sRANK Ligand | PeproTech | 400-30 | |
| Red Blood Cell Lysis Buffer | Absin | abs47014932 | |
| Scanning Electron Microscopy | FEI | Quanta 200 | |
| Toluidine Blue | Sigma-Aldrich | 89649 |
Odniesienia
- Boyle, W. J., Simonet, W. S., Lacey, D. L. Osteoclast differentiation and activation. Nature. 423 (6937), 337-342 (2003).
- Kular, J., Tickner, J., Chim, S. M., Xu, J. K. An overview of the regulation of bone remodelling at the cellular level. Clinical Biochemistry. 45 (12), 863-873 (2012).
- Park, S. J., et al. Apoptosis of the reduced enamel epithelium and its implications for bone resorption during tooth eruption. Journal Of Molecular Histology. 44 (1), 65-73 (2013).
- Boyde, A., Ali, N. N., Jones, S. J. Resorption of dentine by isolated osteoclasts in vitro. British Dental Journal. 156 (6), 216-220 (1984).
- Chambers, T. J., Revell, P. A., Fuller, K., Athanasou, N. A. Resorption of bone by isolated rabbit osteoclasts. Journal of Cell Science. 66, 383-399 (1984).
- Takahashi, N., et al. Osteoclast-like cell formation and its regulation by osteotropic hormones in mouse bone marrow cultures. Endocrinology. 122 (4), 1373-1382 (1988).
- Takahashi, N., et al. Osteoblastic cells are involved in osteoclast formation. Endocrinology. 123 (5), 2600-2602 (1988).
- Suda, T., Takahashi, N., Martin, T. J. Modulation of osteoclast differentiation. Endocrine Reviews. 13 (1), 66-80 (1992).
- Yoshida, H., et al. The murine mutation osteopetrosis is in the coding region of the macrophage colony stimulating factor gene. Nature. 345 (6274), 442-444 (1990).
- Yasuda, H., et al. Osteoclast differentiation factor is a ligand for osteoprotegerin/osteoclastogenesis-inhibitory factor and is identical to TRANCE/RANKL. Proceedings of The National Academy of Sciences of The United States of America. 95 (7), 3597-3602 (1998).
- Lacey, D. L., et al. Osteoprotegerin ligand is a cytokine that regulates osteoclast differentiation and activation. Cell. 93 (2), 165-176 (1998).
- Marino, S., Logan, J. G., Mellis, D., Capulli, M. Generation and culture of osteoclasts. BoneKEy Reports. 3, 570 (2014).
- Pei, J. R., et al. Fluoride decreased osteoclastic bone resorption through the inhibition of NFATc1 gene expression. Environmental Toxicology. 29 (5), 588-595 (2014).
- Arnett, T. R., Dempster, D. W. Effect of pH on bone resorption by rat osteoclasts in vitro. Endocrinology. 119 (1), 119-124 (1986).
- Arnett, T. R., et al. Hypoxia is a major stimulator of osteoclast formation and bone resorption. Journal of Cellular Physiology. 196 (1), 2-8 (2003).
- Orriss, I. R., Arnett, T. R. Rodent osteoclast cultures. Methods in Molecular Biology. 816, 103-117 (2012).
- Quinn, J. M., et al. Calcitonin receptor antibodies in the identification of osteoclasts. Bone. 25 (1), 1-8 (1999).
Przedruki i uprawnienia
Zapytaj o uprawnienia na użycie tekstu lub obrazów z tego artykułu JoVE
Zapytaj o uprawnieniaThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. Wszelkie prawa zastrzeżone