Method Article
Identification of Kinase-substrate Pairs Using High Throughput Screening
W tym Artykule
Podsumowanie
Protein phosphorylation is a central feature of how cells interpret and respond to information in their extracellular milieu. Here, we present a high throughput screening protocol using kinases purified from mammalian cells to rapidly identify kinases that phosphorylate a substrate(s) of interest.
Streszczenie
We have developed a screening platform to identify dedicated human protein kinases for phosphorylated substrates which can be used to elucidate novel signal transduction pathways. Our approach features the use of a library of purified GST-tagged human protein kinases and a recombinant protein substrate of interest. We have used this technology to identify MAP/microtubule affinity-regulating kinase 2 (MARK2) as the kinase for a glucose-regulated site on CREB-Regulated Transcriptional Coactivator 2 (CRTC2), a protein required for beta cell proliferation, as well as the Axl family of tyrosine kinases as regulators of cell metastasis by phosphorylation of the adaptor protein ELMO. We describe this technology and discuss how it can help to establish a comprehensive map of how cells respond to environmental stimuli.
Wprowadzenie
Protein post-translational modifications (PTMs) are essential for intracellular communication. Perhaps the best studied of all PTMs is phosphorylation, catalyzed by protein kinases, which regulate a myriad of protein functions, including their biochemical activity, subcellular localization, conformation, and stability. The identification of phosphorylation sites on target proteins can be accomplished by tryptic phosphopeptide mapping or by now-standard proteomic techniques using samples enriched for phosphorylated peptides 1,2. While three quarters of the expressed proteome are expected to be phosphorylated 3 and an identified 200,000 phosphorylation sites 5, with estimates up to 1 million 6, many of these have no assigned biology, signaling pathway, or protein kinase.
While identification of phosphorylated sites is relatively straightforward, a comparatively greater challenge is to identify the cognate kinase(s) that targets these sites, a process we refer to as mapping kinase:substrate pairs. Several approaches for identifying kinase:substrate pairs have been described, either starting with a kinase of interest and looking for its substrates or starting with a substrate of interest and attempting to find a modifying kinase experimentally 7-11 or computationally 12. To identify kinases for a known phosphorylated substrate, bioinformatics can be used to identify proteins that contain a short conserved sequence of amino acids flanking the phosphorylated residue (the consensus site), as well as identifying kinases that form a precipitable complex with the substrate. However, these approaches are time-consuming and often do not meet with success.
We developed a systematic functional approach to rapidly identify kinases that can phosphorylate a given substrate 13. The screen assay produces excellent specificity, with very clear selection for potential cognate kinases. Given the centrality of phosphorylation to biological signaling, the screen is useful for discovery in virtually all cell signaling pathways 14-16. The screen involves performing a large-scale kinase assay with a library of human protein kinases. The kinases have been tagged with bacterial glutathione S-transferase (GST) protein and are purified from mammalian cell extracts, which means that the recombinant enzymes — unlike those prepared from bacteria — are generated in the presence of the upstream protein kinases often required for the recombinant enzymes to have activity in vitro. Indeed, while serine, threonine, and tyrosine kinase activity required for downstream kinase activation are present in yeast 10, the yeast genome encodes 122 protein kinases, indicating that the mammalian kinome, with over 500 genes 17, has become significantly more complex in order to regulate the processes unique to higher order organisms. Moreover, the effect of different stimuli relevant to cell biology and human disease (such as small molecules, growth factors, hormones, etc.) can be used to 14,15modulate kinase activity in an appropriate context.
Protokół
1. Preparation of Reagents, Plates, and Cells
- Make 500 ml lysis buffer: 25 mM Tris pH 7.5, 150 mM NaCl, 50 mM NaF, 0.5 mM EDTA pH 8.0, 0.5% TritonX-100, 5 mM beta-glycerophosphate, 5% glycerol. Store at 4 °C. Immediately prior to use, add 1 mM dithiothreitol (DTT), 1 mM phenylmethyl sulfonyl fluoride (PMSF), and 1 mM sodium vanadate. After this step, PMSF is not required in any rinse buffer.
- Make 20 ml of 10x Kinase Buffer: 200 mM Tris pH 7.5, 50 mM beta-glycerophosphate (FW 216), 2 mM sodium vanadate. Aliquot in 1 ml tubes and store at -20 °C. Immediately prior to use, add 5 mM DTT.
- Make 20 ml of 10x M-ATP buffer: 300 µM adenosine triphosphate (ATP), 66 mM MgCl2, 33 mM MnCl2. Aliquot in 1 ml tubes and store at -20 °C.
- Make 100 ml of 2x sodium dodecyl sulfate (SDS) lysis buffer: 1.5 g TRIS base, 20 ml glycerol, 30 ml H2O. Dissolve and adjust pH to 6.8 with HCl. Add 40 ml 10% SDS, adjust volume to 100 ml. Add 25 mg bromophenol blue.
- Spot 4 µl of 25 ng/µl mammalian expression plasmid encoding a GST-kinase 14 per well in sets of 96 well plates and label plates accordingly. Seal and freeze plates at -20 °C until use.
- 1-2 days prior to transfection, culture HEK293T cells in complete Dulbecco’s modified Eagle medium (DMEM) + 10% fetal bovine serum (FBS) and antibiotics in a 37 °C incubator supplemented with CO2 (final 5%). Passage with trypsin and do not allow the stock to become >80% confluent during expansion. A minimum of 6 x 107 cells are required for the screen (approximately 3 x 15 cm dishes of HEK293T cells at 80% confluence).
2. Transfection
Note: See Figure 1 for a flowchart of entire protocol.
- If plates containing kinase plasmids have been frozen, thaw at room temperature and centrifuge at 1,900 x g for 3 min to collect any moisture at the bottom of wells.
- Mix 8.6 ml of reduced serum medium (e.g., OPTI-MEM) with 312.7 µl of lipid-based transfection reagent. Let sit for 5 min.
- Add 10 µl of reduced serum medium to each well using an automated liquid dispenser fitted with a small volume cassette.
- Add 10 µl per well of the reduced serum medium/transfection reagent mix from step 2.2 using an automated liquid dispenser fitted with a small volume cassette. Let sit for 20 to 45 min.
- Resuspend 293T cells at 7.5 x 105 cells/ml in 80 ml of complete DMEM. Add 100 µl of cell suspension (i.e. 7.5 x 104 cells) per well using an automated liquid dispenser fitted with a standard volume cassette.
- Check wells under microscope for uniform cell distribution and return to incubator for 24 hr.
3. GST-kinase Pulldown
- Make fresh: 4 mM pervanadate solution by mixing 60 µl of 0.2 M sodium vanadate with 540 µl of H2O. In a second tube mix 2.7 µl of 30% peroxide and 1.4 ml of PBS. Add the two solutions together and let sit for 15 min before use.
- Using a multichannel pipette (do not use an automated liquid dispenser as it creates too much turbulence in the well) dispense 2 µl of 0.25 M CaCl2 in each well, followed by 2.5 µl of the pervanadate prepared in step 3.1. Incubate each plate at 37 °C for 10 min and then place on ice.
- Prepare 35 ml of lysis buffer by adding DTT, PMSF, and sodium vanadate as indicated in Section 1 (Preparation of Reagents).
- Keeping plates on ice, remove medium from each well using a vacuum aspirator. Immediately add 50 µl/well of ice cold lysis buffer using an automated liquid dispenser fitted with a standard cassette. Let sit 30 min on ice to lyse (optional: can stop here if necessary by sealing plate and storing at -80 °C. To continue, thaw plates on ice).
- Spin plates at 1,900 x g for 3 min at 4 °C.
- Scrape cells from each well using a multichannel pipette and transfer all contents to appropriately labeled V-bottom 96 well plates. Spin plates at 1,900 x g for 10 min at 4 °C.
- During spin, fill glutathione coated plates (one for each 96-well plate) with 100 µl/well of ice cold lysis buffer (without PMSF) as a rinse. Keep plates on ice.
- Remove V-bottom plates from the centrifuge. One at a time, invert the glutathione plates over a sink to shake out the lysis buffer and blot on a paper towel. Transfer lysis buffer from the V-bottom plates to the glutathione plates by tilting the plate and using a multichannel pipette, being careful to not disturb the pellet at the bottom. Cover plates and leave on ice for minimum 2 hr to bind.
- Close to the end of the 2 hr binding step, prepare a radioactivity workstation, ensuring the necessary safety precautions are in place for radioactive work. Set hybridization oven to 30 °C.
- Prepare 200 ml of lysis buffer by adding DTT and sodium vanadate as indicated in Section 1. PMSF is not required at this stage.
- Invert the glutathione plates over a sink to shake out the lysis buffer and blot on a paper towel. Rinse wells 3x with 100 µl lysis buffer (without PMSF). Do not let wells sit dry — keep them in rinse until ready to proceed.
- Prepare 55 ml of 1x kinase buffer (KB) by diluting the 10x stock and adding DTT as indicated in Section 1. Rinse plates once with 50 µl of 1x KB using an automated liquid dispenser fitted with a standard volume cassette. Leave 1x KB in wells until solution A is ready:
- Prepare Solution A by adding 500 to 530 µg of the substrate of interest, 500 µg of myelin basic protein (MBP), 2.65 ml of 10x KB, 13.25 µl of 1 M DTT, and H2O up to 15.9 ml.
- One at a time, invert the plates over a sink to remove 1x KB rinse, blot on paper towel, and immediately add 30 µl of Solution A using an automated liquid dispenser fitted with a small volume cassette. Keep plates on ice.
- Prepare Solution B in the radioactivity work area by adding 2.5 ml 10x M-ATP, 500 µCi gamma-32P ATP, and H2O to 10 ml.
- Add 20 µl of Solution B per well using a repeater pipette which aids in mixing due to ejection force. Cover and incubate in 30 °C hybridization oven for 30 min.
- After 30 min, transfer plates back to ice. Add 50 µl of 2x SDS lysis buffer to each well using a multichannel pipette. Can proceed at this point to the next step, or seal the plates with aluminum foil and store at -20 °C until convenient.
4. Running, Staining, and Drying Gels
Note: All work should be performed in an area designated for radioactivity.
- Turn on hybridization oven and set to 85 °C. Thaw plates at room temperature. Once oven has reached temperature, transfer plates to oven and incubate for 10 min to denature samples.
- Load 26-well pre-cast gels with 15 µl of each reaction using a multichannel pipette to fill several wells at once. Care must be taken that all tips align with corresponding well before adding samples. Run gel at 150 V. Do not let the tracking dye (blue line) run off of the bottom of the gel as this contains the unincorporated ATP.
- Dismantle the gels and cut off the unincorporated ATP (blue line) using a scalpel or straight edge as it will overexpose the films. Place the gels in labeled containers and cover with Coomassie stain for 15 min.
- Remove the Coomassie stain, briefly rinse the gels with water, and add destain solution. Destain the gels until the proteins are clearly visible. A band for MBP and a band for the substrate should be visible for each sample.
- To dry the gels:
- Cut a large sheet of filter paper and place it on the dryer.
- Wet a cellophane sheet in distilled water until it is smooth and wrinkle free, and place it on top of the paper.
- Lay the gels on top of the cellophane sheet, making a note of the order of gels. Wet a second cellophane sheet and place on top of the gels.
- Roll out all bubbles (a plate sealing roller works well for this) for a nice uniform surface. Close the flap, turn on the vacuum, and dry the gels for 3 hr at 80 °C.
- Once gels are dry, expose them to XAR film using a screen to intensify the signal. Wrap the cassette with saran wrap or a plastic bag and seal with tape to keep out frost. Store the cassette at -80 °C overnight.
5. Developing XAR Films
- The following day, remove cassette from freezer and let thaw at room temperature. Develop the film in a darkroom using a film processor according to manufacturer’s instructions.
Note: Examine the films for evidence of kinase-substrate pairs. A second longer exposure can also be useful to detect weaker phosphorylation events.
Wyniki
Representative results from a screen are shown in Figure 2. 180 kinases were screened using a GST-tagged peptide substrate corresponding to aa 268-283 from CRTC2 as well as the classic kinase assay substrate myelin basic protein (MBP). Only two kinases, MARK2 and the highly related kinase MARK3 phosphorylated the CRTC2 peptide. MBP is included as an internal control in all assays, as it contains many phosphorylatable residues and runs at 18 kDa, toward the bottom of the gel. This allows for an interpretation of specificity: some kinase will robustly phosphorylate a substrate and MBP. Of note here is that the wells containing GST alone (i.e. the no kinase controls) always purify some endogenous kinase activity, thus there is always background phosphorylation in the assay. While this does not rule out that the phosphorylation of the substrate is real, it does suggest that in the in vitro setting the kinase may be less selective. It is particularly informative to include multiple substrates of differing MW to draw conclusions regarding kinase-substrate specificity.
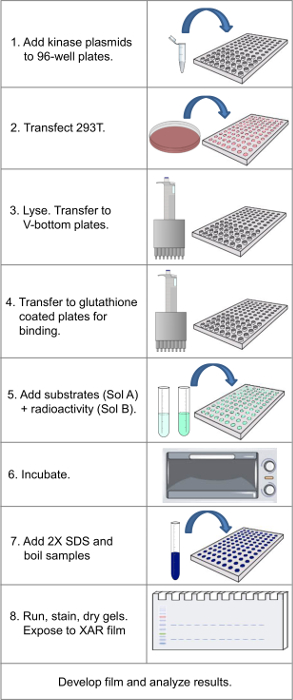
Figure 1. Flowchart showing key steps in protocol. Please click here to view a larger version of this figure.

Figure 2. Example of screen outcome. Autoradiographs of a screen of Version 1 of the library (180 human protein kinases) performed using GST-peptide substrate corresponding to aa 268-283 of mouse CRTC2 (reproduced from Jansson et al., 2008). MBP, myelin basic protein is indicated. The high degree of substrate selection by related kinases is a feature of the screen. Each lane represents the reaction products from a distinct kinase assay. MARK3 (Gel 1) and MARK2 (Gel 16), the only kinases to phosphorylate TORC2 268-283 peptide, are indicated. Please click here to view a larger version of this figure.
Dyskusje
Since the original publications describing the approach 14,15, the original library of 180 GST-kinases has been expanded to 420 members, or ~80% of the human protein kinome. With the expanded library, the protocol as described takes 4-5 days and then 1-4 days to develop films (as deemed necessary), which could be shortened by use of phosphorimaging and digital signal enhancement. There are several key steps where care must be taken (See Figure 1 for overview of protocol). First, is the health of the stock of cells (mycoplasma free, never allowed to reach confluence during expansion, and treated with trypsin at each passage) during the batch expansion stage and in the 96 well plates at the time of transfection. Cells should be uniformly seeded, and 80% confluent when transfection takes place (Step 1.5).
Second, it is ideal during the transfection to use an automated liquid dispenser to minimize volume transfer errors, thus limiting coefficient of variations (“cvs”) between wells. For small volumes, fitting the dispenser with a smaller volume cassette limits reagent loss due to reduced dead volumes and increases dispensing accuracy. The treatment step with sodium pervanadate, a pan tyrosine phosphatase inhibitor, is used as a general stimulus to increase phosphorylation status of target kinases. Inclusion of calcium at this step increases cell-matrix adhesion, preventing cell loss due to rounding elicited by prolonged pervanadate treatment. This step should be done with strict adherence to the 10 minute incubation time.
Third, for the kinase assay itself, there are several considerations when selecting the recombinant substrate: peptides, protein domains, or intact proteins as large as 120 kDa can all be used. If a specific phosphorylation site is known, a peptide substrate is the most straight-forward approach, but needs to be large enough to detect on a SDS-PAGE gel. Thus, peptide substrates can be fused to GST and purified from bacteria to give a MW of >25. Larger proteins have the advantage that they are likely folded and their phosphorylatable residues exposed as they would be in the cell, yet come with the drawback that they will include additional residues that could be phosphorylated and give signal in the assay. Our preference is to run a screen using a peptide substrate composed of 15-20 amino acids surrounding a residue that is known to be phosphorylated in vivo and is known to regulate a biological event, as this renders validation of the candidate kinases from the screen both in vitro and in vivo much faster.
Last, ideally the amount of protein approaches or exceeds 1 µg per well; this of course varies with the MW of the substrate. Less protein per well can yield meaningful hits, but the ‘more is better’ rule applies as it increases signal:noise. Standard, non-gradient gels are recommended as gradient gels crack too often during drying. After drying the gels, detection of a radioactive signal over background with a Geiger counter gives an excellent indication of success of the assay.
As the screen is in vitro and additional levels of complexity exist in vivo, a candidate kinase(s) for a given substrate must be validated in cells. Specifically, the screen may identify a family member that has the biochemical capacity to phosphorylate the substrate in vitro, yet is not expressed in the same cell type or in the same subcellular compartment as the substrate. For example, while MARK2 and MARK3 were both hits in a screen for kinase that could phosphorylate CRTC2 on Ser275, only MARK2 formed a complex with the substrate in cells 14. The following are a series of additional experiments that can be used to confirm the physiological relevance of a candidate kinase. First, mobility shifts of the substrate on SDS-PAGE following coexpression of the candidate kinase can be used as a confirmation control. Appropriate controls such cotransfection of a catalytically inactive kinase can confirm specificity. Second, the substrate can be immunoprecipitated from cell extracts that have been incubated with 32P-orthophosphate to confirm incorporation of phosphate into the substrate in the presence of the wildtype and not a catalytically inactive kinase. Third, a phosphospecific antibody can be generated against the phosphoacceptor sequence (if known) and used to confirm an increase in substrate phosphorylation when the kinase is overexpressed. A secondary screen using a mutant substrate carrying a non-phosphorylatable residue at the target site can confirm specificity prior to generation of a phosphoantibody, a particularly important control with larger domain and full-length protein substrates. Pharmacological inhibition approaches to inhibit a candidate kinase can also be used, yet caution must be exercised as inhibition of related kinases is an underappreciated reality. Last, RNAi-mediated silencing of the candidate kinase(s) in cells followed and western blotting to monitor loss of target site phosphorylation with a phosphospecific antibody can be performed.
Ujawnienia
The authors have nothing to disclose.
Podziękowania
This work was supported by NSERC grant 386634. We would like to thank members of the Screaton Lab for helpful discussions.
Materiały
| Name | Company | Catalog Number | Comments |
| Lysis buffer | Made in house | See Protocol step 1.1 | |
| 10x kinase buffer | Made in house | See Protocol step 1.2 | |
| 10x M-ATP | Made in house | See Protocol step 1.3 | |
| Human kinase plasmids | Orfeome, Invitrogen, Origene | GST-tagged in house | |
| 96 well plates | Fisher Scientific | CS003595 | |
| 293T cells | ATCC | CRL-11268 | |
| DMEM | Fisher Scientific | SH3002201 | supplement with 100 U/ml penicillin, 100 μg/ml streptomycin, 10% fetal calf serum. |
| CO2 incubator | Sanyo | MCO-17AIC | |
| 15 cm cell culture dishes | Fisher Scientific | 877224 | |
| Reduced serum medium | Invitrogen | 22600-050 | |
| Lipid-based transfection reagent | Invitrogen | 11668-019 | |
| Automated liquid dispenser | Thermo Scientific | 5840300 | |
| Small cassette attachment | Thermo Scientific | 24073295 | |
| Standard cassette attachment | Thermo Scientific | 14072670 | |
| 4 mM pervanadate | Made in house | See Protocol step 3.1 | |
| 0.25 M CaCl2 | Made in house | ||
| Multichannel pipette (20-200 μl) | Labnet | p4812-200 | |
| Multichannel pipette (1-10 μl) | Thermo Scientific | 4661040 | |
| V-bottom 6-well plates | Evergreen Scientific | 290-8116-01V | |
| Glutathione coated 96-well plates | Fisher Scientific | PI-15240 | |
| Hybridization oven | Biostad | 350355 | |
| GST tagged substrate | Made in house | ||
| Myelin Basic Protein (MBP) | Sigma | M1891 | |
| Repeater pipette (1 ml) | Eppendorf | 22266209 | |
| 32P gamma-ATP | Perkin Elmer | BLU502Z500UC | |
| 2x SDS lysis buffer (100 ml) | Made in house | See Protocol step 1.4 | |
| 26-well precast TGX gels | BioRad | 567-1045 | gel percentage required is dependent on the molecular weight of the substrate of interest |
| Coomassie stain | Made in house | 0.1% Coomassie R250, 10% acetic acid, 40% methanol | |
| Coomassie destain | Made in house | 10% acetic acid, 20% methanol | |
| Labeled gel containers | Made in house | Used plastic lids from empty tip boxes, just big enough to contain one gel | |
| Whatman filter paper | Fisher Scientific | 57144 | |
| Cellophane sheets (2) | BioRad | 165-0963 | |
| Gel dryer | Labconco | 4330150 | |
| Double emulsion autoradiography film | VWR | IB1651454 | |
| Film cassette | Fisher Scientific | FBAC-1417 | |
| Intensifying screen | Fisher Scientific | FBIS-1417 | |
| Plate sealing rubber roller | Sigma | R1275 |
Odniesienia
- Meisenhelder, J., Hunter, T., van der Geer, P. Phosphopeptide mapping and identification of phosphorylation sites. Curr Protoc Mol Biol. 18, Unit 18 19 (2001).
- Doll, S., Burlingame, A. L. Mass spectrometry-based detection and assignment of protein posttranslational modifications. ACS chem. 10, 63-71 (2015).
- Sharma, K., et al. Ultradeep human phosphoproteome reveals a distinct regulatory nature of Tyr and Ser/Thr-based signaling. Cell rep. 8, 1583-1594 (2014).
- Cohen, P. The regulation of protein function by multisite phosphorylation--a 25 year update. Trends Biochem Sci. 25, 596-601 (2000).
- Walsh, C. T. . Posttranslation Modification of Proteins: Expanding Nature's Inventory. , (2006).
- Boersema, P. J., et al. In-depth qualitative and quantitative profiling of tyrosine phosphorylation using a combination of phosphopeptide immunoaffinity purification and stable isotope dimethyl labeling. Mol Cell Proteomics. 9, 84-99 (2010).
- Hutti, J. E., et al. A rapid method for determining protein kinase phosphorylation specificity. Nat Methods. 1, 27-29 (2004).
- Johnson, S. A., Hunter, T. Kinomics: methods for deciphering the kinome. Nat Methods. 2, 17-25 (2005).
- Pawson, T., Nash, P. Assembly of cell regulatory systems through protein interaction domains. Science. 300, 445-452 (2003).
- Zhu, H., et al. Analysis of yeast protein kinases using protein chips. Nat Genet. 26, 283-289 (2000).
- Shah, K., Shokat, K. M. A chemical genetic approach for the identification of direct substrates of protein kinases. Methods Mol Biol. 233, 253-271 (2003).
- Zou, L., et al. PKIS: computational identification of protein kinases for experimentally discovered protein phosphorylation sites. BMC bioinform. 14, 247 (2013).
- Varjosalo, M., et al. Application of active and kinase-deficient kinome collection for identification of kinases regulating hedgehog signaling. Cell. 133, 537-548 (2008).
- Jansson, D., et al. Glucose controls CREB activity in islet cells via regulated phosphorylation of TORC2. Proc Natl Acad Sci U S A. 105, 10161-10166 (2008).
- Fu, A., Screaton, R. A. Using kinomics to delineate signaling pathways: control of CRTC2/TORC2 by the AMPK family. Cell Cycle. 7, 3823-3828 (2008).
- Abu-Thuraia, A., et al. Axl phosphorylates elmo scaffold proteins to promote rac activation and cell invasion. Mol Cell Biol. 35, 76-87 (2015).
- Manning, G., Whyte, D. B., Martinez, R., Hunter, T., Sudarsanam, S. The protein kinase complement of the human genome. Science. 298, 1912-1934 (2002).
Przedruki i uprawnienia
Zapytaj o uprawnienia na użycie tekstu lub obrazów z tego artykułu JoVE
Zapytaj o uprawnieniaThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. Wszelkie prawa zastrzeżone