Method Article
Three-dimensional Location Approach with Silk Thread Guided Laparoscopic Segmentectomy for Liver Tumor
In This Article
Summary
The utilization of 3D reconstruction and virtual simulations in preoperative planning for liver resections represents a significant advancement in surgical oncology. Our team's 3D-LAST (three-dimensional location approach with the silk thread) technique enables safe, efficient, precise tumor removal with practical intraoperative navigation, promising broad medical adoption.
Abstract
When performing hepatectomy to treat liver tumors, accurately determining the resection margin and ensuring the adequacy of residual liver parenchyma are of utmost importance. At present, intraoperative ultrasound and indocyanine green fluorescence navigation are frequently utilized methods. However, certain technical constraints prevent their extensive application. We have developed the 3D-LAST technique for precise liver tumor resection. This technique makes use of computer post-processing to extract features from computed tomography (CT) scans and generate volumetric images, creating three-dimensional (3D) visualizations. This provides a valuable resource for clinical decision-making, as it can vividly display complex internal anatomical structures in an intuitive and stereoscopic manner. In this study, preoperative 3D positioning was carried out on patients with a single liver tumor to identify anatomical landmarks and calculate the resection range. During the surgical procedure, margin lines of lengths calculated by preoperative 3D software were set up, and silk thread was used to mark the edges. This approach offers a time-saving and accurate way to determine the optimal cutting plane. The objective of this article is to demonstrate the viability of applying 3D-LAST in laparoscopic segmentectomy for liver tumors. The results of the research indicate that 3D-LAST is a safe, effective, and practical new method for intraoperative liver navigation, and it has great potential for widescale promotion.
Introduction
Hepatectomy remains a cornerstone treatment for liver tumors. Over recent decades, surgical approaches have evolved from irregular resections to precise anatomical resections, driven by advancements in assistive technologies such as intraoperative ultrasound (IOUS) and indocyanine green (ICG) fluorescence imaging1,2. Despite these innovations, achieving optimal resection margins while preserving sufficient functional liver volume remains a critical challenge. The overall goal of our proposed 3D-LAST (three-dimensional location approach with silk thread) technique is to provide a precise, cost-effective, and universally accessible intraoperative navigation method for liver tumor resection, minimizing reliance on specialized equipment while improving spatial accuracy.
The rationale for developing 3D-LAST stems from the limitations of current techniques. IOUS, while valuable for real-time tumor localization, requires skilled sonographers for image interpretation and struggles with two-dimensional (2D) spatial visualization, often prolonging operative time3,4. ICG fluorescence navigation, though effective for superficial tumors, is constrained by its limited tissue penetration depth (5-10 mm), rendering it unreliable for deeper lesions5,6. Both methods depend on costly, specialized hardware, limiting their adoption in resource-constrained settings.
The advantages of 3D-LAST over existing techniques are multifaceted. Three-dimensional visualization, derived from preoperative computed tomography (CT) reconstructions, overcomes the spatial ambiguity of 2D imaging by providing stereoscopic anatomical guidance. Unlike ICG, which lacks depth resolution, 3D-LAST enables precise volumetric resection planning, reducing the risk of positive margins or excessive parenchymal loss. Furthermore, the use of silk thread for intraoperative marking eliminates the need for real-time imaging devices, streamlining workflow and reducing costs.
3D-LAST is particularly suited for centers lacking advanced imaging infrastructure or expertise in complex intraoperative navigation. It is ideal for single-tumor resections where anatomical landmarks are identifiable on preoperative CT and where minimizing procedural complexity is prioritized. By addressing the limitations of current methods and leveraging validated 3D technologies, 3D-LAST represents a pragmatic advancement in achieving precision liver surgery with broad clinical adaptability.
Case Presentation:
A 59-year-old man with upper abdominal discomfort was diagnosed with a 2.7 cm x 1.6 cm liver tumor in the right liver. The patient was previously diagnosed with gastric adenocarcinoma and underwent radical gastrectomy for gastric cancer, followed by routine chemotherapy. No extrahepatic metastasis was found on the preoperative contrast-enhanced CT scan. CA19-9, CA15-3, CA72-4, AFP, and CEA were normal.
Protocol
The study was approved by the review committee of West China Hospital of Sichuan University. Informed consent was obtained from the patient before the surgery.
1. Preoperative preparation
- Obtain high-resolution CT scans of the patient liver in DICOM format (Figure 1A).
- Launch the Mimics software, create a new project, and import the DICOM files to ensure all image data is correctly loaded for 3D reconstruction and analysis.
- Reconstruct the three-dimensional structure of the liver, vessels, and tumor. Mark the liver in pink, the portal vein in blue, the artery in red, the hepatic vein and inferior vena cava in blue, and the tumor in yellow (Figure 1B).
- Place three small virtual sticks on the 3D liver model and obtain four key points (S1, S2, S3, S4) on the surface of liver (Figure 2A-B).
- These virtual sticks act as digital landmarks, guiding the surgeon in determining the precise location and orientation of the intended cut. Place the virtual stick marking of the resection point at 1 cm from the tumor margin and the midpoint of the edge of the liver at the bottom of the gallbladder.
NOTE: By marking these key points, surgeons can ensure they remain focused on the target area during surgery, minimizing the risk of unnecessary tissue damage.
- These virtual sticks act as digital landmarks, guiding the surgeon in determining the precise location and orientation of the intended cut. Place the virtual stick marking of the resection point at 1 cm from the tumor margin and the midpoint of the edge of the liver at the bottom of the gallbladder.
- Connect the four key points with lines on the liver surface and measure their lengths (S1-S2 = 9.8 cm, S1-S3 =7.2 cm, S2-S3 = 10.4 cm, S4-S2 = 8.2 cm, S4-S3 = 6.5 cm) as shown in Figure 2C-D. These lines represent the proposed resection path, guiding the surgeon in visualizing the shape and contour of the liver after the resection.
- Measure the length of each line meticulously to ensure accuracy and consistency. This measurement is crucial as it allows surgeons to prepare silk threads of the exact same length, which will be used intraoperatively to guide the liver cut.
2. Operative procedure
- Position the patient supine, with their legs spread and inclined to the right (Figure 3A). Administer standard general anesthesia, including tracheal intubation and controlled ventilation.
- Disinfect the skin with 0.5% iodine-based scrub 3x on the area of inter-nipple connection, symphysis pubis, right midaxillary, and left midclavicular lines.
- Arrange the surgical team with the surgeon on the right and the assistant on the left. Position the camera centrally among the trocar sites, with the camera holder standing in the middle as well.
- Make a 12 mm incision below the umbilicus as the laparoscopic hole (H1), one 12 mm and one 5 mm curved incision (H2 and H3) as the main operating holes, another one 12 mm and one 5 mm (H4 and H5) as the auxiliary operating holes (Figure 3B).
- Insert five trocars (12 mm, 12 mm, 12 mm, 5 mm, and 5 mm) into incisions as depicted in Figure 3B. Turn on the pneumoperitoneum system and inject 100% carbon dioxide gas through the trocar to maintain the pneumoperitoneum at a pressure of about 12 mmHg.
- Conduct an exploratory examination of the abdominal cavity, starting from the left epigastrium and moving rightwards down to the hypogastrium, to detect any presence of ascites, cirrhosis, and metastasis.
- Free abdominal adhesions, detach the round ligament of the liver, free the right hepatic ligament and adhesions, and fully expose the liver segment V using an ultrasonic knife.
- Use an ultrasonic knife to dissect the gallbladder triangle, revealing the gallbladder duct and artery. Subsequently, ligate these structures with small hemo-lock clips before excising the gallbladder.
- Prepare three silk threads with lengths of S1-S2 = 9.8 cm, S1-S3 = 7.2 cm, and S2-S3 = 10.4 cm respectively. Point S4 is located at the neck of the gallbladder, so prepare no silk threads for S4-S2 = 8.2 cm and S4-S3 = 6.5 cm.
- Place the three silk threads at the anatomical position on the liver surface, corresponding to the preoperatively planned resection path, as shown in Figure 4. By using silk threads as a physical guide, surgeons can visualize and follow the planned resection path with greater precision during surgery.
NOTE: This step bridges the gap between the virtual planning and the actual surgical procedure. - Use an electric knife to cauterize the liver by cutting markers along the silk thread on the surface of the liver (Figure 4D) and sew a rubber band on the liver for traction (Figure 5A).
- Use an ultrasonic knife to transect the liver parenchyma along the marker lines. Cut vessels that are encountered by home-o-lock clipping, use an aspirator to suction the blood, and use bipolar forceps to stop bleeding (Figure 5B).
- Place the specimen in a bag and make a 4cm incision by scalpel at the upper abdomen to remove the specimen. Confirm no active bleeding in the abdominal cavity, place hemostatic materials on the liver transection surface and one drainage tube exiting from the right lower abdomen.
- Remove the trocar and suture incisions by 3-0 absorbable sutures layer by layer. Examine the specimen to verify the integrity of the tumor capsule and measure the size of the tumor (Figure 5C-D).
3. Post-hepatectomy management
- Transfer the patient to the ward following successful recovery from anesthesia and observe the patient's vital signs using uninterrupted cardiac monitoring during the initial 24 h recovery period following surgery.
- Implement prophylactic antimicrobial therapy by intravenous infusion throughout the 1st postoperative day to reduce potential septic complications.
- Remove urinary catheterization at 24 h post-procedure. Conduct a plain computed tomography scan 72 h after surgery (Figure 6). Evacuate surgical drains 4 days after the operation without massive ascites and bile leakage.
Results
The total operation time was 150 min, with 50 mL of blood loss that did not require a blood transfusion. Intraoperative urine volume was 500 mL, and intraoperative infusion volume was 800 mL. On the 1st day after surgery, the blood test results showed a mild increase in transaminase levels. CT scan of the abdomen showed complete resection of the liver tumor and no significant ascites 3 days after surgery. The drain was removed on postoperative day 4. The patient had an uneventful postoperative course and was discharged on the 5th day after the operation. Postoperative gross pathological specimen demonstrated that the tumor size was 1.5 cm x 1.5 cm, confirming R0 resection as shown in Table 1.
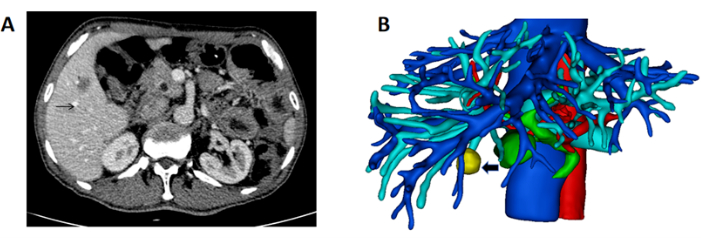
Figure 1: CT showing the mass and 3D reconstruction of the liver and mass. (A) The CT scan showing the tumor located in the right liver (black arrow indicating mass). (B) 3D reconstruction of liver, vessel, and mass (black arrow indicating mass). Please click here to view a larger version of this figure.

Figure 2: Mark the resection line on the 3D model. (A-D) Reconstruct the intrahepatic duct structure and tumor in 3D, and mark the key points (S1, S2, S3, S4) of the cutting edge with a small stick. Draw liver lines along the key points on the surface of the virtual liver model and measure the length of each line (S1-S2 = 9.8 cm, S1-S3 = 7.2 cm, S2-S3 = 10.4 cm, S4-S2 = 8.2 cm, S4-S3 = 6.5 cm). Please click here to view a larger version of this figure.

Figure 3: Intraoperative layout of the surgeon, patient, and trocar placement. (A) The operating surgeon is on the right, the assistant on the left, and the camera man between the legs. (B) The laparoscopic hepatectomy procedure is performed using a five-port technique. Please click here to view a larger version of this figure.

Figure 4: Silk thread marking the resection line. (A-D) During liver surgery, use silk thread to mark the cutting edge, prepare silk threads of the same length, and place them at the anatomical position on the surface of the liver. The shape enclosed by the silk thread is the cutting line for the liver. Please click here to view a larger version of this figure.

Figure 5: Liver resection and specimen. (A) Sew a rubber band on the liver for traction. (B) Expose the middle hepatic vein in the liver resection plane (black arrow indicating the middle hepatic vein). (C-D) Complete resection of the liver mass (black arrow), the cross-sectional view showing the tumor margin is intact and matching the preoperatively planned margin. Please click here to view a larger version of this figure.

Figure 6: Postoperative CT scan. The CT scan indicated successful tumor removal without perihepatic fluid accumulation on postoperative day 3. Please click here to view a larger version of this figure.
| Parameters | Results |
| Duration of surgery | 150 min |
| Blood loss | 50 mL |
| Postoperative liver function | ALT 222 IU/L, AST 217 IU/L |
| Postoperative CT re examination | POD 3 |
| Rmovement of drainage tube | POD 4 |
| Day of discharge | POD 5 |
| Tumor size | 1.5 cm x 1.5 cm |
| Pathological type | Adenocarcinoma |
Table 1: The patient's surgical outcomes. Abbreviations: ALT = Alanine Aminotransferase; AST = Aspartate Aminotransferase; POD = Postoperative Day.
Discussion
With the development of technology and the accumulation of experience, laparoscopic liver resection has become more and more common, and its indications are almost as extensive as those of open surgery. Compared with laparotomy, laparoscopic liver resection has many advantages, such as less pain, fewer perioperative complications, and faster recovery7,8,9. However, laparoscopic liver resection also faces some inherent difficulties. The lack of tactile and depth perception, limited operating space, and restricted visual field pose challenges to its widespread use10,11,12. To address these issues, IOUS and ICG fluorescence imaging have been used as real-time navigation tools in recent years. IOUS, when applied directly on the liver surface, can improve the accuracy of lesion detection and localization13,14.
However, since it is usually operated by sonographers, surgeons often need to pause the operation to wait for them, which not only prolongs the operation time but also increases the dependence on ultrasound technology. In addition, in cirrhotic livers, IOUS may misinterpret regenerative nodules as tumors, leading to over-diagnosis15,16,17. ICG, a harmless water-soluble near-infrared fluorescent agent, can help visualize anatomical structures during the operation through special scopes. Its high sensitivity and clear contrast make it a popular tool for surgical navigation in various liver surgeries. However, due to the limited tissue penetration of near-infrared light (up to 10 mm), its application in detecting deep-seated liver lesions is restricted. Moreover, an excessive dose of ICG may cause false-positive results, and the success of tumor staining is related to factors such as blood supply, liver cirrhosis, and necrosis18,19,20. Therefore, developing an innovative, efficient, and accurate method for localizing deep-seated liver tumors is of great clinical significance.
In this study, preoperative 3D positioning was used to identify anatomical landmarks and calculate the resection area for patients with solitary liver tumors. During the operation, margin lines of lengths calculated by preoperative 3D software were arranged, and silk thread was used to mark the edges. This method provides a time-efficient and accurate way to navigate the optimal cutting plane21. The operation time in our study was significantly shorter than that reported in studies using IOUS and ICG-guided hepatectomy. This approach reduces the reliance on IOUS, saving surgical time and lowering technical and conditional requirements. It can be applied to various liver tumor navigation situations, regardless of the size and depth of the tumor.
Despite its advantages, this approach has limitations. First, its accuracy depends on preoperative imaging quality; motion artifacts or low-resolution scans may compromise 3D model fidelity. Second, the technique assumes static liver anatomy, whereas respiratory movements or surgical manipulation can shift tumor positions, necessitating real-time adjustments. Third, the learning curve for 3D software operation and intraoperative spatial translation may limit adoption in surgery without specialized training. In addition, the sample size of this study is relatively small, and future large-scale prospective studies are needed to verify the effectiveness of this method further. Developing a simple and widely applicable 3D reconstruction program is also an important goal for future research.
Disclosures
The authors report no conflict of interest.
Acknowledgements
This work was supported by the Guizhou Provincial Health Commission Science and Technology Fund Project(gzwkj2025-300), the Project of Guizhou Provincial Department of Science and Technology (Qian Ke He Cheng Guo, LC[2024]109 ).
Materials
| Name | Company | Catalog Number | Comments |
| BiClamp LAP | ERBE Company | No.20195-132 | |
| Laparoscopic system | Olympus | VISERA OTV-S400 | |
| Ultrasonic knife | Johnson and Johnson MedTech | ETHICON HARMONIC |
References
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved