Method Article
Optimizing Extracellular Vesicle Delivery Using a Core-Sheath 3D-Bioprinted Scaffold for Chronic Wound Management
In This Article
Summary
This study presents a protocol for fabricating core-sheath 3D-bio-printed scaffolds for chronic wound healing. Extracellular vesicles are isolated from mesenchymal stem cells, and loaded into the core (alginate) with the sheath made from carboxymethyl cellulose and alginate lyase. This design allows controlled scaffold degradation and efficient EVs release.
Abstract
This study outlines a detailed protocol for the fabrication of core-sheath 3D-bioprinted scaffolds designed to enhance chronic wound healing. The protocol involves isolating extracellular vesicles (EVs) from mesenchymal stem cells (MSCs), known for their regenerative and immunomodulatory properties. These EVs are then incorporated into a unique scaffold structure. The scaffold features a core composed of alginate loaded with EVs, surrounded by a sheath made of carboxymethyl cellulose and alginate lyase. This innovative design ensures controlled scaffold degradation while promoting efficient and controlled release of EVs at the wound site. The protocol covers key steps, including the preparation and characterization of the EVs, the formulation of bio-inks for 3D bioprinting, and the optimization of printing parameters to achieve the desired core-sheath architecture. By combining structural integrity and bioactivity, the scaffold aims to address the limitations of conventional wound dressings, offering a targeted approach to accelerate tissue regeneration and reduce inflammation in chronic wounds. This method provides a reproducible and scalable strategy for developing advanced biomaterials with potential clinical applications in chronic wound management. The protocol also highlights critical considerations for achieving consistent results, ensuring adaptability for future therapeutic applications.
Introduction
Chronic wounds, often linked to excessive inflammation, require timely management to prevent serious complications like infections and tissue necrosis, which can lead to amputations. Despite advancements, current treatments remain costly, inconvenient, have side effects, and have limited efficacy, highlighting the need for more curative dressings1,2,3. The development of a new generation of wound dressings specifically designed for chronic wounds is essential to address these challenges. Moreover, the complex nature of wound healing demands dressing materials with a range of properties, including moisturization, flexibility, adhesion, bioactivity, and biodegradability4. This study aims to develop a bioengineered wound dressing that integrates extracellular vesicles (EVs) with a core-sheath 3D-bioprinted scaffold to provide a controlled therapeutic environment and accelerate chronic wound healing.
EVs derived from stem cells aid chronic wound healing by promoting anti-inflammatory responses, cell growth, migration, and blood vessel formation5. Additionally, EVs can deliver bioactive molecules, including small molecule drugs, gene and protein constructs for chronic wound management6. Moreover, their ability to protect cargo from enzymatic degradation improves the stability and bioavailability of therapeutic agents, offering distinct advantages over conventional growth factors and small molecule drugs, which often degrade rapidly in vivo7. Despite these advantages, the efficient and sustained delivery of EVs to target tissues remains a significant challenge.
3D-bioprinting scaffolds can serve as a delivery platform for EVs to boost their therapeutic effects8. These scaffolds mimic natural cellular environments and allow for the controlled release of EVs9,10. They also protect EVs from degradation, enhancing the stability of their microRNAs and proteins11. Han et al. demonstrated that EVs can be effectively released from 3D bioprinted GelMA scaffolds. This release led to improved cell attachment and enhanced gene expression related to mechanotransduction pathways in human buccal fat pad mesenchymal stem cells (hBFP-MSCs) seeded onto the scaffolds12. Born et al., by optimizing the concentration of the crosslinker, achieved a controlled release of the EVs. This approach has demonstrated efficacy in promoting angiogenesis and offers a promising method for the regulated delivery of EVs13.
Core-sheath 3D-bioprinting enables the creation of complex, multi-material structures by printing a core material encased in a sheath. The core can include cells, growth factors, or drugs, while the sheath offers mechanical support and protection or acts as a barrier. This method has applications in tissue engineering and regenerative medicine, such as developing vascular networks, mimicking natural tissue structures, and creating drug delivery systems. It allows precise control over material distribution and composition, enhancing the functionality and biological relevance of the constructs. Compared to alternative techniques, core-sheath 3D bioprinting provides precise control over material distribution and composition, improving the functionality and biological relevance of the constructs14,15.
Engineered degradation in wound dressings offers benefits such as reduced discomfort during changes, a moist environment for healing and infection control, timely therapeutic delivery, and optimal tissue regeneration16,17,18. Alginate (Alg) and carboxymethyl cellulose (CMCh) hydrogels are biocompatible and effective for delivering extracellular vesicles (EVs) to wounds, enhancing healing through cellular communication and inflammation reduction18. In this study, EVs were integrated into a core of Alg, while a sheath of CMCh and AlgLyase (AlgLyase) was used to enable rapid dressing degradation and EVs delivery. This core-sheath design facilitates the rapid release of EVs in response to scaffold degradation, enhancing their therapeutic efficacy and addressing the limitations of existing chronic wound treatments. The primary objective of this study is to develop a bioengineered dressing that enhances wound healing by integrating controlled EVs release with a responsively degradable scaffold, ultimately improving the treatment outcomes for chronic wounds.
Protocol
The animal research was conducted in full accordance with the ethical standards established by the National Committee of Bioethics and the Animal Ethics Committee of the University of Nizwa. Ethical approval for this study was granted under clearance ID: VCGSR, AREC/01/2023. All animals were housed under standard laboratory conditions, ensuring optimal environmental controls, proper nutrition, and comprehensive care to safeguard their welfare throughout the study. All procedures involving animals adhered strictly to institutional policies, international animal care standards, and the ARRIVE guidelines.
1. Cell culture
- Retrieve a vial of MSCs (passage #2) from liquid nitrogen storage and handle it with aseptic techniques to prevent contamination. Rapidly thaw the cells in a 37 °C water bath, ensuring to remove them as soon as a small ice fragment remains.
- Prepare a complete medium including MSC SFM Basal Medium, supplemented with 1% (v/v) MSC SFM XenoFree supplement, 1% (v/v) GlutaMAX, and 0.01% (v/v) Gentamicin. Add 1 mL pre-warmed (37 °C) complete medium at a rate of 3 to 5 drops every 5 s, to the vial and then gently mix it. Transfer the entire contents of the MSCs vial into a 15 mL conical tube containing 4 mL pre-warmed complete medium, under aseptic conditions in a Class II biosafety cabinet.
- Centrifuge the cells at 200 x g for 5 min at room temperature. Ensure the centrifuge is balanced properly.
- Aspirate the supernatant and resuspend the cells in 1 mL of pre-warmed complete media. Then, transfer the cells into a T25 flask containing 4 mL of complete medium.
- Gently tilt the flask to ensure the cells are evenly distributed. Incubate the flask at 37 °C with 5% CO2.
- Change the medium every 2 days, replacing it with fresh, pre-warmed, complete medium. Use gentle pipetting to avoid cell disruption. Upon reaching 70%-80% confluence, aspirate the spent medium from the T25 flask.
- Wash the cells with 3 mL of fresh PBS to remove residuals. Ensure complete coverage of the flask during the wash.
- Add 1 mL of 0.25% trypsin solution to the T25 flask, incubate it at 37 °C for 3-7 min, and carefully monitor the detachment under the microscope at 4x magnification.
- Tap the flask gently if required to ensure complete cell detachment. Add pre-warmed complete medium to the flask and pipette up and down over the surface to help detach the cells. Then, transfer the cell suspension into a 15 mL centrifuge tube.
- Centrifuge the tube at 200 x g for 5 min at room temperature. Suspend the cell pellet in a fresh, complete medium and count the cells using a Neubauer hemocytometer. Transfer the cells to a T75 flask. Ensure a seeding density of 5,000 cells/cm2 for optimal growth.
- Incubate the cells at 37 °C with 5% CO2. After 72 h of cell incubation, collect the conditioned media from cells for EVs isolation. Use immediately after collection.
2. EVs isolation
- Centrifuge the 13 mL of the collected conditioned media at 800 x g for 15 min to remove cellular debris. Filter the supernatant gently using a 0.22 µm syringe filter to remove large particles.
- Add 5 mL of precipitation buffer to the filtered conditioned media and vortex thoroughly to mix. Ensure the solution is homogenous.
- Incubate the mixture overnight at 4 °C to allow the EVs to precipitate. Centrifuge the mixture at 3,220 x g for 30 min at 20 °C. Check the balance of the tubes.
- Carefully discard the supernatant without disturbing the pellet. Centrifuge the pellet once again at 3,220 x g for 5 s to remove residual liquid.
- Gently pipette the EV pellet in 200 µL of PBS to avoid damage to the EVs. Measure the EVs protein concentration following the manufacturer's instructions (BCA Protein Assay Kit).
- Perform Western blotting to detect EV-specific markers (CD63, CD81, and CD9), to verify EV phenotype18. The analysis confirmed the presence of these markers, validating the EVs identity19.
- To minimize the risk of RNase contamination, it is recommended to use it directly without additional storage. In case it is needed, store the EVs at -80 °C for further use. Aliquot the EVs suspension into 40 µL volumes to avoid repeated freeze-thaw cycles.
3. EVs labeling with PKH-26
- Buffer preparation
- Dissolve 0.238 g of HEPES in approximately 90 mL of sterile ultra-pure water in a sterile container. Use freshly prepared HEPES solution. Add 0.85 g of NaCl to the HEPES solution.
- Adjust the pH to 7.4 using 1 M NaOH or HCl, as required, using a calibrated pH meter. Add deionized water to bring the total volume to 100 mL. Filter the solution through a 0.22 µm filter to sterilize it.
- Dilute 4 µL of PKH-26 dye in 1 mL of prepared buffer. Mix thoroughly by pipette to ensure homogeneity.
- Resuspend purified EVs in 1 mL of PBS at a concentration of 700 µg/mL. Add 1 mL of the EVs suspension to 1 mL of the prepared PKH-26 solution. For the dye-only control group, add 1 mL of complete medium without EVs to 1 mL of the PKH-26 solution. All subsequent steps are performed for the control group as well to account for potential nonspecific aggregation or micelle formation.
- Mix the EVs thoroughly by gently inverting the tube 10x-15x. Incubate the mixture for 3-5 min at room temperature, ensuring even exposure of EVs to the dye.
- Prepare a 2 mL fresh 1% BSA solution using sterile ultrapure water and add it to the 2 mL of the resultant mixture to quench the labeling reaction. Mix gently to prevent aggregation.
- Concentrate the PKH-26 labeled EVs according to the abovementioned protocol (steps 2.2-2.7). Resuspend the labeled EVs in 300 µL of PBS. Pipette the PKH-26 labeled EVs sample into a 30 kDa filter tube.
- Centrifuge the sample at 3,220 x g for 30 min at 4 °C. During this step, the free PKH-26 dye and small molecules will pass through the membrane into the filtrate, while the PKH-26 labeled EVs will be retained in the retentate.
- After the initial centrifugation, discard the filtrate and add 300 µL of PBS to the retentate. Gently resuspend the EVs in the PBS by pipetting up and down.
- Centrifuge the sample again at 3,220 x g for 30 min at 4 °C to wash away any remaining free dye or small molecules.
- Confirm the lack of PKH-26 in the filtrate solution by a fluorescent microscope. If any dye is detected, repeat the washing steps.
- Collect the top solution by micropipette and remove the filter from the collection tube. Carefully invert the filter (turn it upside down).
- Centrifuge the inverted filter at 800 x g for 5 min at 4 °C. This will help collect the retained PKH-26-EVs from the filter membrane into the new collection tube. Directly use the PKH-26-EVs for the next step.
4. 3D Bio-printing
- Bioinks preparation
- Prepare a fresh 4.5% (w/v) sodium Alg solution using sterile ultra-pure water. Stir overnight at 60 °C to allow the solution to dissolve completely.
- Dissolve CMCh in sterile ultra-pure water to achieve a 3.8% (w/v) solution. Prepare fresh. Stir overnight at 60 °C to dissolve completely.
- Centrifuge the prepared bioinks at 3,220 x g for 10 min at 25°C to remove any bubbles that may interfere with the printing process.
- Mix the prepared 3 mL of Alg solution with the labeled EVs or the dye-only control using a syringe mixer to achieve the 0.01% (w/v) concentration of EVs. Ensure even distribution by gentle mixing.
- Using a syringe mixer, combine 1 mL of CMCh with a freshly prepared AlgLyase solution in sterile ultra-pure water to achieve a final concentration of 0.5 mU/mL.
- As depicted in Figure 1A, simultaneously load the Alg/Exo bioink into the core part and the CMCh/AlgLyase bioink into the sheath part of the core/sheath syringe setup.
- Allow the bioinks to rest for 15 min before printing.
- 3D-bio-printer setup
- Using the 3D-bioprinter software, create the scaffold structure by selecting a Cylindrical Shape with a Diagonal Infill Pattern. For this purpose, set the cylinder diameter and height to 20 mm, and 1.1 mm, respectively. Configure the pore size to 1 mm.
- Set the core and sheath nozzle pumping speeds to 1 mm/s with a thickness of 0.25 mm per layer and set the moving speed to 6 mm/s. Print four layers with a thickness of 0.25 mm per layer at room temperature.
- Start to print on polyester film.
- Use the humidifier with aerosol calcium chloride (2.2%) to cross-link the scaffold during the extrusion process. Position the humidifier nozzle approximately 20 cm away from the extrusion head to ensure effective cross-linking without compromising the scaffold structure. For further-crosslinking, immerse the scaffold in calcium chloride solution (2.2%) for 10 min.
- Rinse the scaffold 3x with sterile ultra-pure water to remove any excess calcium chloride and non-bound bioink.
- Ensure that the scaffold is stored in a sterile environment at 4 °C to maintain the integrity and functionality of the scaffold for up to three months.
5. Tracking EVs release
- Creation of circular cutaneous wound model
- Anesthetize male Akita heterozygous diabetic mice (8 weeks) by administering an intraperitoneal injection of Ketamine (70 mg/kg) and Xylazine (10 mg/kg). Confirm proper anesthetization by assessing the absence of reflex responses (e.g., toe pinch) and monitor the respiratory rate. To prevent corneal dryness during anesthesia, apply sterile veterinary ophthalmic ointment to the eyes.
- Prepare the dorsal skin area by first shaving it using an electric clipper. Avoid skin irritation or injury. Sterilize the shaved area using a povidone-iodine solution.
- Using a sterile seizer, create a 6 mm circular full-thickness cutaneous wound on the dorsal surface of each mouse.
- Gently place the 3D bioprinted scaffold containing PKH-labeled EVs directly onto the wound bed. Ensure the scaffold fully covers the wound surface with minimal air pockets by pressing lightly using sterile forceps. Ensure the animal is closely observed after the procedure and remains attended until it has fully regained consciousness and can maintain sternal recumbency.
- Fluorescent imaging
- At 2 h, 4 h, 8 h, 24 h post-implantation, anesthetize the mice using isoflurane. For induction of anesthesia, place the mice in the chamber of the in vivo imaging system (IVIS) and expose them to 2%-3% isoflurane in oxygen. Apply ophthalmic ointment to the mice's eyes to prevent dryness. Once anesthetized, transfer the mice to the IVIS system and maintain them with 1%-2% isoflurane in oxygen delivered through the nose channels. Verify that the animals are fully anesthetized and stable before proceeding with imaging.
- Use the IVIS system to capture the fluorescent signals from the PKH-labeled EVs released from the scaffold. In the imaging wizard, select the Fluorescence Imaging option and activate the excitation and emission filters for the PKH dye. Adjust the camera settings, including the field of view and subject height, to optimize signal detection. Ensure consistent positioning across all imaging time points to enable accurate comparisons. Start acquiring images and save the resulting data.
- Quantify the fluorescent signal intensities using the IVIS software. This will allow for the tracking of EVs release over time.
Results
The in vivo release of EVs from both the Alg-EVs/CMCh and Alg-EVs/CMCh-AlgLyase scaffolds is depicted in Figure 1B,C. As anticipated, the Alg-EVs/CMCh-AlgLyase scaffold exhibited a more rapid release profile compared to Alg-EVs/CMCh, particularly at the 2 h and 4 h time points. The release of EVs from hydrogels is governed by a combination of physicochemical mechanisms, including diffusion, swelling, erosion, and degradation20. By leveraging the Alglyase, the scaffold facilitates the breakdown of Alg, accelerating the release of EVs to efficiently control chronic inflammation in the early stages of wound healing. The early release of EVs is critical in mitigating excessive inflammation, which can delay healing21,22,23. To account for potential nonspecific aggregation or micelle formation, the control group was also analyzed, revealing negligible fluorescent signals. This observation confirms that the detected signals predominantly originate from the labeled EVs.
These results reflect the critical role of scaffold composition in modulating EVs release rates. The faster release observed in the Alg-EVs/CMCh-AlgLyase scaffold is attributed to the enzymatic breakdown of Alg by Alglyase, which enhances hydrogel degradation. This rapid EVs release is particularly advantageous for addressing the inflammatory phase of wound healing24. Chronic wounds often suffer from prolonged inflammation, which impairs healing; the early and controlled release of EVs can mitigate this by modulating inflammatory responses and promoting a pro-regenerative environment22,25.
To analyze these outcomes, researchers should quantify the fluorescence intensity captured using the IVIS system, correlating signal strength with EVs concentration over time. The data should be plotted to illustrate release kinetics, enabling comparisons between different scaffold formulations. Variations in release profiles can also be assessed concerning scaffold integrity and enzyme activity, providing further insight into the underlying release mechanisms.
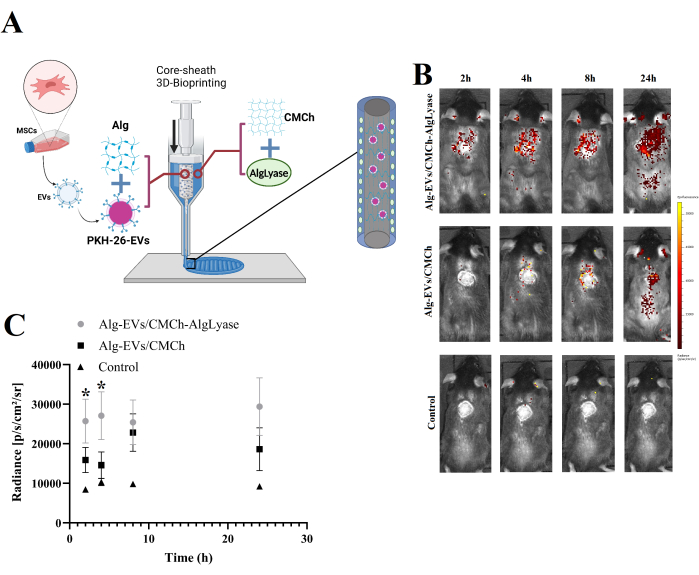
Figure 1: Overview of scaffold fabrication and in vivo EVs release profile. (A) Schematic representation of the fabrication process for the Alg-EVs/CMCh-AlgLyase 3D-bioprinted scaffold. (B) In vivo release profile of PKH-labeled EVs from the Alg-EVs/CMCh-AlgLyase scaffold compared to the Alg-EVs/CMCh scaffold and dye-only control scaffold (control). Color bars indicate fluorescence intensity levels. (C) Quantitative analysis of PKH-EVs release intensity (n=3). Error bars represent standard deviation, and one-way ANOVA was used to assess statistical significance. Please click here to view a larger version of this figure.
Discussion
A pivotal aspect of the protocol is the core-sheath scaffold design, which is essential for achieving efficient EVs delivery. The design incorporates Alg as the core material and a combination of CMCh with Alglyase as the sheath. This setup facilitates controlled and rapid release of EVs. The core material, Alg, encapsulates the EVs, ensuring their protection and localized delivery. The sheath, composed of CMCh and Alglyase, enables the accelerated degradation of the Alg core, which is critical for the timely release of the EVs. In our previous publication18, we assessed the in vitro release kinetics of EVs from a similar scaffold system and demonstrated a controlled release profile over an extended period, highlighting the potential of this approach for sustained therapeutic effects. This controlled degradation and release are achieved through precise formulation and encapsulation techniques. These data will enable the correlation of EVs release dynamics with wound healing progression, offering deeper insights into the scaffold's clinical potential.
A slower release system offers advantages in therapeutic applications where sustained, lower concentrations of a drug or growth factor may improve efficacy while reducing the risk of side effects. For example, a gradual release can ensure continuous stimulation of cellular processes over a longer period26, avoiding the high concentrations that might lead to toxicity27. However, the specific needs of the application dictate the ideal release profile. In some cases, a rapid release may be preferred to provide an immediate therapeutic effect28.
Critical steps within the protocol include the preparation and layering of the bioink materials for the core-sheath scaffold. Maintaining the precise concentrations of Alg, CMCh, and Alglyase during bioink preparation ensures structural stability and effective EVs encapsulation. Another critical step is the optimization of the 3D-bioprinting parameters, including nozzle speeds and cross-linking conditions, to ensure uniform scaffold geometry and functional performance. Optimization of the PKH-26 labeling process is essential to get rid of free dye and unwanted particles. This process would improve the EVs tracking accuracy.
The protocol has been optimized to enhance the stability of the scaffold and the consistency of the EV release. For example, excessive free PKH-26 dye during labeling was mitigated by optimizing the dye-to-EV ratio and using alternative PKH-26-labled EVs isolation method. Regular adjustments for 3D-bioprinting parameters were employed to maintain the effectiveness of the scaffold.
The viscosity of the bioinks should be optimized to prevent clogging during printing. Tuning the AlgLyase concentration is required to control the degradation rate. Troubleshooting the scaffold's mechanical properties involved balancing calcium chloride cross-linking durations to avoid brittle or overly soft scaffolds. During EVs labeling, filtration and centrifugation steps mitigated the free-dye contamination and improved the accuracy of imaging results.
In addition to the direct benefits of using degradable dressings for chronic wound healing, the scaffold's degradation rate significantly influences the release kinetics of EVs. This degradation rate can be adjusted to meet the specific needs of the wound. By optimizing the concentration of AlgLyase in the sheath, the degradation rate of the Alg core can be fine-tuned, allowing for a customized release profile29. However, the method's effectiveness under varying physiological conditions is still not fully understood, as factors like pH and enzymatic activity in different types of wounds could affect the scaffold's performance. Therefore, variability in scaffold performance under different physiological conditions should be considered. Factors like pH, enzymatic activity, and temperature variations in chronic wounds may affect the scaffold's degradation and EVs release kinetics, potentially reducing therapeutic efficacy.
While hydrogel-based wound dressings are widely used for their biocompatibility and ability to maintain a moist wound environment, they often lack the ability to provide controlled and localized delivery of bioactive molecules such as EVs. The incorporation of EVs into the scaffold system offers a unique advantage by combining structural support with a biologically active, cell-derived therapeutic component that can modulate immune responses and enhance tissue regeneration.
Similarly, nanoparticle-mediated delivery systems are highly efficient in targeting specific cellular pathways; however, they may face challenges in retention at the wound site and often require additional stabilizing agents to maintain bioactivity30. In contrast, the presented scaffold system provides a protective microenvironment that preserves the bioactivity of EVs during delivery24.
While this protocol is offering promising outcomes, there are some limitations that should be considered. One challenge lies in achieving a uniform distribution of EVs within the scaffold. Variations in the mixing process may lead to inconsistencies in EVs concentration across the scaffold, which could affect the reproducibility. To overcome this challenge, the syringe mixing technique is an advantage. Additionally, precise control over gelation and crosslinking conditions, such as calcium ion concentration for alginate scaffolds, is critical to ensure optimal mechanical properties and structural integrity. Deviations in these parameters may compromise scaffold performance. Another limitation is the potential loss of EVs during scaffold preparation, as they can adhere to plasticware during washing steps. Lastly, the shelf life of the prepared scaffolds is relatively short, as hydrogel matrices may degrade or lose functionality over time. So, immediate use or short-term storage at 4 °C is recommended. A periodic bioactivity testing of the encapsulated EVs is required to confirm their stability18. By addressing these limitations and employing the suggested troubleshooting strategies, researchers can improve the reproducibility and effectiveness of this protocol in their applications.
The core-sheath scaffold design method presents a significant advancement for drug delivery over traditional scaffold designs that lack controlled release mechanisms31. Unlike one-phase scaffolds that may not provide adequate or timely release of therapeutic agents, this method offers a dynamic approach to EVs delivery with enhanced precision. The use of a rapid-degrading core material and a functional sheath allows for a more targeted and effective therapeutic intervention compared to conventional methods, which often require manual or sequential adjustments.
While the scaffold materials used in this study, such as Alg and CMCh, are known for their biocompatibility, a more thorough evaluation of their long-term biocompatibility in chronic wound environments is necessary for clinical translation. In our previous work, we investigated the in vivo effects of the scaffold in a preclinical animal model and observed no toxic effects or significant immune responses, suggesting that the materials are well-tolerated in the short term18. However, long-term biocompatibility, including the potential immune responses and the effects of degradation byproducts, requires further exploration. The degradation of the scaffold materials could potentially release byproducts that may influence the wound healing process or provoke chronic inflammation, particularly in the context of diabetic or aged models, where the immune response may be altered. Therefore, it is crucial to conduct additional studies to assess how degradation products affect the wound microenvironment and overall tissue regeneration. We acknowledge that further biocompatibility testing, including long-term in vivo studies focusing on immune responses and scaffold degradation, is essential to understanding the safety and clinical relevance of this approach for chronic wound healing.
This scaffold design is particularly important in the fields of tissue engineering and regenerative medicine. Its ability to deliver EVs rapidly and in a controlled manner makes it valuable for applications such as chronic wound healing, cartilage repair, and other tissue regeneration efforts32. By addressing issues related to scaffold degradation and EVs release, the method holds promise for improving patient outcomes in these areas. Additionally, its potential for customization and adaptation to different tissue types underscores its versatility and relevance in advancing personalized medicine.
The use of the Akita diabetic mouse model in this study provides a valuable platform for investigating chronic wound healing under diabetic conditions. This model mimics key pathophysiological characteristics of human diabetic wounds, including persistent hyperglycemia, delayed angiogenesis, and impaired tissue repair33,34. Moreover, it offers the advantage of genetic consistency and spontaneous onset of diabetes without the need for external chemical induction and hence, reducing variability in experimental outcomes33. However, it is important to acknowledge the limitations of this model when translating findings to human chronic wounds. Murine skin architecture differs from human skin, particularly in its thinner epidermis, looser dermal structure, and the predominant role of wound contraction rather than re-epithelialization during healing35. Additionally, murine immune responses and inflammatory profiles are not fully representative of those observed in humans, which may affect the interpretation of immune modulation therapies36. Despite these differences, the Akita model remains a widely accepted and effective tool for preclinical studies due to its ability to recapitulate systemic diabetic complications central to chronic wound pathogenesis. To further validate the translational potential of the proposed therapeutic approach, future studies are warranted to complement the Akita model findings. Additional human-relevant systems, such as ex vivo human skin models or diabetic large-animal models, would be highly supportive.
In conclusion, the proposed scaffold design with controlled release of EVs marks a significant advancement in the delivery of therapeutic agents. Despite the technical complexities, this innovative approach offers significant advantages over existing methods and holds great potential for improving chronic wound dressings.
Disclosures
The authors declare that they have no conflicts of interest.
Acknowledgements
Special thanks to Said Al-Hashmi and Abdulrahman Almharbi from Happy Production for their excellent work in filming. We also extend our gratitude to the Ministry of Higher Education, Research and Innovation and the University of Nizwa for their financial support and for providing the required resources.
Materials
| Name | Company | Catalog Number | Comments |
| 23 G Purple precision conical Nozzle | Cellink | KT0000002000 | To provide precise extrusion of bioinks with minimal clogging |
| Alginate lyase (AlgLyase) | Sigma Aldrich | A1603-100MG | Algyase is an enzyme that degrades alginate. |
| Amicon Ultra Centrifugal Filter, 30 kDa MWCO | Merck | UFC9030 | Used to wash PKH-26 labeled-EVs |
| BCA assay Kit | Thermo Scientific | 10678484 | To determine the protein/EVs concentration |
| Bioprinting System | Regemat | V1 | To fabricate core-sheath scaffold |
| Bovine serum albumin (BSA) | sigma-aldrich | 05470-5G | To stop PKH 26 reaction |
| Calcium chloride | Sigma Aldrich | C3306-100G | To crosslink and stabilize bioinks in tissue engineering |
| Centrifuge | Sigma | 2-16P | Used for EVs isolation |
| Centrifuge 5810 R | Eppendorf | 22625101 | Used for cell culture |
| Class II Biological Safety Cabinet | Telstar | Bio II Advance | Cell culture |
| CryoCube F570 Series - ULT Freezer | Eppendorf | F571240035 | To store EVs |
| fluorescent microscope | OLYMPUS | IX73P1F | Used to check the residual PKH-26 in the filtrate |
| Gentamicin (50 mg/mL) | Thermofisher | 15750 | Antibiotic for cell culture media |
| GlutaMAX-I CTS, (100X), liquid | Thermofisher | A12860 | Cell culture media supplement |
| HCl | Sigma Aldrich | 7647-01-0 | Buffer preparation |
| HEPES | Carl Roth | Art. No. 6763.3 | Buffer preparation |
| High viscous carboxymethyl cellulose (CMCh) | BDH | 27929 4T | CMCh is a water-soluble cellulose derivative. |
| Incubator | New Brunswick | NB-170R | Cell culture |
| Invivo imaging | PerkinElmer | IVIS Lumina XRMS Series III | To track EVs release, in vivo |
| Magnet stirer | SalvisLAB | MC35 | For Bioinks preparation |
| miRCURY Exosome Kits for Exosome Isolation | Qiagen | 76743 | Evs isolation |
| NaOh | Daejung | 1310-73-2 | Buffer preparation |
| phosphate buffered saline(PBS) | Thermo Scientific | J61196.AP | Cell culture |
| PKH 26 | MCE | 154214-55-8 | Red fluorescent dye for labeling theEVs |
| Sodium alginate (Alg) | Sigma Aldrich | A0682-100G | Natural polysaccharide derived from brown seaweed. |
| Sodium chloride (NaCl) | Carl Roth | Art-Nr-P029.1 | Buffer preparation |
| StemPro BM Mesenchymal Stem Cells | Thermofisher | A1382901 | Mesenchymal stem cells |
| StemPro MSC SFM XenoFree | Thermofisher | A1067501 | Cell culture media |
| Trypsin 0.25% | Thermofisher | 25050014 | Cell dissociation |
| Vortex-Mixer | Daihan Scientific | VM-10 | Used to mix precipitation buffer with the conditioned media |
References
- Falanga, V., et al. Chronic wounds. Nat Rev Disease Primers. 8 (1), 50 (2022).
- Tran, H. Q., Shahriar, S. S., Yan, Z., Xie, J. Recent advances in functional wound dressings. Adv Wound Care. 12 (7), 399-427 (2023).
- Shao, M., et al. Emerging trends in therapeutic algorithm of chronic wound healers: Recent advances in drug delivery systems, concepts-to-clinical application and future prospects. Crit Rev Ther Drug Carrier Syst. 34 (5), 385-452 (2017).
- Rezvani Ghomi, E., Khalili, S., Nouri Khorasani, S., Esmaeely Neisiany, R., Ramakrishna, S. Wound dressings: Current advances and future directions. J Appl Poly Sci. 136 (27), 47738 (2019).
- Ding, J. Y., et al. Mesenchymal stem cell-derived extracellular vesicles in skin wound healing: Roles, opportunities and challenges. Military Med Res. 10 (1), 36 (2023).
- Sharma, D., Kumar, A., Mostafavi, E. Extracellular vesicle-based biovectors in chronic wound healing: Biogenesis and delivery approaches. Mol Ther Nucleic Acids. 32, 822-840 (2023).
- Vader, P., Mol, E. A., Pasterkamp, G., Schiffelers, R. M. Extracellular vesicles for drug delivery. Adv Drug Delivery Rev. 106, 148-156 (2016).
- Han, P., Ivanovski, S. 3d bioprinted extracellular vesicles for tissue engineering-a perspective. Biofabrication. 15 (1), 013001 (2022).
- Annabi, N., et al. 25th anniversary article: Rational design and applications of hydrogels in regenerative medicine. Adv Mater. 26 (1), 85-124 (2014).
- Zheng, Y., Pan, C., Xu, P., Liu, K. Hydrogel-mediated extracellular vesicles for enhanced wound healing: The latest progress, and their prospects for 3d bioprinting. J Nanobiotechnol. 22 (1), 57 (2024).
- Keener, A. B. How extracellular vesicles can enhance drug delivery. Nature. 582 (7812), S14-S14 (2020).
- Han, P., et al. 3d bioprinted small extracellular vesicles from periodontal cells enhance mesenchymal stromal cell function. Biomater Adv. 158, 213770 (2024).
- Born, L. J., et al. Sustained released of bioactive mesenchymal stromal cell-derived extracellular vesicles from 3d-printed gelatin methacrylate hydrogels. J Biomed Mater Res A. 110 (6), 1190-1198 (2022).
- Kjar, A., Mcfarland, B., Mecham, K., Harward, N., Huang, Y. Engineering of tissue constructs using coaxial bioprinting. Bioact Mater. 6 (2), 460-471 (2021).
- Gao, Q., He, Y., Fu, J. Z., Liu, A., Ma, L. Coaxial nozzle-assisted 3d bioprinting with built-in microchannels for nutrients delivery. Biomaterials. 61, 203-215 (2015).
- Deshayes, S., Kasko, A. M. Polymeric biomaterials with engineered degradation. J Poly Sci A Poly Chem. 51 (17), 3531-3566 (2013).
- Xu, Q., et al. Injectable hyperbranched poly (β-amino ester) hydrogels with on-demand degradation profiles to match wound healing processes. Chem Sci. 9 (8), 2179-2187 (2018).
- Vakilian, S., et al. Engineered local delivery of extracellular vesicles loaded with si-tnf-α, via a core-sheath 3d-bio-printed scaffold as an effective wound dressing. J Drug Delivery Sci Technol. 101, 106189 (2024).
- Mirsanei, Z., et al. Synergistic effects of mesenchymal stem cell-derived extracellular vesicles and dexamethasone on macrophage polarization under inflammatory conditions. Inflammopharmacology. 32 (2), 1317-1332 (2024).
- Ma, Y., Brocchini, S., Williams, G. R. Extracellular vesicle-embedded materials. J Controlled Release. 361, 280-296 (2023).
- Jia, Q., Zhao, H., Wang, Y., Cen, Y., Zhang, Z. Mechanisms and applications of adipose-derived stem cell-extracellular vesicles in the inflammation of wound healing. Front Immunol. 14, 1214757 (2023).
- Lou, P., et al. Extracellular vesicle-based therapeutics for the regeneration of chronic wounds: Current knowledge and future perspectives. Acta Biomater. 119, 42-56 (2021).
- Cai, Y., Chen, K., Liu, C., Qu, X. Harnessing strategies for enhancing diabetic wound healing from the perspective of spatial inflammation patterns. Bioactive Mater. 28, 243-254 (2023).
- Li, Z., et al. Multifunctional hydrogel-based engineered extracellular vesicles delivery for complicated wound healing. Theranostics. 14 (11), 4198 (2024).
- Cabral, J., Ryan, A. E., Griffin, M. D., Ritter, T. Extracellular vesicles as modulators of wound healing. Adv Drug Delivery Rev. 129, 394-406 (2018).
- Moghadasi Boroujeni, S., Mashayekhan, S., Vakilian, S., Ardeshirylajimi, A., Soleimani, M. The synergistic effect of surface topography and sustained release of tgf-β1 on myogenic differentiation of human mesenchymal stem cells. J Biomed Mater Res A. 104 (7), 1610-1621 (2016).
- Jayaraman, P., et al. Controlled release of drugs in electrosprayed nanoparticles for bone tissue engineering. Adv Drug Delivery Rev. 94, 77-95 (2015).
- Huang, X., Brazel, C. S. On the importance and mechanisms of burst release in matrix-controlled drug delivery systems. J Controlled Release. 73 (2-3), 121-136 (2001).
- Smith, A. M., Senior, J. J. Alginate hydrogels with tuneable properties. Adv Biochem Eng Biotechnol. 178, 37-61 (2021).
- Yan, X., Sha, X. Nanoparticle-mediated strategies for enhanced drug penetration and retention in the airway mucosa. Pharmaceutics. 15 (10), 2457 (2023).
- Pant, B., Park, M., Park, S. -. J. Drug delivery applications of core-sheath nanofibers prepared by coaxial electrospinning: A review. Pharmaceutics. 11 (7), 305 (2019).
- Pinheiro, A., et al. Extracellular vesicles: Intelligent delivery strategies for therapeutic applications. J Controlled Release. 289, 56-69 (2018).
- Yoshioka, M., Kayo, T., Ikeda, T., Koizuni, A. A novel locus, mody4, distal to d7mit189 on chromosome 7 determines early-onset niddm in nonobese c57bl/6 (akita) mutant mice. Diabetes. 46 (5), 887-894 (1997).
- Fang, R. C., et al. Limitations of the db/db mouse in translational wound healing research: Is the noncnzo10 polygenic mouse model superior. Wound Repair Regen. 18 (6), 605-613 (2010).
- Zomer, H. D., Trentin, A. G. Skin wound healing in humans and mice: Challenges in translational research. J Dermatol Sci. 90 (1), 3-12 (2018).
- Sellers, R. S. Translating mouse models: Immune variation and efficacy testing. Toxicol Pathol. 45 (1), 134-145 (2017).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved