Method Article
Assessing Insulin Clearance in Mice via In Situ Liver Perfusion
In This Article
Summary
Hepatic insulin clearance is critical for regulating glucose homeostasis. This article describes a user-friendly hepatic perfusion procedure for directly evaluating the hepatic insulin clearance rate in situ in mice.
Abstract
Hepatic insulin clearance is essential for maintaining glucose homeostasis and is closely linked to metabolic disorders such as obesity, insulin resistance, and diabetes. Accurate measurement of insulin clearance is vital for understanding the underlying mechanisms of these conditions. This protocol presents a straightforward and user-friendly hepatic perfusion procedure in mice, specifically designed to directly evaluate the hepatic insulin clearance rate. The method involves precise cannulation of the portal vein and suprahepatic inferior vena cava to create an in situ perfusion system that mimics physiological conditions. The protocol guides researchers through every stage of the procedure, from surgical preparation and setting up the perfusion system to sample collection and analysis. Detailed instructions are provided, along with representative results and important tips for optimizing the procedure. A video tutorial accompanies the written protocol, offering visually in-depth instructions and illustrations, making it an accessible and comprehensive reference for scientists exploring the molecular mechanisms behind hepatic insulin metabolism and clearance.
Introduction
The discovery of insulin has become one of the milestones of the last century. Much is known about the regulation of insulin synthesis, secretion, and its physiological functions in metabolic tissues. However, there has been less focus on insulin degradation and its regulatory mechanisms. Insulin metabolism can be understood as the interplay between beta cell function, insulin resistance (IR) or sensitivity, and insulin clearance. Alongside insulin secretion, hepatic insulin clearance plays a crucial role in maintaining the homeostatic level of insulin necessary for reaching peripheral target tissues and facilitating proper insulin action1. Multiple studies have identified impaired insulin clearance as a crucial factor in the pathogenesis of hyperinsulinemia in metabolic syndrome, as well as in other conditions such as type 2 diabetes2,3, nonalcoholic steatohepatitis4, and polycystic ovarian syndrome5. Thus, hyperinsulinemia secondary to reduced clearance may play a role in the pathogenesis of metabolic disease. Strategies that improve insulin clearance have the potential to reverse the unfavorable impacts of hyperinsulinemia in these individuals.
Insulin has a unique pattern of distribution. The level of circulating plasma insulin depends on the equilibrium between insulin secretion and removal. The pancreas secretes insulin into the portal vein in a pulsatile manner, directing it to the hepatocytes. As the first organ to encounter insulin secretion, the liver degrades the majority of insulin during its first passage, accounting for 60%-70% of total insulin6. The remaining insulin exits the liver through the hepatic vein, entering the systemic circulation, where it is partially utilized by peripheral tissues (primarily muscle, adipose tissue, and kidneys) before being further extracted by the liver during its second pass through the hepatic artery7.
The precise measurement of insulin clearance is crucial. Directly measuring hepatic insulin clearance in human studies is challenging because obtaining blood samples from the portal and hepatic veins is difficult. Both direct and indirect methods are used to estimate insulin clearance in humans and animal models. Approximately three strategies are employed to measure insulin clearance indirectly. The assessments most frequently utilized in clinical practice involve methods based on the C-peptide/insulin molar ratio8. This approach is grounded in the equimolar secretion of both peptides and the absence of C-peptide extraction by the liver9. The second group of methods depends on the mathematical analysis of plasma decay curves of insulin after a known and specific input of the hormone into the circulation2,10,11. The third method is based on the fact that the infusion of insulin at a constant rate leads to stable levels of the hormone in the blood, where the removal rate matches the administration rate12. These indirect methods primarily reflect overall insulin clearance in the body. Given that the liver is the primary site of insulin clearance and plays a crucial role in this process, it is essential to directly evaluate hepatic insulin clearance.
Previous studies have directly measured hepatic insulin extraction in healthy dogs13,14. Studies have also used an isolated perfused rat liver model to assess insulin extraction from the liver15,16. Due to the high availability of genetically modified strains, mice serve as valuable models for investigating molecular pathways. A few studies17 have utilized liver perfusion to directly assess hepatic insulin clearance in a mouse model. In these studies, a perfusate containing human insulin is infused into the portal vein and collected from the inferior vena cava. The proportion of insulin absorbed by the liver indicates its clearance. The liver perfusion technique maintains the liver under near-physiological conditions by circulating a warm, oxygenated, and nutrient-enriched perfusate through the liver vasculature. However, there is insufficient practical guidance and essential tips for advancing and disseminating this technique.
Thus, while hepatic insulin clearance has received increasing attention, its role in disorders, as well as its molecular mechanisms, remains unclear18. Therefore, advanced techniques are greatly needed in the field of scientific research. This protocol establishes a detailed modified hepatic perfusion procedure in mice for evaluating hepatic insulin clearance. Additionally, this method can also be used to study the effects of drugs on the liver, including the first-pass effect, drug transport processes, and various other aspects.
Protocol
This protocol was approved by the Nanjing Medical University Animal Care and Use Committee (IACUC-2105018) and followed the guidelines of the Institutional Animal Care and Use Committee. All C57BL/6N mice were maintained on a 12-h light/dark cycle with free access to food and water. Six-week-old mice were randomly divided into a Chow diet (CD) group and a High-fat diet (HFD) group. The HFD group was fed a 60% high-fat diet and continued on this diet until 10 weeks of age. The average body weight was 28.55 g ± 1.2 g for the HFD group and 24.3 g ± 0.48 g for the control group. The details of the reagents and equipment used in this study are listed in the Table of Materials.
1. Preparation
- Perform requisite sterilization of surgical instruments and consumables through autoclaving.
- Place the surgical instruments, 6-0 silk suture, sterile small cotton applicator, sodium chloride injection (500 mL), cotton swabs, and sponges appropriately on the operation table.
- Prepare 30 mL of heparinized saline with a final concentration of 200 IU/mL.
- Prepare two silicone tubes with an inner diameter of 0.31 mm and an outer diameter of 0.64 mm; one 4 cm in length for use as the portal vein catheter, and the other 10 cm in length for use as the inferior vena cava catheter.
- Prepare the Krebs-Henseleit (KRBH) perfusion buffer containing 5.0 mmol/L glucose and 0.25% BSA.
- Prepare the Krebs-Henseleit (KRBH) perfusion buffer containing 5.0 mmol/L glucose, 0.25% BSA, and 4.0 ng/mL human insulin.
- Set up the liver perfusion system. Figure 1 shows the main components of the liver perfusion system.
2. Surgical catheterization
- Prepare the anesthetic mixture by following the steps below:
- Dilute Zoletil 50 (250 mg/5 mL) 10 times with 0.9% sodium chloride solution.
- Dilute Xylazine hydrochloride (200 mg/2 mL) 10 times with 0.9% sodium chloride solution.
- Mix the 0.5% Zoletil 50 solution with the 1% Xylazine hydrochloride solution in a 1:1 ratio.
- Anesthetize the mice.
- Check and record the mouse's body weight. Administer the anesthetic mixture via intraperitoneal injection at a dose of 5 mL/kg body weight (2.5 mg/mL Zoletil 50; 5 mg/mL Xylazine hydrochloride). The onset of anesthesia typically occurs within 5-10 min post-injection, indicated by the loss of the righting reflex and reduced response to external stimuli.
- Transfer the mouse to the operation table. Secure the limbs using adhesive tape. Administer 2.5 U/g heparin intraperitoneally to achieve heparinization.
- Use an electric shaver to trim the fur on the abdominal skin and disinfect the area with a povidone-iodine solution.
- Perform portal vein catheterization.
- Make a 4 cm longitudinal incision from the lower abdomen toward the xiphoid process along the mid-abdominal line. Carefully cut the peritoneum with scissors to avoid damaging visceral organs. Insert the mouse abdominal retractor to expose the surgical field.
- Move the intestines to the right to reveal the portal vein, the right kidney, and the inferior vena cava (Figure 2A). Use artery forceps to clamp the vena cava at the upper edge of the kidney.
- Isolate the portal vein (Figure 2A) and ligate the distal end with a 6-0 silk suture. Loosely tie another suture on the proximal end of the exposed vessel.
- Make an incision near the ligated end with spring scissors and insert the catheter. Advance the catheter through the incision up to the level of the portal bifurcation.
- Secure both ligatures around the catheter and confirm proper sampling by connecting the free end of the catheter to a sampling syringe. Flush with heparinized saline and clamp the catheter (Figure 2C).
- Remove the traction device and reset the intestines. Cover the surgical area with saline-soaked sterile gauze or cotton.
- Perform suprahepatic inferior vena cava catheterization.
- Make an incision along the sternum from the xiphoid process, exposing the sternum.
- Vertically cut open the sternum and cut through the diaphragm along the rib margin to expose the thoracic cavity.
- Expose and isolate the suprahepatic inferior vena cava (Figure 2B). Carefully ligate the distal end with a 6-0 silk suture. Loosely tie another suture on the proximal end of the vessel.
- Make an incision just below the ligated end with spring scissors and insert a 10 cm catheter. Advance the catheter until the tip of the catheter is close to the liver and tie both ligatures securely. Confirm proper sampling and clamp the free end of the catheter (Figure 2D).
- Rinse the surgical area with saline solution. Cover the surface with saline-soaked sterile gauze.
3. Liver perfusion
- Euthanize the mouse by using an overdose of anesthetic and thoracotomy in accordance with institutional guidelines for animal care and use, ensuring that all procedures are performed in a manner that minimizes suffering.
- Set up the liver perfusion system, which includes an oxygenator, a temperature modulation device, an infusion pump, and infusion tubes, as shown in Figure 1.
- Provide a continuous gas flow of 95% oxygen and 5% carbon dioxide to the oxygenator.
- Turn on the water bath and prewarm the organ chamber to 37 °C.
- Prepare the KRBH perfusion buffer with and without insulin. Prime the tubing system with the perfusion buffer incubated in a water bath at 37 °C.
NOTE: The KRBH is free of BSA and glucose. The KRBH perfusion buffer without human insulin contains 5.0 mmol/L glucose and 0.25% BSA, while the KRBH perfusion buffer with human insulin contains 5.0 mmol/L glucose, 0.25% BSA, and 4.0 ng/mL human insulin. - Place the mouse in a container with the environmental temperature maintained at approximately 37 °C. Use a warming pad to maintain the body temperature at 37 °C.
- Infuse the KRBH buffer through the portal vein catheter. Set the infusion rate at 0.2 mL/min via a mini pump.
- Observe that the liver turns pale within seconds, indicating that the perfusion buffer is flowing through the liver. To flush out more remaining blood cells in the liver, pause the infusion for 1 min at the 4-min and 8-min time points, starting the timing at the beginning of the perfusion.
- Perfuse the liver with KRBH buffer for a total of 10 min (excluding the two 1-min pauses), representing the equilibration period. Collect the basal sample from the inferior vena cava catheter.
- Perfuse the liver with the same solution enriched with insulin (4.0 ng/mL human insulin) for an additional 30 min.
- Collect all samples from the inferior vena cava tube every 2 min.
- Record the liver weight after perfusion. Collect liver samples from different lobes, immediately freeze them in liquid nitrogen, and then transfer to -80 °C for storage.
- Centrifuge all collected perfusion samples at ~1,000 x g for 10 min at 4 °C. Collect the supernatants and transfer them to -80 °C for storage.
NOTE: The insulin concentration in perfusion samples is measured using human insulin enzyme-linked immunosorbent assay (ELISA) kits. - Post-procedure, ensure that all biological waste is disposed of according to safety regulations.
4. Data analysis
- Present the data in XY graphs showing the insulin concentration output over time.
- Calculate the average hepatic insulin clearance rate (HICRAVE) using the following formula:
HICRAVE = (1−Cf/Ci) × 100%
where Ci = initial insulin concentration of infusion buffer, Cf = final average insulin concentration in the last 10 min from suprahepatic inferior vena cava.
Results
This protocol outlines the procedure for liver infusion to calculate hepatic insulin clearance directly. This model is reliable and reproducible. An example of the results obtained from an experiment is shown in Figure 3. After a 10-min equilibration period, KRBH buffer supplemented with 4.0 ng/mL human insulin was perfused through the portal vein for 30 min. Perfusion fluid was collected from the catheter in the suprahepatic inferior vena cava at 2-min intervals, and the concentration of human insulin in the perfusion fluid was measured. The results are presented herein as the mean ± SEM. An example of a liver perfusion experiment comparing mice on a control diet (CD) to mice on a high-fat diet (HFD) is shown in Figure 3. The hepatic insulin clearance rate (HICRAVE) was 55.57% ± 4.43% for the CD group and 25.37% ± 6.83% for the HFD group (Figure 3B). The results indicate that high-fat feeding leads to impaired insulin clearance.
After perfusion, liver samples were obtained for hematoxylin and eosin (HE) staining and whole slide scanning; a well-perfused liver, a poorly perfused liver, and a control liver sample from a normal mouse were collected. Under better perfusion conditions, the liver tissue structure was normal, the hepatic cords were arranged radially and neatly, the hepatocytes were intact in shape, the cytoplasm was uniformly stained, and the nucleus was clear and round, almost indistinguishable from that of the normal liver (Figure 4A,B). When perfusion was poor, the hepatocytes were edematous and markedly loose, with punctate necrosis around the central veins, vacuolar degeneration in the cytoplasm, nuclear pyknosis, and vascular detachment (Figure 4C). The levels of alanine aminotransferase (ALT) and aspartate aminotransferase (AST) in the perfusate were measured at baseline and after perfusion to assess hepatocyte function. The results indicated that there was no significant difference in the ALT and AST levels (Figure 4D).
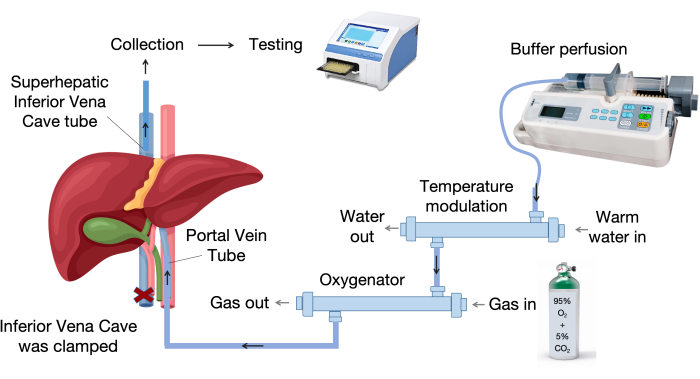
Figure 1: Perfusion system components. The perfusion system consists of key components, including an infusion pump, a temperature control unit, and an oxygenator. The variable temperature device maintains the perfusion liquid at 37 °C, while the membrane oxygenator utilizes polypropylene hollow fibers for effective gas exchange. Please click here to view a larger version of this figure.

Figure 2: Anatomical location and catheterization. (A,B) Depicts the anatomical locations of the portal vein and inferior vena cava. (C) The portal vein catheter is positioned just below the bifurcation into the left and right hepatic branches, with a vessel clamp applied to the intrahepatic inferior vena cava. (D) The catheter is inserted into the suprahepatic inferior vena cava and placed near the upper margin of the liver. Please click here to view a larger version of this figure.
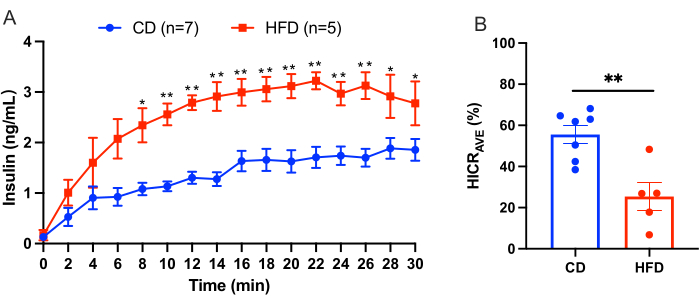
Figure 3: Liver perfusion experiment results. Results from a liver perfusion experiment comparing mice on a control diet (CD) to mice on a high-fat diet (HFD). (A) Insulin concentrations during liver perfusion in CD mice (blue, n = 7) versus HFD mice (red, n = 5). (B) Average hepatic insulin clearance rate (HICRAVE). All data are presented as mean ± SEM. Statistical significance is indicated as *p < 0.05, **p < 0.01 verse the CD mice, analyzed using an unpaired t-test. The results suggest that high-fat feeding leads to impaired insulin clearance. Please click here to view a larger version of this figure.

Figure 4: Impact of perfusion on hepatic histology and function. (A) Normal mouse liver morphology (control). (B) Liver morphology under enhanced perfusion conditions. (C) Liver morphology under suboptimal perfusion conditions. The red arrow indicates vessel detachment, the black arrow denotes vacuolization, and the green arrow highlights pyknosis of the nucleus. Magnification: 40x. (D) Levels of alanine aminotransferase (ALT) and aspartate aminotransferase (AST) in the perfusate, measured at baseline and following perfusion. The paired t-test method was employed for analysis. Please click here to view a larger version of this figure.
Discussion
Critical steps in the protocol
The above-described surgical procedures should be performed with gentle care to avoid creating any lesions in the liver. Moreover, the fragile structure of the liver vein vessel wall renders it vulnerable to puncture and subsequent bleeding if not handled with care during cannulation. Softer silicone tubes are utilized in this protocol to minimize damage to blood vessels. It is recommended that catheterization be performed by an experienced surgeon who should practice frequently to increase the success rate of intubation and minimize the duration of the procedure.
The surgical field should be occasionally irrigated with warm saline to maintain hepatocyte viability. During perfusion, the surgical area should be shielded with sterile gauze or cotton. This practice prevents prolonged exposure to air, which can potentially impair hepatocyte function.
Challenges and solutions
It is crucial to position the tip of the catheter just before the point at which the portal vein branches into the left and right hepatic portal veins. If the catheter is inserted too deeply, uneven perfusion between the liver lobes may occur. In addition, air bubbles must be carefully avoided during the infusion process, as they have the potential to induce air embolism in the liver. This can significantly impact the perfusion of fluid reaching hepatocytes and subsequently affect insulin clearance.
The first 10 min of KH buffer infusion constitutes the equilibrium period, during which the perfusion can be suspended 1-2 times for 2 min each time to flush residual blood cells into the hepatic sinusoid as much as possible. If blood is observed in the collected specimen after 15 min of continuous perfusion, this may indicate that the vascular forceps on the hepatic inferior vena cava are not properly positioned and should be checked19. If the infusion is paused, red blood cells may appear in the collected specimen when the infusion is restarted due to residual blood cells in the hepatic sinusoids. The red blood cells should be separated via centrifugation prior to insulin measurements, as the presence of insulin-degrading enzymes within these cells may potentially impact insulin levels.
The duration of surgical catheterization, liver ischemia-reperfusion time, and the amount of gas entering the portal vein during perfusion can all affect the function of hepatocytes and, thus, the rate of insulin clearance by the liver. The extent of liver dysfunction can be monitored by measuring the levels of alanine aminotransferase (ALT), aspartate aminotransferase (AST), lactate dehydrogenase (LDH), and alkaline phosphatase (ALP) content, as well as by H&E staining. Research has shown that after a successful operation, the liver continues to function and respond for at least 3 h19.
Importance and potential applications of mouse liver perfusion
Mice are ideal research subjects due to their genetic similarity to humans, short reproductive cycles, and the availability of advanced genetic engineering tools20. In mouse models, insulin clearance can be assessed using several methods. During the intraperitoneal glucose tolerance test (IPGTT), hepatic insulin clearance is measured as the ratio of the area under the curve (AUC) (calculated by the trapezoidal method) of C-peptide to insulin21. During the euglycemic-hyperinsulinemia clamp, the ratio between the exogenous insulin infusion rate and the resulting steady-state plasma insulin concentration can be used as a method to estimate insulin clearance indirectly22. However, these methods all indirectly assess hepatic insulin clearance.
Some researchers also use cell-based assays to assess insulin degradation. HepG2 cells or primary hepatocytes from mice are seeded in culture plates, and an appropriate concentration of human insulin is added. Samples of the culture medium are collected at specific intervals to measure the human insulin concentration and evaluate its degradation over time17. In this protocol, a user-friendly in situ hepatic perfusion procedure in mice is described for evaluating the hepatic insulin clearance rate directly. Compared with in vitro studies using isolated hepatocytes, liver perfusion has the benefits of preserving hepatic architecture, zonal division, polarity, and vascular integrity.
The mouse liver perfusion system is a valuable tool for investigating the dynamics and molecular mechanisms of hepatic insulin metabolism. In addition, this protocol could be widely used not only in preinduced disease models but also in acute challenge stimulus testing. However, this technique is limited in that it should be performed in situ. This protocol involves a modified surgical catheterization technique that minimizes injury and preserves the anatomical integrity of the liver to the greatest extent possible. Further efforts are needed to make the best use of this technology to elucidate the mechanisms of insulin clearance, especially in metabolic diseases associated with insulin resistance.
Disclosures
No conflicts of interest were declared.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (82200948, 82270921, 82170882).
Materials
| Name | Company | Catalog Number | Comments |
| 60% high-fat diet | Research Diets, USA | D12492 | |
| Alanine aminotransferase Assay Kit | Nanjing Jiancheng Bioengineering Institute | C009-2-1 | |
| Anhydrous Glucose | Sangon Biotech | 50-99-7 | 500 G |
| Aspartate aminotransferase Assay Kit | Nanjing Jiancheng Bioengineering Institute | C010-2-1 | |
| Bovine Serum Albumin | GeminiBio | 700-107P | Fatty Acid-Free |
| Contour TS Blood Glucose Meter | Bayer | PH220800019 | |
| Contour TS Blood Glucose Test Strips | Bayer | DP38M3F05A | |
| Heparin Sodium | Changzhou Qian hong Bio-pharma | H32022088 | 12500 U/2mL |
| Human insulin | Novo Nordisk | S20191007 | 300 U/3mL |
| Human insulin immunoassay kit | Ezassay Biotechnology | HM200 | |
| KRBH buffer (Sugar, BSA free) | coolaber | SL65501 | 500 mL |
| Membrane oxygenator | Xi'an Xijing Medical Appliance | 5 | |
| Microscopic scissors | Shanghai Jinzhong | YBC020 | |
| Micro-serrefine clamp | Ningbo Medical Needle | 180709 | |
| Microsurgery forceps | Shanghai Jinzhong | WA3010, WA3020 | |
| Needle type filter | N-buliv | LG05-133-2 | |
| Povidone-iodine Solution | Shanghai likang Disinfectant Hi-Tech | 20231016J | |
| pump 11 Elite | Harvard Apparatus | PC5 70-4500 | |
| Retractor | Globalebio (Beijing) Technology | GEKK-10mm | 10 mm |
| Silicone Tubing | scientific commodities | #BB518-12 | 0.31 mm × 0.64 mm |
| Silicone Tubing | Fisher Scientific | #11-189-15A | ID 0.5 mm |
| Sodium Chloride Injection | Baxter | S2402023 | 4.5 g/500 mL |
| Surgical silk suture | Yangzhou Huanyu Medical Equipment | 6-0 | |
| Temperature modulation | Xi'an Xijing Medical Appliance | 6 | |
| Thermostatic water bath | Jiaxing Junsi Electronics | HIH-1 | 220 V 50 HZ |
| Three-way Joint | YISAI | AQTCY1.6 | ID 0.4 mm |
| Xylazine Hydrochloride Injection | ShengXin | 20240106 | 200 mg/2mL |
| Zoletil 50 | Virbac | WK001 | 250 mg/5mL |
References
- Najjar, S. M., Perdomo, G. Hepatic insulin clearance: Mechanism and physiology. Physiology (Bethesda). 34 (3), 198-215 (2019).
- Fu, Z., et al. Impaired insulin clearance as the initial regulator of obesity-associated hyperinsulinemia: Novel insight into the underlying mechanism based on serum bile acid profiles. Diabetes Care. 45 (2), 425-435 (2022).
- Koh, H. E., Cao, C., Mittendorfer, B. Insulin clearance in obesity and type 2 diabetes. Int J Mol Sci. 23 (2), 596 (2022).
- Bril, F., et al. Relationship between disease severity, hyperinsulinemia, and impaired insulin clearance in patients with nonalcoholic steatohepatitis. Hepatology. 59 (6), 2178-2187 (2014).
- Herman, R., Sikonja, J., Jensterle, M., Janez, A., Dolzan, V. Insulin metabolism in polycystic ovary syndrome: Secretion, signaling, and clearance. Int J Mol Sci. 24 (4), 3140 (2023).
- Najjar, S. M., Caprio, S., Gastaldelli, A. Insulin clearance in health and disease. Annu Rev Physiol. 85, 363-381 (2023).
- Polidori, D. C., Bergman, R. N., Chung, S. T., Sumner, A. E. Hepatic and extrahepatic insulin clearance are differentially regulated: Results from a novel model-based analysis of intravenous glucose tolerance data. Diabetes. 65 (6), 1556-1564 (2016).
- Castillo, M. J., Scheen, A. J., Letiexhe, M. R., Lefebvre, P. J. How to measure insulin clearance. Diabetes Metab Rev. 10 (2), 119-150 (1994).
- Rubenstein, A. H., Pottenger, L. A., Mako, M., Getz, G. S., Steiner, D. F. The metabolism of proinsulin and insulin by the liver. J Clin Invest. 51 (4), 912-921 (1972).
- Terris, S., Steiner, D. F. Binding and degradation of 125i-insulin by rat hepatocytes. J Biol Chem. 250 (21), 8389-8398 (1975).
- Ooms, H. A., Arnould, Y., Rosa, U., Pennisi, G. F., Franckson, J. R. Total metabolic clearance of crystalline insulin and radio-iodide substitued insulin. Pathol Biol. 16 (5), 241-245 (1968).
- Defronzo, R. A., Tobin, J. D., Andres, R. Glucose clamp technique: A method for quantifying insulin secretion and resistance. Am J Physiol. 237 (3), E214-E223 (1979).
- Asare-Bediako, I., et al. Assessment of hepatic insulin extraction from in vivo surrogate methods of insulin clearance measurement. Am J Physiol Endocrinol Metab. 315 (4), E605-E612 (2018).
- Kim, S. P., Ellmerer, M., Kirkman, E. L., Bergman, R. N. Beta-cell "rest" accompanies reduced first-pass hepatic insulin extraction in the insulin-resistant, fat-fed canine model. Am J Physiol Endocrinol Metab. 292 (6), E1581-E1589 (2007).
- Kotsis, T., et al. Insulin metabolism and assessment of hepatic insulin extraction during liver regeneration. A study in a rat model. J Invest Surg. 33 (1), 69-76 (2020).
- Mondon, C. E., Olefsky, J. M., Dolkas, C. B., Reaven, G. M. Removal of insulin by perfused rat liver: Effect of concentration. Metabolism. 24 (2), 153-160 (1975).
- Tamaki, M., et al. The diabetes-susceptible gene SLC30A8/ZnT8 regulates hepatic insulin clearance. J Clin Invest. 123 (10), 4513-4524 (2013).
- Ghadieh, H. E., Gastaldelli, A., Najjar, S. M. Role of insulin clearance in insulin action and metabolic diseases. Int J Mol Sci. 24 (8), 7156 (2023).
- Winther-Sorensen, M., Kemp, I. M., Bisgaard, H. C., Holst, J. J., Wewer Albrechtsen, N. J. Hepatic glucose production, ureagenesis, and lipolysis quantified using the perfused mouse liver model. J Vis Exp. 200, e65596 (2023).
- Rydell-Tormanen, K., Johnson, J. R. The applicability of mouse models to the study of human disease. Methods Mol Biol. 1940, 3-22 (2019).
- Zaidi, S., et al. Loss of ceacam1 in hepatocytes causes hepatic fibrosis. Eur J Clin Invest. 54 (7), e14177 (2024).
- Piccinini, F., Bergman, R. N. The measurement of insulin clearance. Diabetes Care. 43 (9), 2296-2302 (2020).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionExplore More Articles
This article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved