Method Article
Integrative Protocol for Generation of iPSC-Derived 3D Human Hepatic Organoids
In This Article
Summary
Human iPSC-derived 3D hepatic organoids constitute a potential tool for understanding the thyroid hormone's action on liver development.
Abstract
Obtaining stable hepatic cells in culture poses a significant challenge for liver studies. Bearing this in mind, an optimized method is depicted utilizing human induced pluripotent stem cells (hiPSCs) to generate 3D cultures of human hepatic organoids (HHOs). The utilization of HHOs offers a valuable approach to understanding liver development, unraveling liver diseases, conducting high-throughput studies for drug development, and exploring the potential for liver transplantation. In the former investigation, through immunofluorescence and quantitative RT-PCR techniques, the progression was monitored, identifying the presence of various cell populations, such as hepatoblasts and the two types of hepatoblast-derived cells: cholangiocytes or hepatocyte-like cells, across different developmental stages. This report presents a straightforward 3D protocol starting from hiPSC to acquire HHOs that mirror the stages of human embryo development. The protocol, spanning 46-50 days, encompasses several steps: (i) meticulous management of hiPSC culture to generate HHOs, (ii) initiation of cell differentiation in 2D and the subsequent transition to 3D, and (iii) an optimized dissociation strategy to break down HHOs into single cells for single-cell RNA sequencing. As an illustration of the broad applications of this approach, the present protocol was previously applied to unravel the role of thyroid hormone signaling in developing liver cells.
Introduction
The liver performs diverse metabolic functions, such as regulating the availability of readily usable energy substrates like glucose and ketone bodies, as well as detoxifying xenobiotic compounds. In recent years, there has been a significant increase in the prevalence of liver diseases, largely attributed to non-alcoholic steatohepatitis (NASH), which, if untreated, can advance to cirrhosis or cancer1. Therefore, it is imperative to understand the metabolic functions of the liver and its related diseases to facilitate the development of effective treatments2,3.
The emergence of three-dimensional (3D) cultures has led to the creation of the organoid model, representing a groundbreaking and innovative approach to addressing the functionality and intricacy of organ development4. Organoids are defined as 3D self-organized aggregates of differentiated cells that mimic the functions and cytoarchitecture of the respective organ5.
Over the past decades, a myriad of human hepatic organoid (HHO) protocols has gained widespread interest, ranging from the utilization of diverse human iPSC-derived cells6 or solely hepatocytes-like cells7 to the incorporation of a variety of intricate microenvironments of growth factors or inhibitors and differentiating progenitor cells in monolayer7 or 3D8. These approaches lend themselves to a multitude of potential objectives, from high throughput drug screening9 to gaining further insights into the mechanisms underlying liver diseases10.
Here, a step-by-step protocol of HHO differentiation based on the chemical cues mentioned11 is performed, with methodological adapted variations. This protocol begins with the appropriate handling and cultivation of human induced pluripotent stem cells (hiPSC), detailing techniques for extracellular matrix gel manipulation, cell passaging, and differentiation into HHOs. The process starts by stimulating the differentiation of hiPSCs into definitive endoderm (DE)12 and subsequently mimicking the in vivo effects of FGF and BMP to promote the development of posterior foregut (PFG) monolayer cells13. The 3D architecture is achieved on day 10 when the PFG cells are differentiated into an immature hepatic phase that will become the hepatoblasts, the fetal precursor cell of cholangiocytes and hepatocytes2. Finally, the 3D structures are dissociated into single cells for RNA sequencing studies. As an example of the applicability of this protocol, it was demonstrated how this HHO model lends itself to the study of thyroid hormone action and the type 2 deiodinase (D2) on the development of hepatocytes and cholangiocytes14.
Protocol
1. Management of hiPSC
NOTE: hiPSCs (CS03iCTR-n3 cell line) were commercially purchased. The appropriate management of the extracellular matrix gel coating and the hiPSC medium is key for attaching the hiPSCs to the plates and feeding them. Here, the volumes needed for one 6-well plate were described. The remaining hiPSCs from the 6-well plate, which will not differentiate into organoids, may be stored in liquid nitrogen for long-term storage.
- Dispensing extracellular matrix gel and hiPSC medium
- Defrost properly the bottle of the extracellular matrix gel by placing in a container with ice at 4 ˚C overnight.
NOTE: Before aliquoting, check the certificate of analysis to verify the volume for the dilution factor. This represents the protein concentration of the extracellular matrix gel; therefore, it varies from one lot to another. - Distribute extracellular matrix gel into suitable aliquots according to the dilution factor (4x, 2x, and 1x) using pre-chilled and labeled tubes. Add a 4x dilution factor of extracellular matrix gel to 25 mL of cold DMEM/F12 to coat four 6-well plates. Promptly seal the lids with laboratory sealing film and freeze them at -80 ˚C. Avoid prolonged exposure to air and the creation of bubbles in the aliquots.
- Thaw 100 mL of hiPSC supplement medium at 4 ˚C overnight. Add it to 400 mL of hiPSC basal medium. Once homogenized with a 50 mL serological pipette, aliquot the hiPSC medium into 40 mL tubes and freeze at -20 ˚C until use.
- Defrost properly the bottle of the extracellular matrix gel by placing in a container with ice at 4 ˚C overnight.
- Plate coating for the hiPSC culture
- Numerate and label the 6-well plate(s). Keep one extracellular matrix gel aliquot nearby, immersed in dry ice.
- For coating one 6-well plate, dilute one extracellular matrix gel aliquot (1x) into 6.25 mL of cold DMEM/F12 (2x into 12.5 mL and 4x into 25 mL of DMEM/F12).
- Pipette a small volume of DMEM/F12 (~500 µL) into the extracellular matrix gel tube. Swirl up and down to defrost and homogenize the extracellular matrix gel with cold DMEM/F12, preventing the creation of bubbles, and return it to the rest of the cold DMEM/F12-containing tube using a serological pipette to mix completely.
- Embed 1 mL of extracellular matrix gel in DMEM/F12 into one well for coating the 6-well plate (6 mL for the whole plate). Distribute the 1 mL evenly over the surface by moving, without shaking, the plate until it is fully covered.
- Allow to sit at room temperature (RT) for 1 h before use. If the 6-well plate is not entirely used, seal it with laboratory sealing film and store it in the refrigerator at 4 ˚C.
NOTE: A coated plate can be stored for a 1 week in the fridge at 4 ˚C.
- Thawing and culture of hiPSC
- Calculate in advance the volume of hiPSC medium and warm at RT along with the 6-well coated plate 1 h before use (2 mL per well).
NOTE: In case of cold or frozen aliquots, immerse them in a 37 ˚C incubator or a water bath 15 min before use. - Split 1 mL of hiPSCs in liquid N2 from a cryovial into 3 wells of a 6-well plate (1:3 ratio). For a whole 6-well plate, use 2 cryovials. Here, the protocol describes the thawing and culture of hiPSCs from one cryovial. Repeat the process for 2 cryovials.
- Introduce the cryovial containing approximately 1 mL of frozen hiPSCs by holding it through the cap and sliding it through the superficial water, with the bottom of the vial immersed in a water bath at 37 ˚C until defrosting.
- Once thawed, spray the cryovial with ethanol and pipette the 1 mL of hiPSCs. Dispense slowly in an empty conical 15 mL tube. Add 5 mL of warm hiPSC medium drop by drop while gently shaking the tube.
- Centrifuge the tube at 300 x g for 5 min at RT. Delicately eliminate the supernatant by aspirating with a glass pipette connected to the vacuum without disrupting the pellet, leaving some residual medium. Carefully resuspend the pellet in 6 mL of medium and then add 2 mL per well in 3 wells of the 6-well plate.
NOTE: Leave small aggregates of hiPSCs for correct growth. - Bend the 6-well plate over and aspirate the extracellular matrix gel from the previously coated plate without touching the tip to the bottom of the well.
- Transfer 2 mL of the medium with hiPSCs per well.Gently shake the plate forward, backward, and side to side to evenly distribute the hiPSC aggregates on the plate.
- Place the 6-well plate into an incubator at 37 ˚C, 5% CO2, and 95% humidity. Change the medium daily (2 mL per well), checking the progressive growth.
- Passage hiPSCs when 80% growth confluence is acquired (after 4-5 days; see Figure 1A). Characteristics of healthy and proliferative hiPSCs include distinct borders and tight packaging of large nuclei in the center of the growing cell aggregations.
- Calculate in advance the volume of hiPSC medium and warm at RT along with the 6-well coated plate 1 h before use (2 mL per well).
- Passaging of hiPSC
- Once the hiPSCs reach 80% confluency, indicating approximately 1.2 x 106 cells per well, passage into a new 6-well plate. Split at a ratio of 1:3 (from 1 well into 3), or adjust the ratio (1:4; 1:6) according to the requirements of the experiment.
- Before passaging, prepare the required extracellular matrix gel embedding for the appropriate number of 6-well plates, as described in step 1.2.
- Remove the supernatant from each well and add 2 mL of PBS per well. Aspirate the PBS, followed by the addition of 1 mL of hiPSC detaching medium per well for 1 min. Then remove the 1 mL of hiPSC detaching medium.
- Place the 6-well plate in an incubator at 37 ˚C for 7 min. Add 1 mL of hiPSC medium per well. Detach and collect by pipetting 1 mL of the hiPSC medium with cells into the 15 mL tube.
- Centrifuge the tube at 300 x g for 5 min at RT. To plate the cells into a 6-well plate, repeat steps 1.3.5-1.3.7. Place the 6-well plate into an incubator at 37 ˚C, 5% CO2, and 95% humidity. Go to step 1.5. to freeze hiPSC if needed.
- Freezing hiPSC (Optional)
NOTE: The number of hiPSCs required for promoting into HOs may result in leftover wells that can be stored long-term. Should there be a need to expand the passage for cryovial storage, the hiPSCs can be maintained for extended periods.- Defrost the required volume of cryopreservation medium (1 mL per well of cells) into ice at 4 ˚C. Label cryotubes with the number of passages, date, and cell line. Detach cells with hiPSC detaching medium as in step 1.4. in a 15 mL conical tube.
- Centrifuge at 300 x g for 5 min at RT. Fully aspirate the supernatant, carefully leaving the cell pellet undisturbed. Prudently resuspend the pellet in 1 mL of cold cryopreservation medium (per well) by leaving hiPSC aggregates.
NOTE: If wells are at low density, less than 50% confluent, 1 mL of cryopreservation medium may be used for every 2 wells. - Transfer 1 mL of cryopreservation medium with the hiPSCs into the cryotube. Gently agitate the cryotube to homogenize the cell content.
NOTE: If preparing more tubes to cryopreserve, leave the already prepared tubes in ice. - Place the tubes in the controlled-rate freezing container at -80 ˚C overnight. Transfer the tubes into the liquid nitrogen tank after 24 h.
2. Step-by-step differentiation from hiPSC into hepatic organoids
NOTE: The reconstitution of the reagents was performed and followed according to the manufacturer's guidelines.
- 2D differentiation from hiPSC into DE (day 0-day 4 (D-0 to D-4); Figure 1B)
- After 3 passages, if cells are healthy and growing well and fast, proceed to the differentiation of the hiPSC. Coat a 6-well plate as described in step 1.2. To differentiate hiPSC into DE, utilize the DE kit by following the manufacturer's instructions.
- Before beginning the differentiation, culture hiPSCs in a passage ratio of 2:1 (from 6 wells to obtain 3 to begin the differentiation) for 24 h to achieve the cell concentration in the well indicated in the DE instructions.
- Perform differentiation as per the manufacturer's protocol with the following modifications.
- Raise the concentration of Y-27632 added to hiPSC from 10 µM to 50 µM (A day before starting the differentiation).
- Increase 1.5 mL per well of the dissociation reagent and also the volume of DMEM/F12 to the same amount per well as the dissociation reagent. Add 1 mL of DMEM/F12 (out of 1.5) to each well, and an extra 0.5 mL accumulated into a 15 or 50-mL tube.
- Differentiation of DE cells into PFG cells (D-4 to D-10; Figure 1C)
- Prepare PFG medium (Advanced DMEM/F12 as basal medium, 1x Glutamax supplement, 1x B27 supplement, 20 ng/mL BMP4, 10 ng/mL FGF2) for the differentiation of DE cells days before the induction into PFG and store it at 4 ˚C.
NOTE: B27 contains T3 as part of its composition. In the case of thyroid hormone (TH) studies, from here onwards, B27 should be replaced with homemade B2615 that does not contain TH. - Warm the PFG medium (2 mL per well) and advanced DMEM/F12 at 37 ˚C for 15 min or RT for 1 h. Bend over the plate and aspirate the DE medium from the wells with a glass pipette connected to a vacuum (or manually with a P1000 pipette) without scratching the cells.
- Add 2 mL of warm advanced DMEM/F12 per well and then aspirate it. Add 2 mL of warm PFG medium per well in the 6-well plate. Change the medium daily for the next 6 days.
- Prepare PFG medium (Advanced DMEM/F12 as basal medium, 1x Glutamax supplement, 1x B27 supplement, 20 ng/mL BMP4, 10 ng/mL FGF2) for the differentiation of DE cells days before the induction into PFG and store it at 4 ˚C.
- Induction from 2D PFG cells to 3D Immature Hepatic Organoids (IHOs; D-10 to D-18)
NOTE: Nearly 30,000-35,000 cells are required in 30 µL added to each well of the 96-well ULA plate. The passage ratio is 2:1, from 2 wells (6-well plate) with a monolayer of differentiated PFG cells; it will provide a full 96-well ultra-low attachment (ULA) plate (Figure 1D).- Before initiating the 3D organoids or in earlier days, prepare previously the IHO medium (Advanced DMEM/F12 as basal medium, 1x N2 supplement, 1x B27, 50 nM A83-01, 30 µM Dexamethasone, 5 µM CHIR99021, 500 nM valproic acid, 50 ng/mL human epidermal growth factor (EGF), 20 ng/mL human hepatocyte growth factor (HGF), 40 ng/mL Jagged-1, 300 ng/mL N-6,20-O-dibutyryladenosine 30,50-cyclic 35 monophosphate sodium salt (dbCAMP), 10 µM Nicotinamide) and store it at 4 ˚C.
- Label a 96-well ultra-low attachment (ULA) plate. For detaching the PFG cells, use the cell dissociation enzyme according to the manufacturer's instructions.
- Warm the dissociation enzyme, advanced DMEM/F12, and PBS at 37 ˚C before use. Aspirate and discard the PFG medium of the wells. Add 2 mL of PBS per well, then aspirate it.
- Add 1.5 mL of cell dissociation enzyme per well at 37 ˚C for 7 min to detach the cells from the well. Without removing the cell dissociation enzyme, add 1 mL of advanced DMEM. Gather all the cells and add them into a 50 mL tube.
- Count the number of cells per mL with a cell counter or a hemocytometer. Centrifuge at 150 x g for 8 min.
- To the pellet, add the appropriate volume (3 mL of IHO medium for an entire 96-well ULA plate) and 1x extracellular matrix gel to adjust to the final number of cells needed (30,000-35,000 cells).
- Place the 96-well plate into an incubator set up to 37 ˚C, 5% CO2, and 95% humidity. After 24 h (D-11), cells will regroup in a circular shape; add 50 µL per well of the IHO medium.
- The next day (D-12), increase to 100 µL of IHO medium per well. At D14, remove ~100-120 µL from each well either with the glass pipette connected to a vacuum or with a pipette one by one. Take care not to aspirate the organoids. Add 100 µL of IHO medium at D-14 and D-16.
NOTE: After D-16, due to not being placed into movement, excessive growth appears that vanishes after D18.
- Hepatoblast organoids (HBOs, D-18 to D-26)
NOTE: In this step, IHOs from the 96-well ULA plate will be relocated into a 6-well ULA plate. For 96 IHOs from the previous stage, 10 organoids were placed per well, representing a total of 1 and ½ 6-well ULA plates to proceed with the maturation (Figure 1E).- As done in previous steps of differentiation, prepare the HBOs differentiation medium in previous days.
- The cocktail of reagents used to differentiate into HBOs is described in step 2.3. Remove valproic acid and incorporate into the cocktail the following biochemicals: BMP7, BMP4, and FGF7. The final composition is Advanced DMEM/F12 as basal medium, 1x N2 supplement, 1x B27, 50 nM A83-01, 30 µM Dexamethasone, 5 µM CHIR99021, 500 nM valproic acid, 50 ng/mL human epidermal growth factor (EGF), 20 ng/mL human hepatocyte growth factor (HGF), 40 ng/mL Jagged-1, 300 ng/mL N-6,20-O-dibutyryladenosine 30,50-cyclic 35 monophosphate sodium salt (dbCAMP), 10 µM Nicotinamide, 25 ng/mL FGF7, 50 ng/mL BMP4, 20 ng/mL BMP7.
- Warm previously HBO medium (3 mL per well) and Advanced DMEM/F12 (3 mL per well) at 37 ˚C. Add 3 mL of warm advanced DMEM/F12 to each well.
- Distribute 10 IHO organoids per well from the 96-well plate with a 200 mL wide bore tip. Remove the advanced DMEM/F12 by bending over the plate and suctioning it with the glass pipette connected to the vacuum (or manually with a P1000).
- Add 3 mL of warm HBO medium per well. Turn on and place the 6-well ULA plates on a multi-platform shaker at 65 rpm inside the incubator set at 37 ˚C, 5% CO2, and 95% humidity. After 4 days (D-22) and 6 days (D-24), replace the HBO medium (3 mL per well).
- As done in previous steps of differentiation, prepare the HBOs differentiation medium in previous days.
- Maturation into hepatic organoids (HO) through 2 different phases (D-26 to D-46)
NOTE: This is the last process of maturation that happens in two periods of differentiation due to the content and the type of reagents in the medium.- Prepare in advance the first of the HO (HO1) medium and store it at 4 ˚C. Remove Jagged1 and decrease the concentration of CHIR99021 compared to the HBO medium. The final composition is: Advanced DMEM/F12 as basal medium, 1x N2 supplement, 1x B27, 500 nM A83-01, 30 µM Dexamethasone, 2 µM CHIR99021, 50 ng/mL human epidermal growth factor (EGF), 20 ng/mL human hepatocyte growth factor (HGF), 300 ng/mL N-6,20-O-dibutyryladenosine 30,50-cyclic 35 monophosphate sodium salt (dbCAMP), 10 µM Nicotinamide, 25 ng/mL FGF7, 20 ng/mL BMP4, 20 ng/mL BMP7.
- Warm Advanced DMEM/F12 and HO1 medium at 37 ˚C before use. In the same 6-well ULA plate, carefully aspirate the previous HBO medium by bending over the plate and placing the tip of the glass pipette connected to a vacuum against the wall (or manually with a P1000 pipette).
- Add 3 mL of warm Advanced DMEM/F12 per well and aspirate it. Add 3 mL of warm HO1 medium per well. Change the HO1 medium every 3 days (D-29, D-32, D-35, D-38) along with sample collection.
- At D 38, modify the cocktail of reagents for the second period of HO (HO2) by removing CHIR99021, BMP4, dbcAMP, and FGF7 and substituting them with FGF19 and DAPT. The final medium composition is Advanced DMEM/F12 as basal medium, 1x N2 supplement, 1x B27, 500 nM A83-01, 30 µM Dexamethasone, 50 ng/mL human epidermal growth factor (EGF), 20 ng/mL human hepatocyte growth factor (HGF), 10 µM Nicotinamide, 20 ng/mL BMP7, 25 ng/mL FGF19, 5 µM DAPT.
- Warm Advanced DMEM/F12 and HO2 medium at 37 ˚C before use. Continue with same the 6-well ULA plate, and carefully aspirate the previous HO1 medium. Add 3 mL of Advanced DMEM/F12 per well and remove it.
- Add 3 mL of warm HO2 medium per well. Change the HO2 medium every 4 days (D-42, D-46, D-50, …) and take samples on the same days as the medium is changed.
3. Single-cell dissociation
NOTE: This step is critical for the single-cell RNA sequencing technique. The number of organoids may vary according to size, and the older the day of dissociation, the greater the quantity of cells in the organoids. In earlier days, the number of dissociated organoids increased, and the dissociation times reduced with equal quantities. Dissociation was performed with 10 organoids at D-14 and D-17, 8 organoids at D-23 and D-26, and 6 organoids at D-30 and D-45. Procedures for one cluster of organoids are detailed (Figure 1F).
- Preparation of reagents
- Prepare Advanced-DMEM with a final concentration of 7.5% Bovine Albumin Fraction V (BSA) and filter after homogenizing.
- Prepare 1 mL of the dissociation enzyme medium with 1 mL of trypsin 0.5% - EDTA (10x) as a basal medium, 50 µM Y-27632, 40 U/µL RNase inhibitor, and 1 U/µL DNase I.
- Prepare the trypsin inactivation medium with 2 mL of Advanced DMEM/F12 + 7.5% BSA as a basal medium, 50 µM Y-27632, 40 U/µL RNase inhibitor, and 1 U/µL DNase I.
- Warm both media and PBS at 37 ˚C before starting.
- Dissociation of HHOs
- Clean the workplace and tools with RNase cleaner. Collect the number of organoids according to the day of differentiation in a 15 mL polypropylene tube with a wide-open tip.
- Wash with PBS 2x-3x. Add 1 mL of dissociation enzyme medium into the 15 mL tube with collected organoids and keep on 37 ˚C in a rocker at 30 rpm for 5 min.
CAUTION: Be careful not to drop the tube from the rocker. - After 5 min, first, check the cap for any remaining organoids, then mechanically disrupt the organoids with a P1000 by swirling up and down 10x per organoid (60 times in total). Repeat the same disruption with a P200. Check the tube cap in case the organoids get stuck on it.
- Place the 15 mL tube again on the rocker into the incubator for another 5 min. Repeat the process of point 4, starting by swirling up and down a P200 and continuing with a 1 mL serological pipette connected to a P10 tip.
- Place the 15 mL tube again on the rocker into the incubator for the last 5 min. In case of no dissociation, leave it for an additional 5 min and repeat manual disruption.
- Add 1 mL of trypsin inactivation medium and homogenize it. Place a new 15 mL polypropylene tube with a 40 µm cell strainer on top. Filter the 2 mL (cells with the dissociation enzyme medium and the trypsin inactivation medium) through a 40 µm cell strainer into the 15 mL tube.
- Add another 1 mL of trypsin inactivation medium to recover any remaining cells from the 40 µm cell strainer.
- Take 5 µL of single-cell medium mixed with 5 µL of a dye solution to count the number of dissociated cells to proceed with their fixation. Count the cells after centrifugation and resuspension with the first reagent of the fixation kit and perform cell filtration using a 40 µm cell strainer.
Results
Each stage of this protocol of stepwise differentiation from hiPSC into HHOs was defined by using quantitative measurements by qPCR and immunofluorescence of stage-specific known markers from the bibliography (Figure 2). The step-by-step of both techniques and the outcomes achieved related to the correct differentiation into HOs were depicted in14. In the previous investigation, hiPSCs were defined through the mRNA levels of POU5F1 (also known as OCT4) and SOX2, two well-known Yamanaka factors16, which decreased over time (Figure 2A). Subsequently, the mRNA levels of DE11 markers were measured such as OTX2, CER1, and FOXA2, and PFG11 markers HNF4A, CDX2, and TBX3, together with the localized expression of OTX2, HNF4A, and TBX3 by immunofluorescence (Figure 2A,B). After 3D promotion, to follow the progression immunofluorescence of albumin and HNF4a (Figure 2C); the proliferative marker, MKI6717, and TBX3, important in the regulation of hepatoblast18 (Figure 2D) was performed from D-18 to D-46. Notably, albumin colocalized with HNF4a (Figure 2C); meanwhile, MKI67 sporadically appeared at D-46 (Figure 2D). Also, hepatocyte- and cholangiocyte-like cells were identified through the mRNA levels and immunofluorescence of HNF4A19 and KRT718 at D-46 (Figure 2E).
The volume of the developing organoids increased progressively from D-14 to D-38, as indicated by the ~2-fold increase in mRNA levels for the proliferative marker MKI67 on D-22 as compared with D-10. The absence of T4 in the medium led to an increase in MKI67 mRNA levels by ~20% and ~75% on D-14 and D-18, respectively, compared to day 10 (Figure 3A). To prove the functionality of the hepatic organoid, levels of albumin, apolipoprotein B, and A114 collected from the media of the hepatic organoids were measured using ELISA. On D-42 and D-46, ALB mRNA levels remained substantially higher in the T4-HOs as compared with T3-HOs or V-Hos by 3.0-fold and 2.5-fold, respectively (Figure 3B). On the other hand, APOB levels were ~10-fold and 3-fold higher (Figure 3C), and APOA1 levels were ~3-fold and ~2-fold higher (Figure 3D) at D-42 and D-46, respectively.
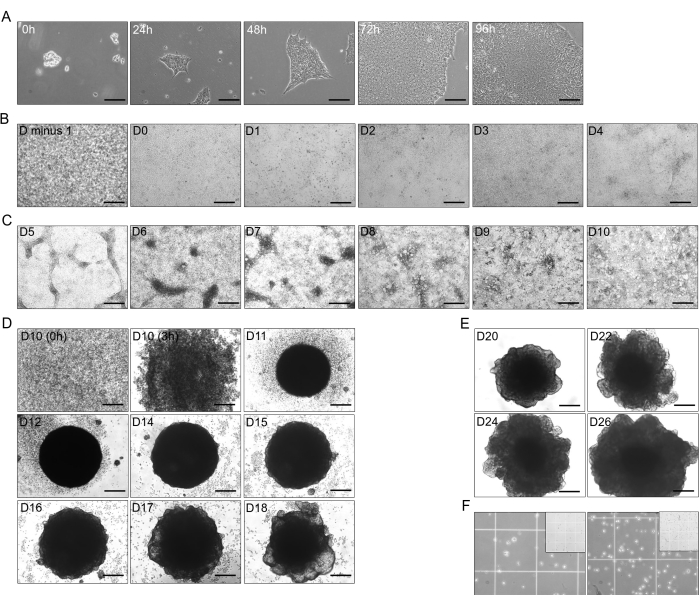
Figure 1: Differentiation stages from hiPSC to hepatic organoids. (A) Transitional growth of hiPSC (CS03iCTR-n3 line) from 0 h to 96 h, reaching approximately 70%-80% confluence in the wells of one plate. Scale bar: 125 µm. (B) Progression of the initial stage of hiPSC differentiation into definitive endoderm (DE), showing 100% confluence on the previous day (D minus 1) after 24 h, and differentiation until D4 (D-0 to D-4). Scale bar: 300 µm. (C) Differentiation from DE (D-4) into posterior foregut (PFG; D-10) with T4 added B26. Scale bar: 300 µm. (D) Transformation of 2D-PFG into 3D immature hepatic organoids (IHO) with T4-added B26 from D-10 as single cells until D-18, excluding D-13. Scale bar: 300 µm. (E) Maintenance and growth of hepatoblast organoids (HBO) with T4-added B26 at D-20, D-22, D-24, and D-26. Scale bar: 300 µm. (F) Dissociation of HBO at D-26 (~2.5 x 105 cells in 750 µL; left), and HO1 at D-30 (~4 x 105 cells in 750 µL; right) into single cells with T4-added B26. The inset illustrates the 1 mm square size of Neuebauer's chamber, where the smaller square measures 250 µm. The images were taken to HHOs whose free concentration of T4 used in the media was ~15 pM. Please click here to view a larger version of this figure.

Figure 2: Monitorization and characterization of the differentiation of the HHOs. (A) Expression of mRNA levels of hiPSC markers (POU5F1, SOX2), definite endoderm (DE; OTX2, CER1, FOXA2) and posterior foregut (PFG; TBX3, HNF4A, CDX2). (iPSC, n=8; DE and PFG n=7; HNF4A in PFG, n=6). (B) Immunofluorescence at hiPSC, DE, and PFG of OTX2 (top, red), HNF4A (middle, green), and TBX3 (bottom, red). (C) “Immunofluorescence at D-18, D-26, and D-46 of albumin (red) and HNF4A (green). (D) Immunofluorescence at D-18, D-26, and D-46 of MKI67 (green) and TBX3 (red). (E) Immunofluorescence at D-46 of albumin (hepatocyte marker; red) and KRT7 (cholangiocyte marker; green). Nuclei are shown with 4′,6-diamidino-2-fenilindol (DAPI). This figure has been modified from14. Please click here to view a larger version of this figure.

Figure 3: Analysis of maturation of the HHO. (A) Relative mRNA levels of the proliferative marker MKI67 in the absence of TH (black) versus free T4 at ~15 pM (red) in the medium from D-10 to D-46 (n=4 except T4-HOs at D-42, n=2; and at D-46, n=3). (B) Albumin levels were measured in the medium from D-35 to D-50 in three conditions: absence of TH (black), with free T4 at ~15 pM (red) and free T3 at ~10 pM (blue; n=4). (C, D) Apolipoprotein B (APOB) and A1 (APOA1) levels were measured in the medium from D-35 to D-50 in three conditions: absence of TH (black); and with free T4 at ~15 pM (red). Data from 10 organoids per well (n=4). Two-tailed Student t-test for comparing V-HOs versus T4-HOs days and one-way ANOVA and Tukey test were used for multiple comparisons. Data are the mean of duplicates, represented as aligned scatter dot plots and their mean. *: p<0.05; **: p<0.01; ***: p<0.001; ****: p<0.0001. The x-axis indicates the days of the differentiation. This figure has been modified from14. Please click here to view a larger version of this figure.
Discussion
The current protocol offers various methodological details on how to handle hiPSCs and the subsequent 3D organoid cultures. This includes the two major critical steps: (i) the detaching of the 2D cultures and then their development into 3D hepatic organoids after 10 days, as well as (ii) the delicate dissociation of a 3D structure into single cells. Based on available information, this is the first report of a 3D HHO model to study thyroid hormone action, demonstrating a peak expression of DIO2 in hepatoblast-like cells14 as originally identified in P1 liver mice20.
As with many other techniques that utilize scaffolds applied to cells, extracellular matrix gel was used to mimic the extracellular matrix21, thereby facilitating the assembly into the characteristic 3D structure of the organoids. The heat-sensitive nature of this reagent underscores the importance of its manipulation keeping it frozen and, during handling, immersed in ice. Consequently, using a different extracellular matrix gel is possible22, although hiPSCs did not attach and proliferate in a scaffold-absence plate in this protocol, making its proper management a crucial aspect at the beginning of the protocol, alongside the generation of 3D organoids.
After completing the differentiation of the HOs, a viable dissociation of organoids into single cells represented another major hurdle to overcome, as mechanical disruption may provoke cell death, by then low-quality RNA-sequencing data23. To ensure optimal results, the present protocol showed that over 70% single-cell viability could be achieved, with the dissociation of over 105 cells before and after fixation for subsequent barcoding.
Unlike other manuscripts describing only one cell type in the organoids7, the differentiation of the HHO includes at least two hepatoblast-derived cells: hepatocytes- and cholangiocytes-like cells. The incorporation of two reagents responsible for their formation, HFG24 and EGF25, and along with dexamethasone26 which promotes the maturation of hepatocytes, and Jagged-118 which activates the Notch signaling leading toward cholangiocytes. This intertwined signal network in the development of HOs allows us to achieve the 3D structure and mimic in vivo experiments, in addition to overcoming the disadvantage of the decline in the differentiation of long-term cultures of primary hepatocytes27. It is notable that despite the expression of typical adult hepatic markers, the presence of fetal markers in advanced phases of organoids28 can still be detected, as this protocol demonstrates. This, in fact, reflects the normal liver development in humans, in whom fetal hepatic markers can be normally detected up to 1 year of life29.
An infrequent occurrence observed during the development of hiPSCs into HHOs (particularly during immature periods of the HOs, such as in the last days of IHO and some HBO and HO1) was the appearance of overdeveloped cystic structures, which eventually faded once the plates were placed on the shaker. This disproportionate progression of cystic structures suggests a necrotic core by then the necessity of higher irrigation30 with more frequent medium changes or the addition of low volumes of extracellular matrix gel to eliminate excessive cyst growth.
In contrast to the original publication, which focused on an increased number of organoids in the last period of differentiation for high-throughput drug screening11, the method now reported introduced the use of ULA plates to promote the early formation of large-size 3D organoids and facilitate the handling of the HHOs. These adjustments demonstrate the flexibility and the vast array of studies that may be accomplished through this straightforward protocol.
Disclosures
Antonio C. Bianco is a consultant for Abbvie, Acella, Aligos, Synthonics. The other authors have no relevant disclosures.
Acknowledgements
This work was supported by the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK -DK58538, DK65066, DK77148; ACB).
Materials
| Name | Company | Catalog Number | Comments |
| 10 µL Universal Pipette Tips Filtered, Low rentention, Pre-sterile | VWR | 613-6462 | All procedures |
| 1000 µL Universal Pipette Tips Filtered, Low rentention, Pre-sterile | VWR | 613-6470 | All procedures |
| 15 mL Polypropilene Conical Tube | Falcon (Corning) | 352097 | Dissociation Hepatic Organoids |
| 200 µL Universal Pipette Tips Filtered, Low rentention, Pre-sterile | VWR | 613-6465 | All procedures |
| 3,3',5-Triiodo-L-thyronine | Sigma | T2877-100 | Hepatic Organoid differentiation |
| 40 μm Cell Strainer | Corning | 431750 | Dissociation Hepatic Organoids |
| 50 mL tube | Falcon (Corning) | 352070 | All procedures |
| 6 well-plate Nunc Cell-Culture Treated Multidishes | Thermo fisher scientific | 140675 | hiPSC maintenance |
| A83-01 | R&D Systems | 2939/10 | Hepatic Organoid differentiation |
| Advanced DMEM/F12 | Gibco | 12634010 | Hepatic Organoid differentiation |
| ART Wide Bore Filtered Pipette Tips | ART | 2069GPK | All procedures |
| B27 supplement | Gibco | 17504044 | Hepatic Organoid differentiation |
| BMP7 | R&D Systems | 354-BP-010/CF | Hepatic Organoid differentiation |
| Bovine Albumin Fraction V (7.5% solution) | Gibco | 15260037 | Dissociation Hepatic Organoids |
| BSA, Fraction V, Fatty Acid Free for Tissue Culture | GoldBio | A-421-100 | Dissociation Hepatic Organoids |
| CHIR99021 | R&D Systems | 4423/10 | Hepatic Organoid differentiation |
| Corning 96-well Clear Flat Bottom Ultra-Low Attachment | Corning | 3474 | Hepatic Organoid differentiation |
| Costar 6-well Clear Flat Bottom Ultra-Low Attachment | Corning | 3471 | Hepatic Organoid differentiation |
| CS03iCTR-n3 human induced Pluripotent Stem Cell line | Cedar-sinai | hiPSC maintenance | |
| DAPT | R&D Systems | 2634/10 | Hepatic Organoid differentiation |
| dbCAMP | Millipore Sigma | D0627-100MG | Hepatic Organoid differentiation |
| Dexamethasone | R&D Systems | 1126/100 | Hepatic Organoid differentiation |
| DMEM/F12 | Gibco | 11320033 | hiPSC maintenance |
| DNAse I, RNase-free, HC | Thermo Fisher scientific | EN0523 | Dissociation Hepatic Organoids |
| Falcon 10 mL Serological Pipet, Polystyrene, 0.1 Increments, Individually Packed, Sterile | Corning | 357551 | All procedures |
| Falcon 5 mL Serological Pipet, Polystyrene, 0.1 Increments, Individually Packed, Sterile | Corning | 357543 | All procedures |
| Falcon 50 mL Serological pipet, Polystyrene, 1.0 Increments, Individually Packed, Sterile | Corning | 357550 | All procedures |
| Gentle Cell Dissociation Reagent (GCDR) | Stemcell Technologies | 100-0485 | Hepatic Organoid differentiation |
| Glutamax supplement | Gibco | 35050061 | Hepatic Organoid differentiation |
| L-Thyroxine | Sigma | T1775-1G | Hepatic Organoid differentiation |
| Matrigel hESC-Qualified Matrix, LDEV-free, 5 mL | Corning | 354277 | Extracellular matrix gel |
| mFreSR | Stemcell Technologies | 5855 | hiPSC cryopreservation medium |
| mTeSR 5x Supplement | Stemcell Technologies | 100-0276 | hiPSC medium |
| mTeSR Plus | Stemcell Technologies | 100-0276 | hiPSC medium |
| Multi Platform Shaker | Fisherbrand (Thermo Fisher technologies) | 88861021 | Hepatic Organoid differentiation |
| N2 supplement | Gibco | 17502048 | Hepatic Organoid differentiation |
| Nicotinamide | R&D Systems | 4106/50 | Hepatic Organoid differentiation |
| PBS, pH 7.4 | Gibco | 10010023 | hiPSC maintenance |
| Recombinant human BMP4 | R&D Systems | 314-BP-010/CF | Hepatic Organoid differentiation |
| Recombinant human EGF | R&D Systems | 236-EG-200 | Hepatic Organoid differentiation |
| Recombinant human FGF basic/FGF2/bFGF | R&D Systems | 233-FB-010/CF | Hepatic Organoid differentiation |
| Recombinant human FGF19 | R&D Systems | 959-FG-025/CF | Hepatic Organoid differentiation |
| Recombinant human HGF | R&D Systems | 294-HG-005/CF | Hepatic Organoid differentiation |
| Recombinant Human Jagged-1 Fc Chimera | R&D Systems | 1277-JG-050 | Hepatic Organoid differentiation |
| Recombinant human KGF/FGF7 | R&D Systems | 251-KG-010/CF | Hepatic Organoid differentiation |
| ReLeSR | Stemcell Technologies | 100-0483 | hiPSC detaching medium |
| RNAse Inhibitor Ambion, cloned, 40 U/μL | Invitrogen | AM2682 | Dissociation Hepatic Organoids |
| RNase Zap | Invitrogen | AM9780 | Dissociation Hepatic Organoids |
| Sorvall Legend XT/XF Centrifuge Series | Thermo Fisher Scientific | 75004539 | All procedures |
| STEMdiff Definitive Endoderm Kit | Stemcell Technologies | 5110 | Hepatic Organoid differentiation |
| Trypan Blue solution (0.4%) | Gibco | 15250061 | Dye solution |
| TrypLE Express Enzyme | Gibco | 12604013 | Cell Dissociation enzyme |
| Trypsin 0.5% - EDTA (10X) | Gibco | 15400054 | Dissociation Hepatic Organoids |
| Valproic acid, sodium salt | R&D Systems | 2815/100 | Hepatic Organoid differentiation |
| Vari-Mix Platform Rocker | Thermo Fisher scientific | M79735Q | Dissociation Hepatic Organoids |
| Y-27632 dihydrochloride | R&D Systems | 1254 | Hepatic Organoid differentiation |
References
- Peiseler, M., et al. Immune mechanisms linking metabolic injury to inflammation and fibrosis in fatty liver disease - novel insights into cellular communication circuits. J Hepatol. 77 (4), 1136-1160 (2022).
- Gordillo, M., Evans, T., Gouon-Evans, V. Orchestrating liver development. Development. 142 (12), 2094-2108 (2015).
- Lemaigre, F. P. Mechanisms of liver development: concepts for understanding liver disorders and design of novel therapies. Gastroenterology. 137 (1), 62-79 (2009).
- He, C., et al. Liver organoids, novel and promising modalities for exploring and repairing liver injury. Stem Cell Rev Rep. 19 (2), 345-357 (2023).
- Lancaster, M. A., Knoblich, J. A. Organogenesis in a dish: modeling development and disease using organoid technologies. Science. 345 (6194), 1247125 (2014).
- Takebe, T., et al. Vascularized and functional human liver from an iPSC-derived organ bud transplant. Nature. 499 (7459), 481-484 (2013).
- Si-Tayeb, K., et al. Highly efficient generation of human hepatocyte-like cells from induced pluripotent stem cells. Hepatology. 51 (1), 297-305 (2010).
- Wu, F., et al. Generation of hepatobiliary organoids from human induced pluripotent stem cells. J Hepatol. 70 (6), 1145-1158 (2019).
- Shinozawa, T., et al. High-fidelity drug-induced liver injury screen using human pluripotent stem cell-derived organoids. Gastroenterology. 160 (3), 831-846.e10 (2021).
- Ouchi, R., et al. Modeling steatohepatitis in humans with pluripotent stem cell-derived organoids. Cell Metab. 30 (2), 374-384.e6 (2019).
- Ramli, M. N. B., et al. Human pluripotent stem cell-derived organoids as models of liver disease. Gastroenterology. 159 (4), 1471-1486.e12 (2020).
- Shen, M. M. Nodal signaling: developmental roles and regulation. Development. 134 (6), 1023-1034 (2007).
- Ang, L. T., et al. A roadmap for human liver differentiation from pluripotent stem cells. Cell Rep. 22 (8), 2190-2205 (2018).
- Hidalgo-Álvarez, J., Salas-Lucia, F., Vera Cruz, D., Fonseca, T. L., Bianco, A. C. Localized T3 production modifies the transcriptome and promotes the hepatocyte-like lineage in iPSC-derived hepatic organoids. JCI Insight. 8 (23), e173780 (2023).
- . B27 Supplement Available from: https://www.weizmann.ac.il/molgen/hanna/sites/molgen.hanna/files/users/user52/HANNA-LAB-B22-B27-PROTOCOL-V3.pdf (2016)
- Takahashi, K., et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 131 (5), 861-872 (2007).
- Zhu, X., Sun, J. CircHIPK3 regulates melanoma cell behaviors by binding with miR-215-5p to upregulate YY1. Mol Cell Probes. 53, 101644 (2020).
- Guan, Y., et al. Human hepatic organoids for the analysis of human genetic diseases. JCI Insight. 2 (17), e94954 (2017).
- Walesky, C., Apte, U. Role of hepatocyte nuclear factor 4α (HNF4α) in cell proliferation and cancer. Gene Expr. 16 (3), 101-108 (2015).
- Fonseca, T. L., et al. Hepatic inactivation of the type 2 deiodinase confers resistance to alcoholic liver steatosis. Alcohol Clin Exp Res. 43 (7), 1376-1383 (2019).
- Kleinman, H. K., Martin, G. R. Matrigel: basement membrane matrix with biological activity. Semin Cancer Biol. 15 (5), 378-386 (2005).
- Kozlowski, M. T., Crook, C. J., Ku, H. T. Towards organoid culture without Matrigel. Commun Biol. 4 (1), 1387 (2021).
- Arceneaux, D., et al. A contamination focused approach for optimizing the single-cell RNA-seq experiment. iScience. 26 (7), 107242 (2023).
- Matsumoto, K., Nakamura, T. Hepatocyte growth factor: molecular structure and implications for a central role in liver regeneration. J Gastroenterol Hepatol. 6 (5), 509-519 (1991).
- Kimura, M., Moteki, H., Ogihara, M. Role of hepatocyte growth regulators in liver regeneration. Cells. 12 (2), 208 (2023).
- Michalopoulos, G. K., Bowen, W. C., Mulè, K., Luo, J. HGF-, EGF-, and dexamethasone-induced gene expression patterns during formation of tissue in hepatic organoid cultures. Gene Expr. 11 (2), 55-75 (2003).
- Kaur, I., et al. Primary hepatocyte isolation and cultures: Technical aspects, challenges and advancements. Bioengineering (Basel). 10 (2), 131 (2023).
- Baxter, M., et al. Phenotypic and functional analyses show stem cell-derived hepatocyte-like cells better mimic fetal rather than adult hepatocytes. J Hepatol. 62 (3), 581-589 (2015).
- Blohm, M. E., Vesterling-Hörner, D., Calaminus, G., Göbel, U. Alpha 1-fetoprotein (AFP) reference values in infants up to 2 years of age. Pediatr Hematol Oncol. 15 (2), 135-142 (1998).
- Liu, Q., Zeng, A., Liu, Z., Wu, C., Song, L. Liver organoids: From fabrication to application in liver diseases. Front Physiol. 13, 956244 (2022).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved