Method Article
Mechanism of Shu-Mu Brain-Kidney Acupoint in Treating Oligoasthenospermia in Rats via the Hypothalamic-Pituitary-Testicular Axis
In This Article
Summary
This study aims to verify the therapeutic effect of the Shu-Mu Brain-Kidney acupoint on oligoasthenospermia in rats and to explore its underlying mechanism through the hypothalamic-pituitary-testicular (HPT) axis.
Abstract
Oligoasthenospermia is a common cause of male infertility. The hypothalamic-pituitary-testicular (HPT) axis regulates gonadal differentiation and maturation through reproductive hormone synthesis and release, playing a vital role in male fertility. Disrupting HPT axis stability impairs sperm production, reducing semen quality. Investigating electroacupuncture's effect on HPT axis regulation may provide insights into treating oligoasthenospermia. Fifty 8-week-old male SD rats were randomly divided into blank, model, Shu-Mu Brain-Kidney, non-acupoint, and L-carnitine groups (n = 10 per group). Except for the blank group, rats received adenine intragastrically for 28 days to establish the model. Post-modeling, the Shu-Mu Brain-Kidney group underwent electroacupuncture at designated acupoints, while the non-acupoint group received sham treatment for 30 min daily. The L-carnitine group received L-carnitine (10 mL/kg) intragastrically once daily. Treatments continued for 28 days. General conditions, organ coefficients, and semen quality were assessed. HE staining analyzed tissue morphology, and ELISA detected serum hormone changes. Compared to the model group, the Shu-Mu Brain-Kidney, and L-carnitine groups exhibited significant improvements in spirit, diet, and bowel movements, with increased body weight, while the non-acupoint group showed no significant change. Renal organ coefficients decreased significantly in the Shu-Mu Brain-Kidney and L-carnitine groups but remained unchanged in the non-acupoint group. Testicular organ coefficients showed no significant differences among treatment groups. Sperm count, density, survival, and motility rates improved significantly in the Shu-Mu Brain-Kidney and L-carnitine groups, but not in the non-acupoint group. H&E staining showed ameliorated kidney and testicular tissue damage in the Shu-Mu Brain-Kidney and L-carnitine groups. ELISA revealed increased T, GnRH, and INHB and decreased LH, FSH, E2, and PRL levels in these groups (p < 0.001), with no significant changes in the non-acupoint group. These findings indicate that Shu-Mu Brain-Kidney acupoint therapy improves sperm quality by regulating the HPT axis, offering a potential treatment for oligoasthenospermia.
Introduction
Oligoasthenospermia, divided into "oligospermia" and "asthenospermia," is one of the common types of male infertility, with an incidence rate accounting for about 75% of male infertility cases1. Oligoasthenospermia is mainly characterized by decreased sperm density and motility in the testes and is mostly clinically manifested as spermatogenesis disorder, reduced sperm count, and low sperm motility2,3. In recent years, the incidence of oligoasthenospermia has increased year by year4. Without effective treatment, it will have long-term adverse effects on patients' physical and mental health and even family harmony5. Studies have shown that genetic problems6, reproductive endocrine disorders7, urogenital tract infections8, and chemical drugs9 can affect sperm production and maturation, leading to a decline in semen quality. Currently, improving the quality of damaged sperm has become a challenging problem in the medical field.
The hypothalamic-pituitary-testicular (HPT) axis is the main pathway of reproductive regulation in the body, which can regulate the differentiation and maturation of gonads by stimulating the synthesis and release of reproductive hormones related to the hypothalamus, pituitary gland, and testes, and plays an important role in maintaining normal male reproductive function10. If the stability of the HPT axis function is disrupted, it will cause the testicular secretion to be insufficient to reach the physiological concentration of testosterone, resulting in a decrease in the number and quality of sperm in the testis11. Therefore, the treatment of oligoasthenospermia mainly aims to regulate the function of the HPT axis and achieve the purpose of maintaining the balance of related endocrine hormones, thereby increasing the number of sperm in semen, enhancing sperm activity, and improving semen quality.
In the clinical treatment of oligoasthenospermia, modern medicine mainly adopts methods such as surgery and drugs of anti-oxidation and enhance sperm motility12,13, while electroacupuncture therapy combines traditional Chinese acupuncture and modern medical bioelectricity. It has the advantages of being fast and accurate, having an obvious curative effect, having few adverse reactions, a wide range of suitable diseases, and low treatment cost, and having a good treatment effect on oligoasthenospermia14,15. "The back is yang, and the abdomen is yin." The Shu-Mu acupoint combination combines the Shu points with the Mu points, one in the front and one in the back, one yin and one yang, working together, also known as the front-back acupoint combination method. Traditional Chinese medicine (TCM) believes that the kidney is the root of prenatal essence, and the disease is located in the kidney. The Front-mu point of the kidney, Jingmen (GB25), and the Back-shu point, Shenshu (BL23), are selected, which has the effect of warming the kidney, assisting yang, and seeking yang within yin16,17; the kidney stores essence and governs the production of marrow, the brain is the sea of marrow and the meeting place of all yang, and Taixi (KI3) is the source point of the foot Shaoyin kidney meridian, which can regulate and tonify the liver and kidney, nourish yin, and generate marrow18. Combined with Epangsanxian (MS4), it connects the kidney and brain, reflecting the idea of "Shu-Mu Brain-Kidney acupoint combination" in TCM19, and the therapeutic effect is more obvious than that of ordinary acupuncture.
Current treatment studies on semen quality primarily focus on improving testicular structure and function, while limited research has been conducted on the regulation of serum hormone levels across different tissues of the hypothalamic-pituitary-testicular (HPT) axis20. In preliminary research, RNA sequencing (RNA-Seq) was used to identify candidate genes and regulatory pathways involved in semen quality regulation within the hypothalamic, pituitary, and testicular tissues of healthy rats and adenine-induced oligozoospermia model rats21. Additionally, semen quality testing and histological analysis of testicular tissues were performed in both groups. These findings provided insights into the molecular mechanisms underlying semen quality in adenine-induced oligoasthenospermia rats. Electroacupuncture therapy with distant-approximal acupoint could improve sperm quality in oligoasthenospermia rats by regulating the HPT axis, and the effect is related to the process of oxidative stress22. However, it is unknown whether the Shu-Mu Brain-Kidney acupoint can improve rats' semen quality by regulating the HPT axis. To further explore the regulatory mechanism of improving semen quality in oligoasthenospermia rats and effective treatment methods, this study used an adenine-induced rat model of oligoasthenospermia and selected four acupoints, Epangsanxian, Shenshu, Jingmen, and Taixi, to explore the related mechanism of the therapeutic effect of Shu-Mu Brain-Kidney acupoint on oligoasthenospermia in rats from the aspect of the HPT axis, providing a more scientific basis for clinical treatment.
Protocol
All animal experiments were reviewed and approved by the Animal Ethics Committee of Ningxia Medical University (approval number: IACUC-NYLAC-2021-130). The study was performed in strict accordance with the ARRIVE Guidelines 2.0 for animal experiments and adhered to the animal welfare principles described in the NIH Guide for the Care and Use of Laboratory Animals. This randomized controlled animal study employed one-way analysis of variance and was conducted at the Animal Experiment Center of Ningxia Medical University from March to May 2023. Eight-week-old SPF-grade male SD rats weighing 180-220 g were used for this study. The details of the reagents and equipment used are listed in the Table of Materials.
1. Animal model establishment and treatment procedures
- Experimental animals and groups
- Obtain fifty 8-week-old SPF-grade male SD rats weighing 180-220 g.
- House all rats under standard conditions, maintaining a room temperature of 22-25 °C, humidity of 50%-60%, and providing free access to standard rodent feed and water.
- After one week of adaptation, randomly divide the rats into a blank (B) group (n = 10) and a modeling group (n = 40) using a random number table method. Administer normal saline to the blank group and adenine to the modeling group by oral gavage.
- After 28 days, randomly divide the modeling group into a model (M) group, Shu-Mu Brain-Kidney (S) group, non-acupoint (N) group, and L-carnitine (L) group (n = 10 per group). Leave the model group untreated.
- Apply electroacupuncture intervention using the Shu-Mu Brain-Kidney acupoint-matching method to the Shu-Mu Brain-Kidney group. Administer non-acupoint treatment to the non-acupoint group and L-carnitine oral liquid by gavage to the L-carnitine group. Treat all groups continuously for 28 days.
- Model construction
NOTE: Studies have shown that high doses of adenine can affect testicular function in rats, leading to abnormal secretion of sex hormones, disruption of the functional stability of the HPT axis, and a decline in sperm quality23. Therefore, adenine is commonly used to establish male animal infertility models.- Administer normal saline (1 mL/100 g body weight) to the blank group and adenine (20 mg/100 g body weight) to the model group by oral gavage (Figure 1A).
- Ensure continuous agitation during gavage to prevent adenine precipitation and blockage of the gavage needle. Administer the treatment once daily for 28 days.
- 1.3 Treatment procedure
- Shu-Mu Brain-Kidney group
- Begin intervention on the first day after modeling. Perform acupuncture with disposable sterile acupuncture needles on bilateral points. After fixing the rat, expose and disinfect the acupuncture site.
- Fix the skin of the acupuncture site with the left hand and quickly pierce the acupuncture point with the disposable sterile acupuncture needle in the right hand. Connect an electrical stimulator. Apply dilatational waves with an electroacupuncture frequency of 2 Hz, a stimulation intensity of 1.5 mA, and a slight tremor of the local muscle.
- Perform electroacupuncture treatment for 30 min each time, once daily, for 28 consecutive days (Figure 1B).
NOTE: Select acupuncture points in rats with reference to Yong Tang's Experimental Acupuncture and Moxibustion Science, Appendix II, "Acupuncture Points Commonly Used in Experimental Animals," combined with a comparative anatomical point selection method19. The following points were selected: (1) Epangsanxian (MS4): Located 3 mm below and 4 mm outside the Touwei point. Operation: Prick 1-2 mm straight; (2) Shenshu (BL23): Located in the spine area, on both sides of the lower spinous process of the second lumbar vertebra, 6 mm beside the median dorsal line. Operation: Prick 6 mm straight; (3) Jingmen (GB25): Located on the side waist, at the free end of the 13th rib on the lower side. Operation: Prick 1-2 mm straight; (4) Taixi (KI3): Located on the inner side of the foot, in the depression between the high point of the medial ankle and the posterior edge of the Achilles tendon. Operation: Prick 3 mm straight.
- Non-acupoint group
- Select and operate non-acupoints 1, 2, and 3 in the same manner as the Shu-Mu Brain-Kidney group (Figure 1B).
NOTE: Because the non-acupoints do not follow the principle of point selection along the meridians, and according to Traditional Chinese Medicine (TCM) theory and long-term clinical observation, these three acupoints have little therapeutic effect on patients with oligoasthenozoospermia24. (1) Non-acupoint 1: Located at the midpoint of the line between the elbow tip and the armpit on the inner side of the elbow. Operation: Prick 1-2 mm straight; (2) Non-acupoint 2: Located at the midpoint between the medial epicondyle of the humerus and the wrist of the ulna, on the ulnar margin. Operation: Prick 1-2 mm straight; (3) Non-acupoint 3: Located at the intersection of the anterior deltoid muscle and the biceps muscle on the inner arm. Operation: Prick 1-2 mm straight.
- Select and operate non-acupoints 1, 2, and 3 in the same manner as the Shu-Mu Brain-Kidney group (Figure 1B).
- L-carnitine group
- Administer levocarnitine oral liquid by oral gavage at a dose of 10 mL/kg body weight, once daily, for 28 consecutive days (Figure 1A).
- Shu-Mu Brain-Kidney group
- Euthanasia
- After treatment, allow all rats to fast for 24 h, anesthetize them by intraperitoneal injection of 2% pentobarbital sodium (40 mg/kg body weight, following institutionally approved protocols), and collect blood from the apex of the heart using a disposable intravenous blood collection needle21,24.
- After death by decapitation, remove one side of the epididymis to measure semen quality. Harvest the kidney and testicular tissues, weigh and record them, and fix them in paraformaldehyde fixative at room temperature for 48 h before embedding them in paraffin24.
2. Observation indices
- General observation: Observe the mental state, diet, and bowel movements of the rats, and monitor their body weight daily.
- Organ coefficients: After death, carefully remove the surface fat and connective tissue from both the kidney and testicular tissues, rinse with normal saline, dry with filter paper, weigh on a balance, and record the measurements for the calculation of organ coefficients. Organ coefficient (%) = (organ weight [g] / body weight [g]) x 100%.
- Semen quality
- After removing excess fat, remove and place one side of the epididymis from each rat in a constant temperature water bath at 37 °C for 5 min. Then, cut, filter, gently blow, and mix the sample well.
- Take a 10 µL aliquot of the diluted sperm suspension and drop it onto the edge of the cover glass of a pre-heated counting plate, allowing the sperm suspension to automatically permeate into the counting chamber.
- Measure sperm count, density, survival rate, and motility rate using a CASA (computer-assisted semen analysis) system21.
- Hematoxylin and Eosin (H&E) staining
NOTE: For details on the procedure, refer to Yang, N. et al.21.- Rinse the fixed tissues with running water, dehydrate, and embed them.
- Cut paraffin sections (5 µm thick) using a microtome and bake at 65 °C for 4 h.
- Dewax the sections and rehydrate them with water.
- Stain the sections with hematoxylin staining solution for 3 min, then rinse with running water.
- Dehydrate the sections with 95% ethanol for 1 min, then stain with eosin solution for 2 min.
- After sealing the slides, perform scanning using a slide scanner, and collect and analyze the images.
- Enzyme-Linked Immunosorbent Assay (ELISA)
NOTE: For details on the procedure, refer to Yang, N. et al.21.- To prepare the serum, add standard substances and serum of the corresponding concentrations to the standard substance wells and sample wells to be measured, followed by the addition of the enzyme-labeled reagent.
- Carry out incubation, washing, color development, and termination steps.
- Determine the absorbance at a wavelength of 450 nm using an enzyme-labeled instrument, and calculate the expression levels of testosterone (T), gonadotropin-releasing hormone (GnRH), inhibin B (INHB), luteinizing hormone (LH), follicle-stimulating hormone (FSH), estradiol (E2), and prolactin (PRL).
3. Statistical analysis
- Analyze the experimental data using statistical software, and present the results of each group as mean ± standard deviation (mean ± SD).
- Use one-way ANOVA to compare sample means between multiple groups that meet the assumptions of normality and homogeneity of variance.
- Use the t-test for comparisons between two groups, and non-parametric tests for data that do not meet the above assumptions.
- Consider differences statistically significant when p < 0.05.
Results
Model evaluation
After modeling, observe the rats for lethargy, crouched posture, cold limbs, reluctance to eat, weight loss, increased urine volume, loose stools, and other symptoms, which generally align with the syndrome of kidney-yang deficiency.
After dissection, observe that the kidneys are tough, white, and heavier. H&E staining reveals that the kidney and testicular tissues of the model rats are seriously damaged, with irregular glomerular shapes, renal tubule interstitial inflammation, and a large number of epithelial cells undergoing degeneration and even fibrosis. More seminiferous tubules in the testes are atrophied and deformed, with disordered or even absent spermatogenic cells (Figure 2), indicating successful model establishment.
General condition of rats
In the blank group, the rats exhibited good spirits, sensitive reactions, glossy hair, and normal eating and defecation. After dissection, their kidneys appeared reddish-brown and soft. In the model group, the rats showed lethargy, crouched backs, cold bodies, loss of appetite, weight loss (p < 0.001), increased urination, and loose stools. After dissection, their kidneys were tough, and a "big white kidney" appearance was observed (Figure 2A). Compared to the model group, the above symptoms significantly improved in the Shu-Mu Brain-Kidney group, did not significantly improve in the non-acupoint group, and also significantly improved in the L-carnitine group. The comparison of body mass among the groups is shown in Figure 3.
Renal and testicular organ coefficients
As shown in Figure 4, compared to the blank group, the renal organ coefficients of rats in the model group significantly increased (p < 0.01), while no significant difference was found in the bilateral testicular organ coefficients (p > 0.05), indicating that the quality of both testes decreased after modeling. Compared to the model group, the renal organ coefficients in the Shu-Mu Brain-Kidney and L-carnitine groups significantly decreased (p < 0.05), but no significant difference was found in the non-acupoint group (p > 0.05). No significant difference was found in the bilateral testicular organ coefficients between the Shu-Mu Brain-Kidney, L-carnitine, and non-acupoint groups compared to the model group (p > 0.05), indicating that the bilateral testicular mass increased in the Shu-Mu Brain-Kidney and L-carnitine groups, while no significant change was observed in the non-acupoint group.
Semen quality analysis
As shown in Figure 5, compared with the blank group, sperm count, density, survival rate, and motility rate significantly decreased in the model group (p < 0.001). Compared to the model group, sperm count, sperm density, survival rate, and motility rate significantly increased in the Shu-Mu Brain-Kidney and L-carnitine groups (p < 0.001), but no significant difference was found in the non-acupoint group.
Morphological changes in the kidney
As shown in Figure 6, the kidney tissue of rats in the blank group had a complete structure and normal shape. The glomeruli were round and full, tightly bound to the renal capsules, and the renal tubules were regular and neatly arranged. Compared to the blank group, the model group exhibited serious renal tissue injury, irregular glomerular morphology, atrophy, reduction, severe renal tubule interstitial inflammation, degeneration of numerous epithelial cells, and severe fibrosis. Compared to the model group, the degree of kidney tissue damage significantly improved in the Shu-Mu Brain-Kidney and L-carnitine groups, with more regular glomerular shapes, slight separation from the renal capsules, denaturation of a few tubular epithelial cells, and mild renal tubule interstitial cell infiltration and fibrosis. Compared to the model group, the degree of renal tissue injury was less apparent in the non-acupoint group, with more regular glomerular shapes, separation from the renal capsules, more denaturation and disorder of renal tubular epithelial cells, moderate inflammatory cell infiltration around the glomeruli and in the renal tubule interstitium, and more fibrosis.
Morphological changes of testes
As shown in Figure 7, the testes of rats in the blank group had a complete tissue structure, normal morphology, neat seminiferous tubules, and regular spermatogenic cells. Compared to the blank group, testicular tissue damage was severe in the model group, with more atrophy and deformation of seminiferous tubules, and disordered or even absent spermatogenic cells. Compared to the model group, the degree of testicular tissue damage significantly improved in the Shu-Mu Brain-Kidney and L-carnitine groups, with most seminiferous tubules arranged neatly and spermatogenic cells arranged regularly. Compared to the model group, the degree of testicular tissue damage did not significantly reduce in the non-acupoint group, with some seminiferous tubules exhibiting atrophy and deformation, cell necrosis, vacuoles, and disordered and absent spermatogenic cells.
Comparison of serum hormone levels
As shown in Figure 8, compared to the blank group, the levels of testosterone (T), gonadotropin-releasing hormone (GnRH), and inhibin B (INHB) in the serum decreased in the model group (p < 0.001), while luteinizing hormone (LH), follicle-stimulating hormone (FSH), estradiol (E2), and prolactin (PRL) were increased (p < 0.001). Compared to the model group, T, GnRH, and INHB increased in the Shu-Mu Brain-Kidney and L-carnitine groups (p < 0.001), while the levels of LH, FSH, E2, and PRL decreased (p < 0.001), but no significant difference was found in the non-acupoint group.
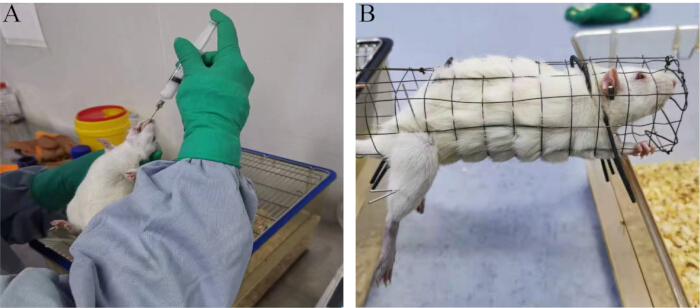
Figure 1: Experimental methods. (A) Oral gavage procedure. (B) Treatment method involving electroacupuncture. Please click here to view a larger version of this figure.
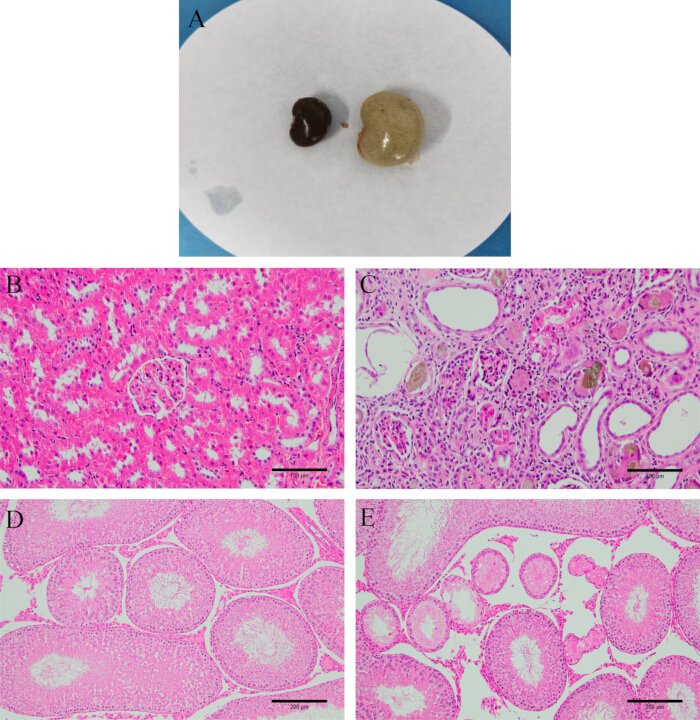
Figure 2: Model verification. (A) Appearance of the kidney before and after modeling. (B) Normal kidney tissue. (C) Kidney tissue after modeling (HE staining, 200x, scale bar: 100 µm). (D) Normal testicular tissue. (E) Testicular tissue after modeling (HE staining, 100x, scale bar: 200 µm). Please click here to view a larger version of this figure.
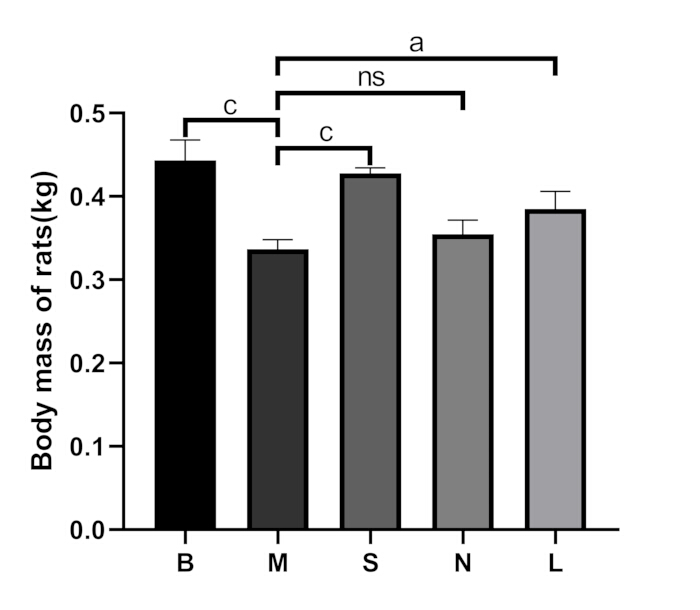
Figure 3: Comparison of body mass among groups. B: blank group, M: model group, S: Shu-Mu Brain-Kidney group, N: non-acupoint group, L: L-carnitine group. Data are presented as mean ± standard deviation (n = 3). ap < 0.05, bp < 0.01, cp < 0.001, nsp > 0.05. Please click here to view a larger version of this figure.

Figure 4: Comparison of renal and testicular organ coefficients among groups. (A) Left renal organ coefficients. (B) Right renal organ coefficients. (C) Left testicular organ coefficients. (D) Right testicular organ coefficients. B: blank group, M: model group, S: Shu-Mu Brain-Kidney group, N: non-acupoint group, L: L-carnitine group. Organ coefficient (%) = organ weight (g) / body weight (g) × 100%. Data are presented as mean ± standard deviation (n = 3). ap < 0.05, bp < 0.01, cp < 0.001, nsp > 0.05. Please click here to view a larger version of this figure.
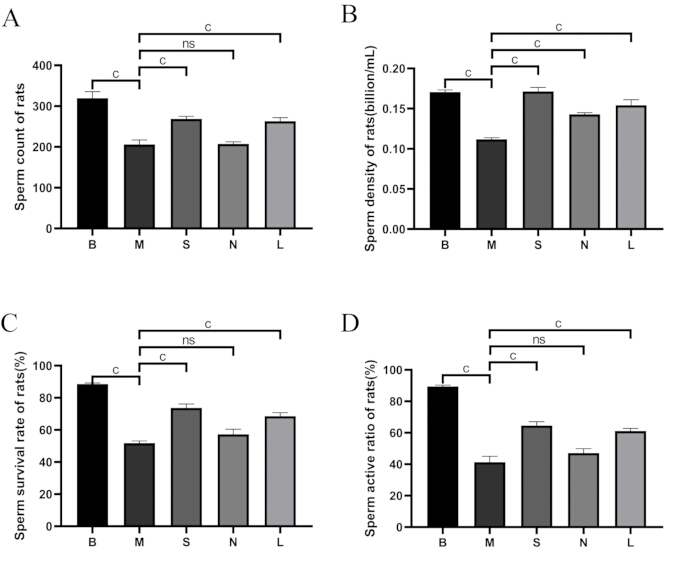
Figure 5: Comparison of sperm quality among groups. (A) Sperm count. (B) Sperm density. (C) Sperm survival rate. (D) Sperm activity ratio. B: blank group, M: model group, S: Shu-Mu Brain-Kidney group, N: non-acupoint group, L: L-carnitine group. Sperm survival rate (%) = motile sperm count / total sperm count × 100%. Data are presented as mean ± standard deviation (n = 3). ap < 0.05, bp < 0.01, cp < 0.001, nsp > 0.05. Please click here to view a larger version of this figure.
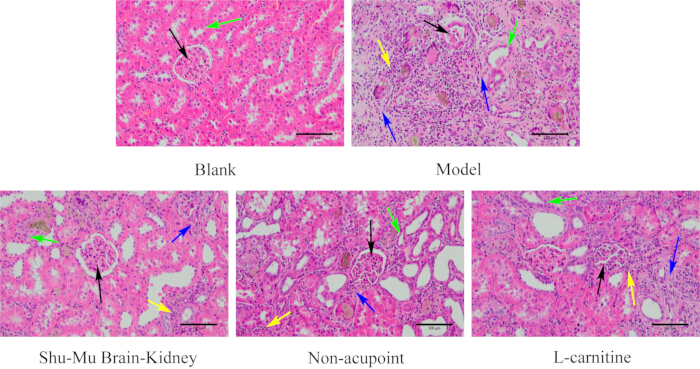
Figure 6: Morphological changes in the kidney in each group. (HE staining, 200×, scale bar: 100 µm). Black arrows indicate glomeruli, green arrows indicate renal tubules, yellow arrows indicate inflammatory cells, and blue arrows indicate fibrosis. Please click here to view a larger version of this figure.
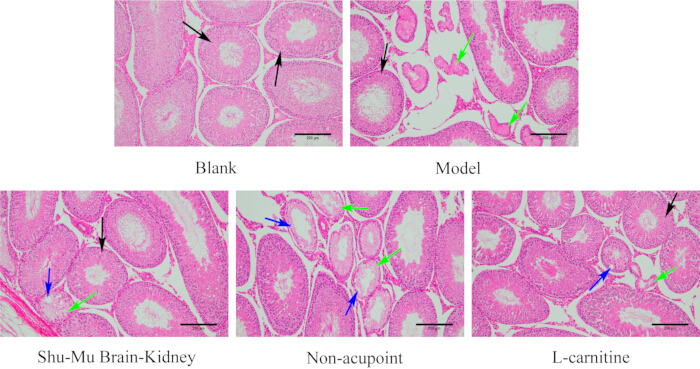
Figure 7: Morphological changes in the testes in each group. (HE staining, 100×, scale bar: 200 µm). Black arrows indicate normal seminiferous tubules, green arrows indicate necrosis, and blue arrows indicate vacuoles. Please click here to view a larger version of this figure.
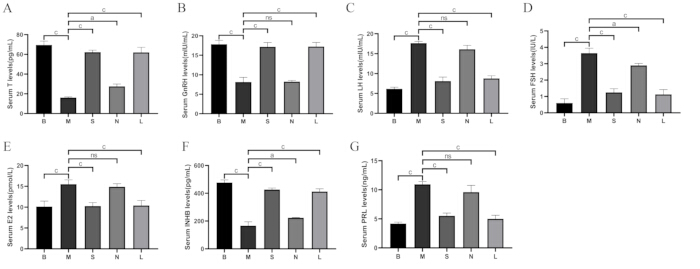
Figure 8: Comparison of serum hormone levels among groups. (A) Serum testosterone (T) levels. (B) Serum GnRH levels. (C) Serum LH levels. (D) Serum FSH levels. (E) Serum E2 levels. (F) Serum INHB levels. (G) Serum PRL levels. B: blank group, M: model group, S: Shu-Mu Brain-Kidney group, N: non-acupoint group, L: L-carnitine group. Data are presented as mean ± standard deviation (n = 3). ap < 0.05, bp < 0.01, cp < 0.001, nsp > 0.05. Please click here to view a larger version of this figure.
Discussion
In TCM, the "kidney" consists of the "inner kidney," referring to the functions of the urinary system in modern medicine, and the "outer kidney," referring to the hypothalamic-pituitary-gonadal axis and the anatomical external reproductive organs25. The male seminal vesicles belong to the "outer kidney," and their components include the testes, epididymides, seminal vesicles, etc26. TCM considers essence to be the material basis of the human body, and "kidney essence" includes the "prenatal essence" inherited from parents and the "postnatal essence" produced by the spleen and stomach's transformation of water and grain essence, which is used to nourish the five viscera and six bowels. Among them, prenatal essence is the original substance that constitutes the embryo and is the foundation of life; postnatal essence enriches the essence of the five viscera and six bowels, and the remaining part is stored in the kidney. The two are interdependent and closely combined in the kidney to form "kidney essence." If kidney essence is insufficient, that is, if the seminal vesicles cannot be full, it will lead to "oligospermia"; if the reproductive essence can be normally discharged, that is, if the seminal vesicles can exert their functions of producing, storing, and discharging sperm, it is the result of the mutual coordination of the kidney yin's astringent nature and the kidney yang's excitatory nature. If the kidney yang is deficient and weak, the astringent function is excessive, while the excitatory function is insufficient, resulting in cold essence and "asthenospermia." Therefore, the manifestations of oligoasthenospermia correspond to the TCM syndrome types of "kidney essence deficiency and kidney yang deficiency." In this experiment, all modeled rats basically met the manifestations of kidney-yang deficiency type oligoasthenospermia, and the treatment should focus on "tonifying the kidney, assisting yang, filling essence, and nourishing the marrow." The combination of Epangsanxian, Shenshu, Jingmen, and Taixi for Shu-Mu Brain-Kidney acupoint can coordinate yin and yang and connect the kidney and brain, ultimately achieving the purpose of "yin-yang balance."
Spermatogenesis is regulated by the endocrine activity of the HPT axis, and the regulation of the HPT axis mainly depends on related hormones and receptor genes expressed in the hypothalamus, pituitary gland, and testes27, which is mainly dominated by the pulsatile release of GnRH from the hypothalamus. This stimulates the anterior pituitary to secrete LH, FSH, and PRL11. Among them, GnRH is the most critical regulator of the HPT axis, and its level can indirectly reflect the status of the HPT axis and the ability of the testes to produce sperm28. LH mediates the release of T from testicular stromal cells, and the occurrence of sperm depends on the stimulation of T. If T secretion is insufficient, the number and quality of sperm will decrease29. FSH binds to receptors on the surface of Sertoli cells and acts together with T to promote the proliferation of spermatogonia and produce INHB to maintain spermatogenesis30. INHB, a glycoprotein synthesized in testicular Sertoli cells and germ cells, is an important regulator of the male gonad, which can, in turn, activate the pituitary gland to produce FSH, and interact with FSH to jointly regulate the HPT axis31,32,33. However, PRL is an important factor affecting sperm motility34. Excessive PRL can inhibit the secretion of the gonadal axis, thus inhibiting spermatogenesis35. E2 is secreted by the gonads and the brain and has a negative feedback effect on LH and FSH36. High E2 levels will damage Sertoli cells and testicular interstitial cells, cause steatosis of surrounding connective tissue, and shrink the diameter of male seminiferous tubules, resulting in impaired fertility of patients37. It has been found that the serum levels of FSH and LH in oligasthenospermia rats induced by Tripterygium wilfordii were significantly increased after modeling. After 60 days of treatment with Qilin Pill, the levels of these two hormones decreased significantly compared to the model group38, which was consistent with the results of this study. Other studies have shown that HPT axis dysfunction occurs in rats with kidney-yang deficiency syndrome caused by adenine, and related sex hormone secretion levels also change39.
In this study, compared to the blank group, the rats in the model group were generally in poor condition, and the semen quality was significantly decreased (p < 0.001). H&E staining showed serious damage of kidney and testicular tissue, indicating that adenine-modulated rats met the criteria for oligonasthenospermia. Compared to the model group, the above conditions significantly improved in the Shu-Mu Brain-Kidney and L-carnitine groups (p < 0.05), while the improvement was not significant in the non-acupoint group, indicating that sperm quality and organ morphology and structure improved in the Shu-Mu Brain-Kidney and L-carnitine groups. Compared to the blank group, T, GnRH, and INHB decreased (p < 0.001) in the model group, indicating that the ability to secrete T, GnRH, and INHB decreased in oligoasthenospermia rats after successful modeling. LH, FSH, E2 and PRL all increased (p < 0.001), indicating that the secretion of LH, FSH, E2 and PRL increased after successful modeling, leading to the weakening of testicular spermatogenic function. Compared to the model group, T, GnRH, and INHB increased (p < 0.001), while LH, FSH, E2 and PRL secretion levels decreased (p < 0.001) in the Shu-Mu Brain-Kidney and L-carnitine groups, but no significant difference was found in the non-acupoint group. This illustrates that both electroacupuncture and L-carnitine can regulate the hormone levels of the HPT axis in rats and have a good effect on improving oligonasthenospermia in rats.
L-carnitine is a natural antioxidant in mammals that can participate in cell energy metabolism and reduce the antioxidant damage of sperm40. Numerous studies have proved that L-carnitine can significantly improve sperm motility, morphology, and concentration while increasing the level of HPT axis-related hormones41. In this experiment, L-carnitine was used as a positive drug control group to compare with the Shu-Mu Brain-Kidney group. The experimental data showed no significant difference in the comparison of various indexes between the two groups, verifying that electroacupuncture at "Epangsanxian, Shenshu, Jingmen, and Taixi" had a good effect on oligoasthenospermia rats. Moreover, acupuncture can coordinate the yin and yang of the viscera and enhance the body's healthy qi to resist pathogenic qi, making it safe, effective, and less toxic with fewer side effects. Electroacupuncture can enhance the curative effect more than ordinary acupuncture and provide a safe and effective treatment for the clinical management of oligoasthenospermia. Since the rats in the non-acupoint group did not follow the principle of point selection along the meridians, various experimental data were significantly different from those in the Shu-Mu Brain-Kidney group, verifying the significance of the compatibility of the four points, "Epangsanxian, Shenshu, Jingmen, and Taixi."
In summary, electroacupuncture at the four points, "Epangsanxian, Shenshu, Jingmen, and Taixi," can improve the function of the HPT axis in rats by regulating the level of related hormones, thereby improving sperm quality in rats and ultimately achieving the purpose of treating oligoasthenospermia. It provides another safe and effective treatment for the clinical management of oligonasthenospermia.
However, due to the lack of technology and funds, this study was limited to the study of serum hormone levels on the HPT axis, and the detection methods were limited. In future experiments, we will consider using various means, such as chromatography, to further verify the changes in hormone levels and use transcriptomics or metabolomics to conduct further studies on the regulation of the HPT axis by electroacupuncture at the gene level and beyond.
Disclosures
The authors have nothing to disclose.
Acknowledgements
This research was supported by the Key Research and Development Project of Ningxia Hui Autonomous Region (2021BEG02041), evidence-based evaluation and clinical study on acupoint compatibility of acupuncture therapy regulating the "hypothalamic-pituitary-testicular axis" in the treatment of male reproductive dysfunction, Project leader: Huisheng Ma; 2024 First-class Discipline Construction Project (ZY0019110305), Project leader: Huisheng Ma; China Academy of Chinese Medical Sciences (YZ-202128), Project leader: Huisheng Ma.
Materials
| Name | Company | Catalog Number | Comments |
| Adenine | Beijing Solarbio Science & Technology Co. Ltd. | A8330 | |
| CASA computer-assisted semen analysis system | Nanning Songjing Tianlun Biotechnology Co., Ltd. | VICOS-SPERM | |
| Centrifuge | Sartorius | A-14C | |
| Differentiation fluid | Shanghai Biyuntian Biotechnology Co., Ltd | C0163M | |
| Disposable sterile acupuncture needles | Hanyi brand | 0.25mm*13mm | |
| Drying machine | Leica | HI1220 | |
| Electrical stimulator | Hua Tuo Brand | SDZ-II | |
| Electronic balance | Hangzhou Youheng Weighing Equipment Co., Ltd | HLD-6002 | |
| ELISA kit | Shanghai Jianglai Biotechnology Co. LTD | JL13251?JL11473?JL11525?JL12505?JL13256?JL12201 | |
| Enzyme-labeled instrument | Rayto | RT-6100 | |
| Eosin staining solution | Zhuhai Beso Biotechnology Co. LTD | BA-4024 | |
| Hematoxylin staining solution | Zhuhai Beso Biotechnology Co. LTD | BA-4041 | |
| Levocarnitine oral liquid | Northeast Pharmaceutical Group Shenyang First Pharmaceutical Co. LTD. | H19990372 | |
| Microtome | Leica | RM2255 | |
| Neutral balsam | Beijing Zhongshan Jinqiao Biotechnology Co., Ltd | Zli-9555 | |
| Paraffin embedding machine | Leica | HistoCore Arcadia H | |
| SD rat, 8-week-old, Male, 180-220 g | 144 Animal Center of Ningxia Medical University | ||
| Slide scanner | Leica | Aperio LV1 | |
| Vortex oscillator | Haimen Qilin Bell Instrument Manufacturing Co., Ltd | QL-902 |
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved