Method Article
Glycemic Impact on Knee Osteoarthritis Symptoms on Physical, Radiographic, and Inflammatory Markers among Individuals Aged 50 and Over with Diabetes
In This Article
Summary
Here, we present a protocol to assess glycemic control using capillary blood glucose (CBG) and glycated hemoglobin A1C (HbA1C) levels. This study investigates the impact of hyperglycemia on knee osteoarthritis (KOA) symptoms, physical performance, physical activity level, radiographic severity, and inflammation in older adults with diabetes.
Abstract
This study explores hyperglycemia's influence on knee osteoarthritis (KOA) related symptoms, physical performance, physical activity level, radiographic severity, and inflammation in older adults. Prolonged hyperglycemic states contribute to advanced glycation end-product (AGE) formation, which worsens KOA symptoms. Capillary blood glucose (CBG) and glycated hemoglobin A1C (HbA1C) levels are commonly used in laboratory tests for glycemic assessment, offering distinct advantages and limitations. Participants were divided into good and poor glycemic control groups based on their CBG and HbA1C levels. KOA clinical severity and physical activity were measured using the knee injury and osteoarthritis outcome score (KOOS) and international physical activity questionnaire. Physical performance was measured with hand grip strength, gait speed, time-up-and-go (TUG), and 5 times sit-to-stand (5STST). Knee X-rays were performed, and serum enzyme-linked immunosorbent assay (ELISA) analysis was conducted for IL-1β, IL-4, CRP, NF-κB, and AGE. Three hundred recruited participants (mean age [SD] = 66.40 years (5.938) with CBG, of fasting blood sugar > 7.0 mmol/L and random blood sugar > 11.1 mmol/L, (N = 254) were compared with KOOS pain (p=0.008) and symptoms (p=0.017) and 5STST (p=0.015); while HbA1c > 6.3% (N = 93) was compared with 5STST (p=0.002), and AGEs (p=0.022) based on Mann Whitney U test. Logistic regression revealed significant associations between glycemic control and lower limb muscle strength, radiological severity, laboratory markers, and between glycemic status and KOOS pain and symptoms. However, these associations did not remain significant after adjusting for BMI. Poor glycemic status alone was associated with better function in sport and recreation domains after antidiabetic medication adjustment, suggesting anti-inflammatory and analgesic effects that masked the effect of high blood sugar. Future studies could explore the predictive ability of glycemic assessment for poor knee function and physical performance while accounting for the effects of the medication.
Introduction
Knee osteoarthritis (KOA) increases in prevalence with age, with the knee being a major weight-bearing joint1. KOA usually manifests with stiffness and chronic pain at the knee joint, which limits mobility, reduces quality of life, and increases the risk of cardiovascular disease2. Diabetes mellitus, which is also related to age, contributes to the risk of KOA development, as elevated glucose and lipids levels promote advanced glycation end product (AGE) formation, leading to chronic joint inflammation and cartilage degeneration3. Despite the availability of healthcare services, two in five Malaysians with diabetes mellitus are unaware of their diagnosis, while 56% of those diagnosed failed to maintain good blood sugar control4. Acute hyperglycemia could lead to a hyperglycemic hyperosmolar state, which is life-threatening, while chronic hyperglycemia leads to peripheral neuropathy, nephropathy, retinopathy, and cardiovascular disease5.
Peripheral neuropathy, which is a microvascular complication resulting from poor glycemic control and leads to altered pain mechanisms, may exaggerate knee pain in KOA6. The presence of diabetes in individuals with KOA is associated with a reduced range of movement at the knee joint, reduced knee function, increased radiographic changes, and poorer quality of life7. The reduced physical performance resulting from the effects of diabetes on KOA is characterized by impaired muscle strength and coordination8. Magnetic resonance imaging evidence of degenerative changes associated with cartilaginous and meniscal damage, such as reduced joint space and malalignment, appears to be more severe in individuals with diabetes9.
Poor glycemic control is linked to upregulated degenerative enzymes and inflammatory factors in knee synovial fluid. Elevated cytokines and proteins in diabetes, such as IL-1β, IL-4, IL-6, nuclear factor-κB (NF-κB), and tumor necrosis factor-alpha (TNF-α), are associated with KOA pathophysiology10,11. While in the chondrocytes, defective glucose transporter leads to upregulated glycolysis, polyol pathways, protein kinase C and pentose pathways, and eventually high production of reactive oxygen species10.
Fasting and random blood glucose provide an estimation of current glycemic status as well as glucose-handling ability related to insulin resistance12. Glycated hemoglobin A (HbA1c) is a measure of glycemic control over the past three months. This does not, however, provide details of acute fluctuations13. Capillary blood glucose testing provides immediate assessments of glycemic status at the bedside or clinic, which has led to debates on their value in determining glycemic control as well as predicting the risk of complications14,15. Thus, this study aims to elucidate the association between glycemic control determined with HbA1c and elevated blood glucose determined with capillary blood glucose (CBG) with the Knee Injury and Osteoarthritis Outcome Scores (KOOS), physical performance, physical activity level, radiographic severity and inflammatory markers in individuals with KOA.
Protocol
The study protocol was in compliance with the Declaration of Helsinki and was approved by the Universiti Kebangsaan Malaysia Ethics Committee (reference number: JEP-2022-001).
1. Participant recruitment
- Through convenience sampling, select the study population from community-dwelling adults with KOA aged 50 years and above in Kuala Lumpur and Selangor. Recruited participants from senior citizens' organizations and diabetes and orthopedic clinics.
NOTE: The presence of KOA is defined with self-reported physician-diagnosed KOA or those in fulfillment of the American College of Rheumatology (ACR) clinical examination criteria16. - Exclude institutionalized older adults or those suffering from major psychological impairment and type 1 diabetes.
- By referring to published literature on cross-sectional cohorts from similar settings, identify effect size, which in this study is the odd ratio. Calculate the sample size, which will provide an 80% power to reject the null hypothesis, using G*Power 3.117.
- Explain research objectives and obtain informed consent before data collection.
2. Data collection - Questionnaire
- Administer questionnaires, which include sociodemographic, KOOS18, and the International Physical Activity Questionnaire (IPAQ)19.
- Calculate the total scores for each of the five KOOS domains derived from the 42 items and transform scores into a 0-100 percentage scale, with zero representing no problems and 100 indicating extreme problems for each domain.
- Calculate the metabolic equivalent of task (MET) for IPAQ domains by multiplying the time taken in minutes and the number of days a week, considering each domain's standard.
NOTE: Total MET indicative of physical activity levels is 3.3 (walking activity MET), + 4 (moderate-intensity activity MET), + 8 (vigorous intensity MET).
3. Data collection - Physical performance
- Measure height with a stadiometer. Obtain body weight and body mass index (BMI) with a body composition analyzer. Ensure participants remove heavy clothing, metallic accessories, and shoes.
- Measure waist, hip, and calf circumference using a measuring tape. Record the measurement in centimeters at the level of the umbilicus at rest for waist circumference and the measurement at the level of maximum posterior protrusion of buttocks for hip circumference20. Measure the calf circumference at the greatest dimension of the long axis with participants sitting with their backs straight and both feet on the floor21.
- Provide instructions to participants on how to carry out the physical performance tests: handgrip strength test (HGS)22, timed up and go test (TUG)23, six-meter walk test24, and five times sit to stand test (5STST)25.
- Ensure the performance setting is clear of obstacles and hazards. Allow 1 min standardized rest periods between tests26.
- Perform the handgrip strength test.
- Instruct the participant to sit with shoulders adducted at the neutral position, with the elbow flexed at 90°.
- Inform them not to perform any rapid wrenching or jerking motion throughout the test.
- Measure the maximum strength with the handgrip dynamometer for each hand thrice and select the greatest measurement in kg.
- Perform the timed up-and-go test.
- Instruct the participant to sit up straight with their back in contact with the back of the chair, arms resting on the armrests, and feet positioned flat on the ground.
- Using a stopwatch, record the time taken to stand up, walk 3 m, make a U-turn, walk back to the chair, and sit back down. Start timing when the participant's back loses contact with the back of the chair and stop timing as soon as the participant's back touches the back of the chair.
- Repeat twice and record the lowest time in seconds taken as the final result.
- Assess gait speed.
- Measure out a 10 m walkway and place markers with adhesive tape at 2 m from each end of the walkway to indicate the points at which measurements will start and finish.
- Have participants walk at their normal pace along a 10 m walkway.
- Start the timer as soon as the participant crosses the first 2 m and stop the timer at 8 m line.
- Calculate gait speed using the velocity (m/s) formula, where 6 m is divided by time taken in seconds.
- Perform the five-times sit-to-stand test.
- Instruct participants to stand up and sit down 5 times as fast as they could with balance.
- Record the time taken to complete 5 repetitions and select the lowest time in seconds from the three trials.
4. Data collection - Knee Xray
- Set an appointment date and time for participants to visit the hospital for a radiographic examination of both knees using the standing anteroposterior weight-bearing view.
- Submit radiographic images to the radiologist, who will determine and assign Kellgren and Lawrence grading to each knee27.
NOTE: The classification system has grades from 0 to 4, with higher grades indicating increasing severity of KOA based on features: osteophyte formation, periarticular ossicles, altered shape of the bone ends, narrowing joint space, and subchondral sclerosis. - Record the assigned grade, ensuring the score is assigned to the correct knee.
5. Data collection - Capillary blood collection for glycemic status assessment
- Wash hands and put on surgical gloves. Clean the participant's finger with an alcohol swab and allow the finger to air dry.
- Prepare the glucometer by inserting the test strip.
- Select a lancet device and ensure that it is unused and sealed.
- Break the seal of the lancet and prick the finger with the new lancet device, squeeze the finger to produce a small bleb of blood, and touch the drop of blood with the test strip.
- Use control solutions for quality assurance, drop the solution on the test strip, and check if it is in the expected range according to the manufacturer.
- Record the blood glucose level displayed by the glucometer. Ask the participant when their last meal was and record whether this was taken more than 8 h before the time of sampling.
NOTE: Fasting blood sugar requires participants to fast for at least 8 h before this procedure, while random blood sugar does not. - Discard the lancet safely into a sharps bin and provide the participant with a cotton swab to apply pressure on the puncture area on the finger to ensure hemostasis.
- Wash hands after the procedure. Clean up any blood spillage.
6. Data collection - Venous blood collection for glycemic control assessment
- Wash hands and put on surgical gloves.
- Identify a suitable vein from either the right or left antecubital fossa. Apply a tourniquet to the upper arm of the selected arm and identify a suitable vein by palpation.
- Clean the skin around the selected vein with an alcohol swab and allow it to air dry.
- Collect venous blood samples with a 23 G butterfly needle using two bottles of 6 mL plain blood tubes. Label the tubes with the participant's unique identification code.
- Discard sharps and clinical waste safely and wash hands.
- Transport blood samples to the laboratory in a cooler with an ice pack. Place the blood samples in their collection tubes in a centrifuge and centrifuge at 604 x g for 10 min.
- Aliquot the serum into 1.5 mL microcentrifuge tubes with a micropipette, and label the tubes with date, identification code, and type of sample before storing at -80 °C.
7. ELISA assay
- Calculate the serum volume needed for the ELISA assay based on the manufacturer's manual. Run an optimization assay to determine the optimal concentration; repeat for IL-1β, IL-4, CRP, NF-κB, and AGEs, respectively.
- Thaw the serum and bring the ELISA reagents to room temperature (RT). Meanwhile, label microcentrifuge tubes for standards, samples, and blank.
- Prepare working solutions per the manufacturer's instructions for the diluent, detection antibodies, substrate, and washing buffer from the stock solutions if required.
- Run two-fold serial dilutions for the standards with the given standard diluent. Reference standard of each marker: IL-1β = 500 pg/mL, IL-4 = 2000 pg/mL, CRP = 25 ng/mL, NF-κB = 10 ng/mL, AGEs = 4800 ng/L. The standard diluent also serves as a blank.
- Dilute serum sample for optimized assay if needed.
- For IL-1β, IL-4, and NF-κB ELISA assay, use neat serum samples. For CRP, dilute by 1000x with reference diluent. Pipette 100 µL samples into the well and duplicate each of them.
- For AGEs ELISA assay, use a serum sample of 2x dilution, pipette 40 µL of sample into the well, and duplicate each.
- Change pipette tips in between different samples or reagents. Use a multichannel pipette to avoid edge effects.
- Incubate according to the manufacturer's manual's suggested time and temperature, and seal the plate with a new adhesive cover for each incubation.
- For this sandwich ELISA, incubate sample and standard to the precoated wells, followed by detection antibody, conjugated secondary antibody, substrate and finally stop solution. Add each solution in the same order as previously.
- Decant and wash wells using wash buffer in between incubation according to the manufacturer's manual. Tap the wells against clean absorbent paper to remove the wash buffer, but ensure the wells do not dry out before the next solution is added.
- Read the wells with a microplate reader at 450 nm. Record and calculate using four parameter logistic (4PL) curve, a quantitative method to plot and determine concentration from symmetrical sigmoidal calibrators28. Use the average for each sample for analysis.
8. Statistical analysis
NOTE: Analyze data using appropriate data analysis software (SPSS Version 20 was used here). Categorize the study population into two groups: 1) good glycemic control, 2) poor glycemic control (Poor glycemic status = Fasting blood sugar more than 7.0 mmol/L or random blood sugar higher than 11.1 mmol/L; Poor glycemic control = HbA1c higher than 6.3%).
- Open the software to create variables based on date, participants' identification code, sociodemographic variables, questionnaire items, and parameters measured.
- Select Variable view. Insert in the column Name, insert description or display name in column Label.
- Select Type > Measure. For coded categorical variables, match representative numeric code and its value in the column Values. Select Ok.
- Key in the data collected into the software where each row represents one participant.
- Select Data View. Key in the representative numeric codes in the column for numeric type and names or descriptions for string type.
- Check the normality of continuous variables to determine parametric test assumptions.
- Select Analyze > Descriptive statistics > Explore. Insert continuous variables into the field Dependent list.
- Select Plots > Normality plots with tests > Continue > OK. For a sample size greater than 50, refer to the p-value in the Kolmogorov-Smirnov test; a significant p-value rejects the null hypothesis where data is normally distributed.
- Run the Mann-Whitney U test for nonparametric variables to test for significant differences between the groups.
- Select Analyze > Nonparametric tests > Settings > Choose Tests > Customize Tests > Mann-Whitney U (2 samples).
- Go to Fields and insert continuous variables into the field Test Fields.
- Insert categorical group for CBG or HbA1c into the field Groups > Run.
- Run a Chi-square test for categorical variables to test for significant differences between the groups.
- Select Analyze > Descriptive statistics > Crosstabs > Statistics > Chi-square > Continue.
- Select Cells Display. Select Observed in the field Counts and select Column in the field Percentage. Then, select Continue.
- Insert categorical variables into the field Row(s) and categorical group for CBG or HbA1c into the field Column(s) > OK.
- Transform continuous dependent variables into binary groups to prepare for logistic regression.
- Select Transform > Recode into Different Variables and insert continuous variables into the field Input Variables > Output Variables.
- Insert new variable name at the field Name. Insert a new label at the field Label > Change > Old and New Values.
- Insert below the threshold value of the cut-off point at the field Range, LOWEST through value, and pair it with zero at the field Value of New Value if this indicates a good outcome.
- Select Add > insert the cut-off point at the field Range, value though HIGHEST and pair it with one at the field Value of New Value > Add > Continue > OK.
- Cut off points for dependent variables:
- KOOS pain domain < 86.1%, Symptoms domain < 85.7%, Activity of daily living domain <86.8%, Sport domains < 85.0%, Quality of life domain < 87.5%
- Poor HGS: Male < 28 kg, Female < 18 kg
- Poor TUG > 8.00 s
- Poor Gait speed < 1.13 ms-1
- Poor 5TSTS >12.80 s
- Low physical activity, IPAQ MET < 3000
- Moderate to severe radiographic KOA, Kellgren, and Lawrence grading scale > 2
- High IL-1β > 11.9 pg/mL
- High IL-4 > 5 pg/mL
- High CRP > 8 ng/mL
- High AGE > 900 ng/L
- High NF-κB > 3 ng/mL - Run multiple logistic regression to obtain odds ratios. Adjust the logistic model with confounding factors based on significant participants' characteristics.
- Select Analyze > Regression > Binary Logistic. Insert binary dependent variable into the field Dependent.
- Insert CBG or HbA1c variable into the field Covariates.
- Select Categorical and transfer categorical variables into the field Categorical Covariates. Then, select Reference Category as First > Change > Continue.
- Select Options > CI for exp(B): 95% > Continue > OK.
- Repeat the procedure for adjusted models but add significant confounding factors into the field Covariates.
- Present variables as mean (standard deviation) for continuous variables or median (interquartile range) if using a nonparametric test, and number (percentage) for categorical variables. Report odd ratios (OR) with 95% confidence intervals (CI) and label the p-value below 0.05 as statistically significant.
Results
Participants' characteristics
Table 1 summarizes participants' characteristics according to glycemic status with FPBS and HbA1c. Figure 1 illustrates the total number of participants included at each stage based on variable inclusion criteria. From the total of 300 recruited participants, capillary blood glucose sampling was obtained from 254 individuals for FPBS, while venous blood sampling was obtained from 93 for HbA1c. Of the 254 capillary samples, 45 (17.7%) fulfilled the criteria for hyperglycemia. While of the 93 venous samples, 42 (45.2%) fulfilled the criteria for poor glycemic control. The mean age of participants was 65.98 ± 5.41 years in those in whom FPBS was available and 66.41 ± 6.02 years for whom HbA1c was available. Significant differences were found in ethnicity, education level, BMI, and chronic kidney disease between euglycemic and hyperglycemic groups based on CBG, while only BMI was significantly higher in those with poor glycemic control compared to those with good glycemic control based on HbA1c (p < 0.05). In addition, the proportion of participants receiving ongoing antidiabetic medication was significantly different between the two groups for both CBG (p < 0.001) and HbA1c, respectively (p < 0.001) (Table 1).
Comparison of Knee osteoarthritis symptoms, physical performance, physical activity level, radiographic severity, and inflammation between euglycemic and hyperglycemic groups
Figure 2 presented a correlation matrix that illustrated the relationships between key variables, providing insight into potential interdependencies. Comparison of KOOS domain scores between euglycemic and hyperglycemic groups using Mann Whitney U revealed differences in pain (p = 0.008) and symptoms (p = 0.017) domain scores. Significant differences were also observed between 5STST and glycemic status (p=0.015), as well as glycemic control (p = 0.002) (Table 2).
Eighteen participants who consented to capillary blood glucose sampling had laboratory markers measured from venous blood samples, of whom only two individuals had hyperglycemia. Statistical conclusions were, therefore, not possible. Laboratory markers were available for a total of 30 individuals from whom HbA1c was measured: 18 with poor glycemic control and 12 with good glycemic control. The glycemic control groups differed significantly in their serum AGE levels (p = 0.022) (Table 2).
Multiple logistic regression analyses
Multiple logistic regression models were used to evaluate associations between glycemic status and glycemic control with KOA severity, physical performance, physical activity, radiographic severity, and laboratory markers. To rule out confounding effects, adjusted models were developed by adding covariates ethnicity, education level, presence of chronic kidney disease, and BMI into the unadjusted glycemic status model and BMI into the unadjusted glycemic control model. Adjusted Model 2 with a second adjustment was applied by adding antidiabetic medications in both confounder lists.
From the five domains of KOOS, pain (OR = 3.56, 95% CI = 1.40, 9.09), symptoms (OR = 2.77, 95% CI = 1.21, 6.32) and sport (OR = 0.27, 95% CI = 0.10, 0.72) were significantly associated with glycemic status only, but this was nullified after adjustment for potential confounders, except the sports domain (OR = 0.19, 95% CI = 0.04, 0.85) (Table 3). No significant variation in score was reported for the ADL domain across the glycemic status groups. Among the physical performance tests, only 5STST was found to be significantly associated with glycemic status (OR = 3.22, 95% CI = 1.62, 6.39); however, the association did not withstand adjustment. Both gait speed (OR = 2.46, 95% CI = 1.05, 5.78) and 5STST (OR = 3.83, 95% CI = 1.10, 13.35) were associated with glycemic control, but the associations were attenuated following adjustment for BMI (gait speed (OR = 2.01, 95% CI = 0.82, 4.88); and 5STST (OR = 3.08, 95% CI = 0.85, 11.13) (Table 3).
Logistic regression was conducted for laboratory markers by glycemic control only. The crude association between AGE and glycemic control (OR = 0.19, 95% CI = 0.04, 0.94) lost significance after BMI adjustment (OR = 0.20, 95% CI = 0.04, 1.09) (Table 3). Likewise, radiographic evidence of KOA was significantly associated with glycemic control before adjustment for BMI (OR = 4.12, 95% CI = 1.33, 12.78). Physical activity level did not report any significant link between glycemic status and glycemic control (Table 3).
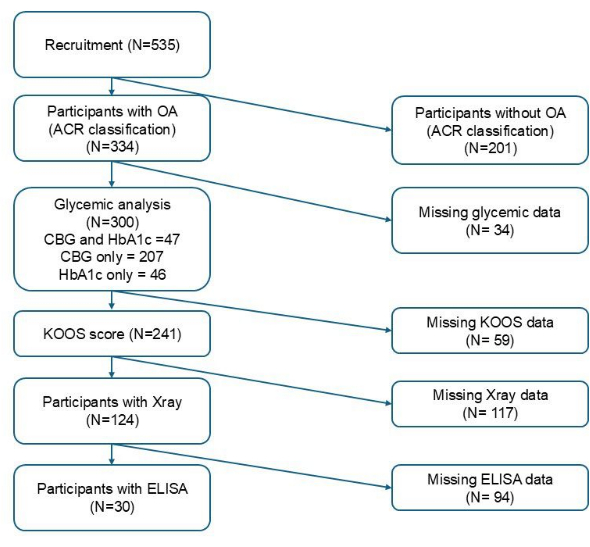
Figure 1: Flow diagram of participants recruitment. Please click here to view a larger version of this figure.
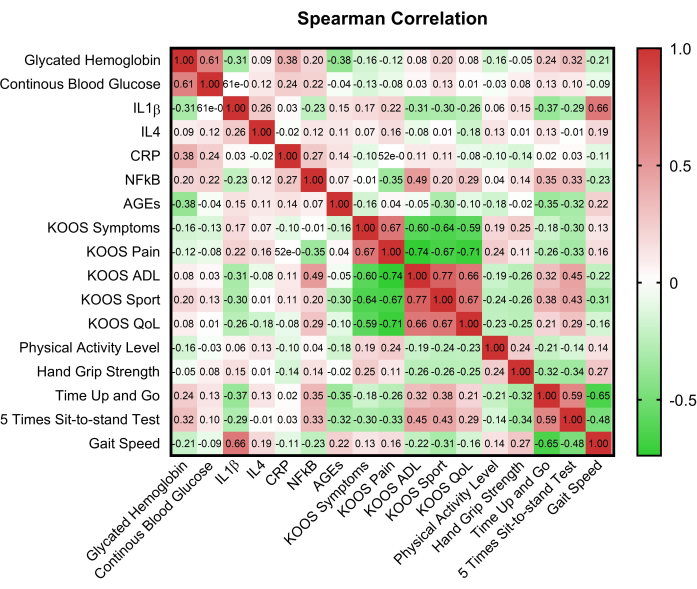
Figure 2: Correlation matrix of the key variables. HbA1c: glycated hemoglobin A1C; CBG: Capillary blood glucose; KOOS: Knee injury and Osteoarthritis Outcome Score; ADL: Activities of daily living; QoL: Quality of life; CRP: C reactive protein; AGEs: advanced glycation end-products; IL-1β: Interleukin-1β; IL-4: Interleukin-4; NF-κB: nuclear factor-κB. Please click here to view a larger version of this figure.
Table 1: Participants' characteristics. P-values were obtained with the Mann-Whitney U test for the continuous variables in the table, and the categorical variables were analyzed with Chi-square between the groups. Asterisk '*' indicates significance at α-value < 0.05. Abbreviations: CBG: Capillary blood glucose; HbA1c: glycated hemoglobin A1C; IQR: Interquartile range; N: Number of cases. Please click here to download this Table.
Table 2: Comparison of knee osteoarthritis symptoms, physical performance, physical activity level, radiographic severity, and inflammation between euglycemic and hyperglycemic groups. P-values were obtained with the Mann-Whitney U test for the variables in the table. Asterisk '*' indicates significance at α-value < 0.05. Abbreviations: IQR: Interquartile range; N: Number of cases; HbA1c: glycated hemoglobin A1C; KOOS: Knee injury and Osteoarthritis Outcome Score; ADL: Activities of daily living; QoL: Quality of life; IPAQ: International Physical Activity Questionnaires; MET: Metabolic Equivalent Task; HGS: Handgrip strength; TUG: Timed-up-and-go; 5TSTS: Five-times sit-to-stand; ELISA: Enzyme-linked immunosorbent assay; CRP: C reactive protein; AGEs: advanced glycation end-products; IL-1β: Interleukin-1β; IL-4: Interleukin-4; NF-κB: nuclear factor-κB. Please click here to download this Table.
Table 3: Multiple logistic regression analyses according to glycemic status and control. Adjusted Model 1: Capillary blood glucose adjusted for ethnicity, education level, chronic kidney disease, and body mass index, HbA1c adjusted for body mass index. Adjusted Model 2: Adjusted Model 1 with antidiabetic medication added to the confounder adjustment. Capillary blood glucose and HbA1c were tested as independent variables on each parameter. Asterisk '*' indicates significance at α-value < 0.05. Abbreviations: IPAQ: International Physical Activity Questionnaires; MET: Metabolic Equivalent Task; ELISA: Enzyme-linked immunosorbent assay; CRP: C reactive protein; AGEs: advanced glycation end-products; IL-1β: Interleukin-1β; IL-4: Interleukin-4; NF-κB: nuclear factor-κB; UTC: Unable to compute. Please click here to download this Table.
Discussion
Venous blood collection is often preferred for laboratory tests over capillary blood sampling in terms of accuracy of results29. The HbA1c is strongly associated with diabetes complications, stable chemical nature and well-standardized laboratory tests. As the HbA1c reflects glycemic control over 3 months it does not require fasting samples, while one-time capillary blood sampling could reflect one-point glycemic status, which is influenced by the timing and the contents of recent meals. Both glycemic assessments, however, do have their pros and cons. Capillary blood glucose results are influenced by impaired microcirculation, hypotension, severe dehydration, edema, and diabetic ketoacidosis, and the function of the commercial glucometer and test strips used30. Nevertheless, capillary blood sampling could provide immediate results, offer higher accessibility, and capture acute glycemic status; this caters to different research needs and potentially enhances research capability within low-resource settings.
In this study, the association between lower limb strength, radiological severity, and laboratory markers with glycemic control was confounded by BMI, which is a marker of obesity. Similarly, the association between glycemic status with KOOS pain and symptoms was partially attributed to the confounding effect of BMI, while the association with lower limb strength was mediated by antidiabetic medications. Antidiabetic medications have also previously been found to affect both muscle mass and muscle strength31,32. On the other hand, poor glycemic status in individuals with KOA was independently associated with better function in sports and recreation. The regained significance in the KOOS sport and recreation function domain after adjusting for the confounder of antidiabetic medications suggests that the treatment may have masked the influence of glycemic status on participants' subjective assessments of their agility and function. This is in line with the improved KOOS score after metformin, meloxicam, and pioglitazone administration in a clinical trial33. Nevertheless, as opposed to HbA1c, glycemic status may reflect short-term glucose spikes, which are less likely to negatively affect functional limitation, leading to higher scores in their responses.
A previous study has also found that KOOS domain scores were negatively correlated with glycemic control7. Similarly, HbA1c significantly predicts KOOS pain domain scores in individuals undergoing total knee arthroplasty. However, the presence of unbearable pain in those who opted for total knee replacements should also be taken into account34,35. Snapshot measures of capillary blood glucose may not be associated with KOOS scores since it reflects glycemic status at one point in time and does not take into account glycemic control over a period of time36. However, the association between hyperglycemia with education level, ethnicity, chronic kidney disease and BMI does suggest that random capillary glucose testing reflects glycemic control, as these are established risk factors for poor glycemic control. In addition, knowledge of knee self-management, lifestyle, and food intake influenced by ethnicity may also affect KOA pain and symptoms37,38,39. Chronic kidney disease in individuals with diabetes is usually an indicator of the presence of microvascular disease, which is a long-term complication of poor glycemic control40,41. Increased BMI leads to increased mechanical load on the knee joint in addition to its well-established association with insulin resistance and, hence, an important role in glycemic control42. The confounding effect of BMI on the relationship between glycemic control and radiological KOA severity may be a reflection of the relationship between obesity and insulin resistance as well as weight-induced mechanical loading on the knee joint43. A previous study has suggested that insulin treatment leads to protection against osteophyte formation, suggesting that better glycemic control may lead to reduced KOA structural changes44. Looking into ELISA markers, the non-enzymatic modification of collagen, which forms AGE is irreversible and reflects cumulative hyperglycemia45; this will potentially explain the relationship between glycemic control and not glycemic status in this study46. The diminished association after BMI adjustment was also found in other studies47.
There are some critical steps outlined in the protocols, such as those during questionnaire administration and steps explanation. Researchers should be mindful of comprehension in older adults' participants due to potential decline in cognitive function; instructions given should be clear and in layman's terms to avoid confusion. During blood sampling, participants should be briefed about the risk of complications and obtain consent before the procedure since it is invasive. After blood centrifugation, serum samples should be aliquoted into multiple microcentrifuge tubes before freezing to prevent repeating freeze-thaw processes that will cause protein degradation and signal loss in ELISA assay48. ELISA step-by-step procedure varies across manufacturers, it is important to read the manual thoroughly and optimize the incubation time, dilution, and temperature according to the study sample48.
The limitations of this study include the presence of unmeasured confounders, such as dietary habits and genetic predispositions. Moreover, the sample collection was restricted to the Kuala Lumpur and Selangor areas, which may not capture variations across different populations to consider the impact of lifestyle and healthcare access towards glycemic status or control. Beyond that, KOOS, as a retrospective self-reported questionnaire, is inherently subjective; future assessments of knee symptoms and functionality could incorporate biomechanical evaluation to mitigate potential biases. For better reflection on the physical performance of participants with KOA with diabetes, repeated measures could be obtained in future study methodology. In terms of inflammatory mediators, this study only measured five biomarkers, but more extensive profiling could better map the underlying mechanism. A small sample size could also be one of the concerns. Missing capillary blood glucose data from the total sample population was due test strips running out during data collection at the community site and reluctance on the part of the participant to attend a second visit. Venous blood collection for the HbA1c test, on the other hand, was conducted during the second visit to the hospital at the same time as the knee X-ray, which required additional time commitment from participants. The sample size was calculated based on 80% power to obtain the odds ratio (OR = 2.010, 95% CI 1.003, 4.026), which was identified from the published literature and yielded an estimated sample size of 7249. In this study, we grouped both fasting blood sugar and random blood sugar into one variable, namely CBG; however, the findings suggested reduced sensitivity, in which the advantage of the individual measurement could be masked by another. Hence, it is preferable to analyze associations of glycemic status with the parameters by fasting blood sugar and random blood sugar, respectively. Future studies should now evaluate long-term glycemic trends to determine the effect of chronic hyperglycemia on KOA.
The protocols involved in this study can be modified for longitudinal study or randomized clinical trial for data collection using questionnaires, physical tests, blood sugar level measurements, and ELISA assay. The glycemic assessment methods, capillary blood sampling, and venous blood collection should be selected based on the parameters investigated and the nature of the study. With diabetes mellitus as a risk factor for cardiovascular disease, retinopathy, peripheral neuropathy, and diabetic kidney disease, glycemic assessment methods are undeniably essential to be adopted in research methodology5,50. On top of that, improvements in diabetes management and increased life expectancy have set the stage for emerging diabetes complications, such as cancer, liver disease, and functional and cognitive disability51. These are anticipated in future research trends.
Disclosures
All authors have no conflict of interest to declare.
Acknowledgements
This study was funded by the Fundamental Research Grant Scheme, Ministry of Higher Education, Malaysia, Grant/Award Number: FRGS/1/2021/SKK0/UKM/02/15.
Materials
| Name | Company | Catalog Number | Comments |
| Butterfly needle | BD Vacutainer | 367282 | |
| G*Power 3.1 | Heinrich-Heine-University | https://www.psychologie.hhu.de/arbeitsgruppen/allgemeine-psychologie-und-arbeitspsychologie/gpower | Heinrich-Heine-University, Düsseldorf |
| Glucometer and test strips | Contour plus | https://www.diabetes.ascensia.my/en/products/contour-plus/ | Basel, Switzerland |
| Human CRP(C-Reactive Protein) ELISA Kit | Elabscience | E-EL-H0043-96T | ELISA kit |
| Human IL-1β(Interleukin 1 Beta) ELISA Kit | Elabscience | E-EL-H0149-96T | ELISA kit |
| Human IL-4(Interleukin 4) ELISA Kit | Elabscience | E-EL-H0101-96T | ELISA kit |
| Human NF-κB-p105 subunit | Bioassay Technology Laboratory | E0003Hu | ELISA kit |
| Human NF-κBp105(Nuclear factor NF-kappa-B p105 subunit) | Elabscience | E-EL-H1386-96T | ELISA kit |
| Manual hand dynamometer | Jamar | 5030J1 | Warrenville, Illinois, USA |
| Portable Body Composition Analyzer | InBody ASIA | https://inbodyasia.com/products/inbody-270/ | Inbody 270, Cheonan, Chungcheongnam-do |
| Portable stadiometer | Seca | 213 1821 009 | SECA 213, Hamburg, Germany |
References
- Allen, K. D., Thoma, L. M., Golightly, Y. M. Epidemiology of osteoarthritis. Osteoarthritis Cartilage. 30 (2), 184-195 (2022).
- Mat, S., et al. Factors influencing quality of life among older persons living with osteoarthritis using 3 different definitions. Top Geriatr Rehabil. 38 (1), 26-34 (2022).
- Berenbaum, F. Diabetes-induced osteoarthritis: From a new paradigm to a new phenotype. Ann Rheum Dis. 70 (8), 1354 (2011).
- Institute for Public Health. . National health and morbidity survey (NHMS) 2023: Non-communicable diseases and healthcare demand - key findings. , (2024).
- Harding, J. L., Pavkov, M. E., Magliano, D. J., Shaw, J. E., Gregg, E. W. Global trends in diabetes complications: A review of current evidence. Diabetologia. 62 (1), 3-16 (2019).
- Eitner, A., Culvenor, A. G., Wirth, W., Schaible, H. -. G., Eckstein, F. Impact of diabetes mellitus on knee osteoarthritis pain and physical and mental status: Data from the osteoarthritis initiative. Arthritis Care Res (Hoboken). 73 (4), 540-548 (2021).
- Aykan, S. A., Kaymaz, S. The association between diabetes mellitus and functionality in knee osteoarthritis: A cross-sectional study. J Health Sci Med. 5 (4), 1114-1118 (2022).
- Alenazi, A. M., Alqahtani, B. A. Diabetes is associated with longitudinal declined physical performance measures in persons with or at risk of knee osteoarthritis: Data from the osteoarthritis initiative. Eur J Phys Rehabil Med. 60 (3), 496-504 (2024).
- Neumann, J., et al. Diabetics show accelerated progression of knee cartilage and meniscal lesions: Data from the osteoarthritis initiative. Skeletal Radiol. 48 (6), 919-930 (2019).
- Courties, A., Gualillo, O., Berenbaum, F., Sellam, J. Metabolic stress-induced joint inflammation and osteoarthritis. Osteoarthritis Cartilage. 23 (11), 1955-1965 (2015).
- Wei, G., et al. Risk of metabolic abnormalities in osteoarthritis: A new perspective to understand its pathological mechanisms. Bone Res. 11 (1), 63 (2023).
- Sriwimol, W., Choosongsang, P., Choosongsang, P., Petkliang, W., Treerut, P. Associations between hba1c-derived estimated average glucose and fasting plasma glucose in patients with normal and abnormal hemoglobin patterns. Scand J Clin Lab Invest. 82 (3), 192-198 (2022).
- Papachristoforou, E., Lambadiari, V., Maratou, E., Makrilakis, K. Association of glycemic indices (hyperglycemia, glucose variability, and hypoglycemia) with oxidative stress and diabetic complications. J Diabetes Res. 2020 (1), 7489795 (2020).
- Matsushita, Y., et al. A comparison of the association of fasting plasma glucose and hba1c levels with diabetic retinopathy in japanese men. J Diabetes Res. 2020 (1), 3214676 (2020).
- Baig, M. A. Comparative evaluation of efficiency of hba1c, fasting & post prandial blood glucose levels, in the diagnosis of type-2 diabetes mellitus and its prognostic outcome. Int J Res Med Sci. 3 (11), 3245-3249 (2015).
- Altman, R., et al. Development of criteria for the classification and reporting of osteoarthritis: Classification of osteoarthritis of the knee. Arthritis Rheum. 29 (8), 1039-1049 (1986).
- Kang, H. Sample size determination and power analysis using the G* power software. J Educ Eval Health Prof. 18, (2021).
- Roos, E. M., Lohmander, L. S. The knee injury and osteoarthritis outcome score (koos): From joint injury to osteoarthritis. Health Qual Life Outcomes. 1, 64 (2003).
- Ipaq Research Committee. . Guidelines for data processing and analysis of the international physical activity questionnaire (IPAQ)-short and long forms. , (2005).
- Perissinotto, E., Pisent, C., Sergi, G., Grigoletto, F., Enzi, G. Anthropometric measurements in the elderly: Age and gender differences. Br J Nutr. 87 (2), 177-186 (2002).
- Sun, Y. -. S., et al. Calf circumference as a novel tool for risk of disability of the elderly population. Sci Rep. 7 (1), 16359 (2017).
- Chen, L. -. K., et al. Asian working group for sarcopenia: 2019 consensus update on sarcopenia diagnosis and treatment. J Am Med Dir Assoc. 21 (3), 300-307 (2020).
- Samah, Z. A., et al. Discriminative and predictive ability of physical performance measures in identifying fall risk among older adults. Sains Malays. 47 (11), 2769-2776 (2018).
- Bohannon, R. W., Williams Andrews, A. Normal walking speed: A descriptive meta-analysis. Physiotherapy. 97 (3), 182-189 (2011).
- Kim, M., Won, C. W. Cut points of chair stand test for poor physical function and its association with adverse health outcomes in community-dwelling older adults: A cross-sectional and longitudinal study. J Am Med Dir Assoc. 23 (8), 1375-1382.e3 (2022).
- Parcell, A. C., Sawyer, R. D., Tricoli, V. A., Chinevere, T. D. Minimum rest period for strength recovery during a common isokinetic testing protocol. Med Sci Sports Exerc. 34 (6), (2002).
- Kohn, M. D., Sassoon, A. A., Fernando, N. D. Classifications in brief: Kellgren-lawrence classification of osteoarthritis. Clin Orthop Relat Res. 474 (8), 1886-1893 (2016).
- Gottschalk, P. G., Dunn, J. R. Measuring parallelism, linearity, and relative potency in bioassay and immunoassay data. J Biopharm Stat. 15 (3), 437-463 (2005).
- Das, S., Swain, M., Pradhan, R. Evaluating the relationship of fasting capillary and venous blood sugar level in self-glucose monitoring device, fasting plasma glucose level and glycosylated hemoglobin (HbA1c). Nurs Care Open Access J. 1 (2), 00011 (2016).
- Bao, Y., Zhu, D. Clinical application guidelines for blood glucose monitoring in china (2022 edition). Diabetes Metab Res Rev. 38 (8), e3581 (2022).
- Wu, C. -. N., Tien, K. -. J. The impact of antidiabetic agents on sarcopenia in type 2 diabetes: A literature review. J Diabetes Res. 2020 (1), 9368583 (2020).
- Kalaitzoglou, E., Fowlkes, J. L., Popescu, I., Thrailkill, K. M. Diabetes pharmacotherapy and effects on the musculoskeletal system. Diabetes Metab Res Rev. 35 (2), e3100 (2019).
- Mohammed, M. M., Al-Shamma, K. J., Jassim, N. A. Evaluation of the clinical use of metformin or pioglitazone in combination with meloxicam in patients with knee osteoarthritis; using knee injury and osteoarthritis outcome score. Iraqi J Pharm Sci. 23 (2), 13-23 (2014).
- Vervullens, S., et al. Preoperative glycaemic control, number of pain locations, structural knee damage, self-reported central sensitisation, satisfaction and personal control are predictive of 1-year postoperative pain, and change in pain from pre- to 1-year posttotal knee arthroplasty. Knee Surg Sports Traumatol Arthrosc. , (2024).
- Eitner, A., et al. Pain sensation in human osteoarthritic knee joints is strongly enhanced by diabetes mellitus. Pain. 158 (9), 1743-1753 (2017).
- Li, H., George, D. M., Jaarsma, R. L., Mao, X. Metabolic syndrome and components exacerbate osteoarthritis symptoms of pain, depression and reduced knee function. Ann Transl Med. 4 (7), 133 (2016).
- Khachian, A., Seyedoshohadaei, M., Haghani, H., Amiri, F. Effect of self-management program on outcome of adult knee osteoarthritis. Int J Orthop Trauma Nurs. 39, 100797 (2020).
- Mat, S., et al. Ethnic differences in the prevalence, socioeconomic and health related risk factors of knee pain and osteoarthritis symptoms in older malaysians. PLoS One. 14 (11), e0225075 (2019).
- Cruz-Almeida, Y., et al. Racial and ethnic differences in older adults with knee osteoarthritis. Arthritis Rheumatol. 66 (7), 1800-1810 (2014).
- Kalantar-Zadeh, K., et al. Patient-centred approaches for the management of unpleasant symptoms in kidney disease. Nat Rev Nephrol. 18 (3), 185-198 (2022).
- Hsu, H. -. J., et al. Factors associated with chronic musculoskeletal pain in patients with chronic kidney disease. BMC Nephrol. 15 (1), 6 (2014).
- Larsen, P., Engberg, A. S., Motahar, I., Ostgaard, S. E., Elsoe, R. Obesity influences the knee injury and osteoarthritis outcome score. Joints. 7 (01), 008-012 (2019).
- Solanki, P., et al. Association between weight gain and knee osteoarthritis: A systematic review. Osteoarthritis Cartilage. 31 (3), 300-316 (2023).
- Al-Jarallah, K., Shehab, D., Abdella, N., Al Mohamedy, H., Abraham, M. Knee osteoarthritis in type 2 diabetes mellitus: Does insulin therapy retard osteophyte formation. Med Princ Pract. 25 (1), 12-17 (2016).
- Suzuki, A., Yabu, A., Nakamura, H. Advanced glycation end products in musculoskeletal system and disorders. Methods. 203, 179-186 (2022).
- Wang, M., Hng, T. M. Hba1c: More than just a number. Aust J Gen Pract. 50 (9), 628-632 (2021).
- Kalousova, M., Skrha, J., Zima, T. Advanced glycation end-products and advanced oxidation protein products in patients with diabetes mellitus. Physiol Res. 51 (6), 597-604 (2002).
- Shah, K., Maghsoudlou, P. Enzyme-linked immunosorbent assay (elisa): The basics. Br J Hosp Med. 77 (7), C98-C101 (2016).
- Nakanishi, S., et al. The impact of hand strength on hba1c, body mass index and body composition by group according to sedentary behaviour: Cross-sectional study in japanese patients with type 2 diabetes mellitus. Malays J Med Sci. 31 (3), 185-193 (2024).
- Joseph, J. J., et al. Comprehensive management of cardiovascular risk factors for adults with type 2 diabetes: A scientific statement from the american heart association. Circulation. 145 (9), e722-e759 (2022).
- Tomic, D., Shaw, J. E., Magliano, D. J. The burden and risks of emerging complications of diabetes mellitus. Nat Rev Endocrinol. 18 (9), 525-539 (2022).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved