Method Article
Patch-Clamp Techniques for Single Endolysosomal Vesicle Analysis
In This Article
Summary
This protocol details a method for directly measuring ion channel activity on intracellular vesicles using a manual endolysosomal patch-clamp system. We illustrate the method that involves enlarging endolysosomes and manually isolating these vesicles. This approach ensures that researchers can accurately replicate and apply the procedure.
Abstract
Endolysosomal ion channels are critical for endolysosomal ion and pH homeostasis, membrane potential regulation, and vesicle trafficking. However, electrophysiologically accessing these channels within small intracellular vesicles has been a challenge. The development of endolysosomal patch-clamp techniques has been instrumental in overcoming this barrier, allowing for the direct measurement of ion channel activity in endolysosomal membranes.
Compared to existing planar patch-clamp techniques, endolysosomal patch-clamp can simultaneously record multiple cells and easily combine with other measurement methods. Manual operation offers the advantage of visualizing targeted vesicles. It also addresses the limitation of the indispensable presence of Ca2+ on one side of the endolysosomal membrane, increasing the flexibility of experimental design. Utilizing endolysosomal patch-clamp techniques enables the direct measurement and analysis of ion channel activity within endolysosomes.
Given the close link between aberrant endolysosomal ion channel function and diseases such as neurodegenerative diseases and metabolic disorders, investigating and modulating these channels may unveil new drug targets. By restoring intracellular ion balance, we may alleviate or cure related diseases. Therefore, this technique is pivotal for discovering new drug targets and developing relevant medications.
Introduction
Ion channels play a crucial role in numerous physiological processes. While surface ion channels have received significant attention, the importance of intracellular channels, particularly those within endolysosomes, is gradually being acknowledged. The endolysosomal system is composed of multifunctional, membrane-bound organelles specialized for fundamental cellular functions, including recycling endosomes (RE), early endosomes (EE), late endosomes (LE), lysosomes (LY), and hybrid organelles with both endolysosomal and other compartment characteristics, such as phagosomes and autophagosomes.
EE, also known as sorting endosomes (SE), are one of the earlier destinations for materials internalized from the plasma membrane (PM). EEs are critical compartments responsible for sorting cargo into various endocytic pathways, such as the maturation pathway to LE/LY for degradation, the rapid recycling pathway back to the PM, and the slow recycling pathway involving the recycling compartment or peripheral RE. Multivesicular bodies (MVBs) derived from endosomes are spherical compartments surrounded by a limiting membrane, which can be filled with intraluminal vesicles (ILVs)1. To maintain the normal function of these organelles, they require membrane ion channels to regulate vesicular pH, osmolarity, and signal transduction. However, measuring the activity of these channels is not straightforward.
For ion channels located on the plasma membrane, the patch-clamp technique developed in the 1970s has long been the gold standard method2. However, accessing channels electrophysiologically within small intracellular vesicles has remained a challenge. Applying the gold standard for measuring ion channels on the plasma membrane to that on intracellular organelles faces three main challenges. First, the size of endolysosomes is typically very small (less than 1 µm in diameter), making them difficult to observe and isolate under a microscope and smaller than the opening diameter of typical glass micropipettes, rendering the experiment inoperable. Second, isolating endolysosomes directly from target cells while maintaining organelle integrity requires special skills. Third, due to the absence of a cytoskeleton in intracellular organelles, forming a seal on the endolysosomal membrane within the patch pipette and then rupturing it to achieve a whole-endolysosome configuration can be challenging, as it compromises the structural integrity of the organelle3.
Several methods have been developed to overcome these issues, including lipid bilayer recording, modifying lysosomal targeting sequences, and solid-supported membrane-based electrophysiology (SSM or SSME) techniques. The lipid bilayer recording method involves reconstructing synthetic phospholipid membranes with purified ion channels, enabling detailed electrophysiological study of membrane protein function under controlled conditions4,5. Modifying lysosomal targeting sequences on ion channels involves the redirection of endolysosomal ion channels to the plasma membrane for measurement using conventional patch-clamp methods6. Solid-supported membrane-based electrophysiology (SSM or SSME) techniques, also known as the endolysosomal planar patch-clamp method, use solid substrate planar glass chips with small apertures (<1 µm in diameter) in microstructured planar borosilicate chips. These small aperture glass chips allow for the analysis of small, even native, endolysosomes using a pressure-suction control system (Nanion). However, in the first two methods, the ion channels are not in their natural physiological environment. Attempts to record lysosomal channels expressed on the plasma membrane or reconstituted into lipid bilayers have largely produced uncertain and contradictory results.
Although planar patch-clamp techniques have effectively addressed the issue of artificial interference and offer the advantage of high-throughput measurements, the solutions used are also limited by this method. The endolysosomal patch-clamp technique introduced in this article can simultaneously record multiple cells and easily combine with other measurement methods. Manual operation provides the advantage of visualizing target vesicles. It also overcomes the unavoidable limitation of Ca2+ in the solution on one side of the endolysosomal membrane, increasing the freedom of experimental design3. Recently, endolysosomal patch-clamp techniques have played a key role in drug development research. For example, in neurodegenerative diseases, this technique has helped identify new drugs targeting endolysosomal ion channels associated with Alzheimer's and Parkinson's diseases7,8. Researchers can also use this technique to explore the role of endolysosomal ion channels in tumor cells9, thereby controlling tumor growth and proliferation. Regarding metabolic diseases, endolysosomal patch-clamp studies are revealing compounds that regulate endolysosomal ion channels, offering new treatment approaches for diabetes and obesity. The endolysosomal patch-clamp technique aids in understanding endolysosomal dysfunction and finding potential therapies6, significantly enhancing our understanding of endolysosomal ion channel functions and promoting the discovery of new drug targets.
Protocol
1. Instrument setup
- Hardware
NOTE: See Figure 1 for a standard electrophysiology rig setup.- Shield the setup from external interference using a table and a Faraday cage.
- Use an inverted microscope with a micromanipulator for stably positioning the microelectrode.
- Set up an amplifier to collect and amplify the acquired signals.
- Use a digitizer to convert the analog signals into digital signals.
- Use data acquisition and analysis software to set up experimental protocols and extract meaningful, analyzable results from the collected data.
- Electrodes
- Use two electrodes: a bath electrode and a pipette electrode.
NOTE: Platinum and silver chloride have the best polarization properties. Silver chloride electrodes consist of silver wire or pellets that have been chlorided, thus having an AgCl layer on the external surface of the wire or pellet (Figure 2).
- Use two electrodes: a bath electrode and a pipette electrode.
- Pipette preparation
- Pulling
- Install the capillary tube into the puller.
NOTE: The parameter used for pipette fabrication below are instrument-specific. The instrument we used in this paper is mentioned in Table of Materials. - Under experimental conditions (room temperature 22 ˚C, through-shape heater filament, borosilicate glass with filament) that do not burn out the heater filament, run a RAMP test on the puller to determine the heat value (HEAT) required to melt the glass capillary.
- Design a new pulling program with six separate pull cycles using the HEAT, velocity (VEL), and time (TIME) parameters (Table 1).
NOTE: HEAT refers to the parameter that heats the heater's filament, allowing the glass filament to melt. Velocity indicates the speed at which the puller pulls the filament in both directions. Time represents the duration of the interval between each cycle. - Press the green Pull button on the keypad.
- Loosen the clamping knob and remove the pipettes from the puller.
- Inspect the pipette tips under the microforge's eyepiece to determine the tip diameter, sharpness, and geometry. Ensure the tip diameter is 0.5-0.9 µm.
NOTE: As the filament is used more frequently, the heat value required to melt the glass capillary will change. - If the tip size does not meet expectations, adjust the HEAT and VEL parameters. Higher temperatures and faster speeds will produce finer, longer tips, and vice versa. Adjust the HEAT by increments of 5 degrees and the speed by increments of 3.
- Install the capillary tube into the puller.
- Polishing
- Place the pulled patch pipettes into the microforge holder.
- Inspect the pipette tips using a 35x objective lens (combined with a 15x eyepiece, resulting in 525x magnification).
- Use the micromanipulator to bring the patch pipettes close to the filament.
- Set the temperature dial to 80.
- Use the footswitch to turn on the heater and apply a brief heat pulse (1 - 2 s), monitoring the polishing process through the microforge eyepiece.
- Repeat the process until all pipettes are polished.
- Place the completed pipettes in a sealed box to prevent dust from entering.
NOTE: The goal is to quickly melt the tip so that the glass reforms the final tip geometry without making it too sharp. This prevents the pipette from penetrating the vesicle membrane it is pressed against and effectively promotes seal formation. Following the final polish, the inner path of the pipette tip should be very narrow, straight, and linear.
The best recording pipettes typically have a resistance of 5 - 8 MΩ after fire polishing. The tip opening diameter should be 2 µm. A perfectly polished patch pipette is essential for successful gigaseal formation, as this is one of the key techniques crucial to the procedure's success.
- Pulling
2. Sample preparation
- Cell Culture (using HEK293 as an example)
- Culture the cells in a standard humidified incubator at 37 °C with 5% CO2.
- Culture the HEK293 cells in low-glucose DMEM supplemented with 10% heat-inactivated fetal bovine serum (FBS), 100 U/mL penicillin, and 100 mg/mL streptomycin.
- Place poly-L-lysine-coated 12 mm coverslips in a 24-well plate.
- Organelle enlargement
- Add 1 µM vacuolin-1 to HEK293 cells in the 24-well plate (from step 2.1) and incubate at 37 °C with 5% CO2 overnight until enlarged vesicles begin to form.
NOTE: The size of the enlarged endolysosomes can reach 1-10 µm, depending on the tool, compound, incubation time, and cell type (Figure 3 and Table 2).
- Add 1 µM vacuolin-1 to HEK293 cells in the 24-well plate (from step 2.1) and incubate at 37 °C with 5% CO2 overnight until enlarged vesicles begin to form.
- Solution preparation
NOTE: The standard solution preparation aims to simulate the ionic concentrations inside the cell and organelles. For example, the standard solutions for measuring ion channels on lysosomes are as follows:- Prepare bath solution (in mM):140 K-methanesulfonate (MSA), 5 KOH, 4 NaCl, 0.39 CaCl2, 1 EGTA, 10 HEPES. Adjust the pH to 7.2 with KOH. Adjust the osmolarity to 300 mosm/L with glucose.
- Prepare pipette solution (in mM):140 Na-MSA, 5 K-MSA, 2 Ca-MSA, 1 CaCl2, 10 HEPES, 10 MES. Adjust the pH to 4.6 with MSA. Adjust the osmolarity to 310 mosm/L with glucose.
NOTE: Pass the solution through a 0.2 µm filter, prepare 45 mL aliquots in 50 mL conical tubes, and store them at 4 °C for up to 2-4 weeks.
3. Organelle isolation
- Remove a coverslip with treated cells (see section 2) from the 24-well plate, transfer it to the microscope chamber, and add 1 mL of bath solution.
NOTE: Cells must not be stored in the bath solution for >1 h for whole-endolysosomal patch-clamp experiments that involve endolysosome isolation, sealing, and recording. After 1 h, the enlarged endolysosomes will shrink, adhere to the pipette, and become difficult to isolate. - Use a homemade plastic filling needle to fill the isolation pipette with pipette solution and install the pipette at the front end of the patch-clamp setup.
- Set the diagonal movement angle of the micromanipulator to be close to 30° (factory default value).
- Under the microscope (40x objective and 10x eyepiece), examine the cells for sufficiently enlarged endosomes or lysosomes located near the cell membrane edge for isolation. Move the isolation pipette close to the selected cell.
- Lower the isolation pipette using the micromanipulator until it touches the edge of the plasma membrane (Figure 4). Quickly move the isolation pipette horizontally to tear off a small piece of the plasma membrane.
- Using the same pipette, press the cell from the opposite side to squeeze out the endosome/lysosomes to a distance of ~2 µm from the cell.
NOTE: If there is residual cell membrane on the organelle, it may prevent the formation of a gigaseal. - Examine the isolated endolysosome under the microscope.
4. Gigaseal formation
- Fill a freshly polished patch pipette with the appropriate pipette solution and install the pipette at the front end of the amplifier.
- Apply 20 - 50 mbar of positive pressure to the pipette (or use 0.03-0.05 mL from a 1 mL syringe for pressure control) and maintain this pressure (lock the valve).
NOTE: 1 mbar (millibar) = 0.001 bar = 0.1 kPa (kilopascal) = 1 hPa (hectopascal) = 1,000 dyn/cm2. - Moving the pipette tip into the bath solution, in the center of the field of view, confirm that the pressure is high enough to see the liquid flowing into the bath solution.
- Apply repeated current pulses (+5 mV; 5 ms) to determine the pipette resistance, seal resistance, and series resistance. Monitor the pipette tip size, seal formation, and the establishment of the whole-endolysosomal configuration.
- Quickly move the pipette close to the top of the target vesicle. Bring the pipette near the vesicle until the vesicle moves or rolls due to the fluid flow from the pipette (Figure 5).
- Adjust the offset voltage of the pipette to 0 mV. Immediately release the positive pressure. Ideally, the vesicle will be drawn toward the pipette and connect to the membrane, forming a gigaseal (1-20 GΩ) within 1 s.Look for a decrease to <10 pA of the current amplitude evoked by the voltage pulse of 5 mV, which indicates successful gigaseal formation.
NOTE: Stable positive pressure is essential for gigaseal formation. Sometimes, leaks in the tubing can prevent gigaseal formation. It is recommended to use a pressure gauge to ensure there are no leaks. If a leak is detected, reattach the tubing or apply a small amount of petroleum jelly to the seams to improve the seal.
5. Current measurement
- Membrane breakage
NOTE: Depending on the experimental needs, the electrode-to-membrane contact can be categorized into four modes: cell-attached mode, whole-cell mode, inside-out mode, and outside-out mode (Figure 6).- For whole vesicle recording, use a high-voltage short pulse (ZAP pulse) of 200 µs or 500 µs to break the membrane at the contact point between the pipette and the organelle. Set the voltage from -500 mV to -1,200 mV, decreasing by 100 mV each time until the membrane is disrupted.
- For inside-out (single channel recording) mode, after forming the gigaseal, pull the pipette away from the organelle, tearing off a small piece of the membrane, exposing the side that was originally facing the vesicle lumen.
- For the outside-out mode, after forming the gigaseal and transitioning to the whole vesicle mode, pull the pipette away from the organelle, tearing off a small piece of the membrane while maintaining a small vesicular structure.
- Current recording
- Record the current across the endolysosomal membrane as the input voltage varies in endolysosomal voltage-clamp patch-clamp experiments.
- Control these voltages by voltage steps or voltage ramps; use a wide range of input voltages (e.g., -100 mV to +100 mV) in endolysosomal voltage-clamp experiments.
- Record the current across the endolysosomal membrane as the input voltage varies in endolysosomal voltage-clamp patch-clamp experiments.
Results
The following describes the current shapes observed during endolysosomal patch-clamp experiments. If the current shape is not as expected, it could be due to poor contact or leakage. Poor contact may occur if the reference electrode is not fully in contact with the bath solution or if the pipette electrode is about to break. Leakage can happen if there is a gap between the chamber and the coverslip allowing fluid to flow onto the objective lens or the stage; having too much or too little pipette solution could also result in such abnormalities.
Needle insertion into solution/needle contact with cell surface/ZAP
During the establishment of the lysosome-attached mode (gigaseal formation), the current response decreases rapidly. Seal resistance can be determined by dividing the command voltage by the low amount of remaining current (Figure 7).
Ramp/leak
Repeatedly applying a continuous input voltage ranging from -100 mV to +100 mV can record the current across the endolysosomal membrane over time (Figure 8). Using standard solutions, the ion composition inside and outside the organelle differs. If the channel exhibits selectivity, the current-voltage curve's intersection point will not be at zero due to the different permeability of ions flowing in and out. This intersection point is referred to as the reverse potential of the channel, and it can be calculated using the following formula10:
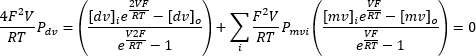
Where dv: divalent ions; mv: monovalent ions; i: internal; o: external; P: permeability of the membrane for ion S measured in m·s−1; V: transmembrane potential in volts; F: Faraday constant, equal to 96,485 C·mol−1 or J·V−1·mol−1; R: gas constant, equal to 8.314 J·K−1·mol−1; T: absolute temperature, measured in kelvins (= °C + 273.15).
There may be passive current components associated with leakage conductance and specific ion channels in endolysosomes. Leakage current is characterized by a linear current (I)-voltage (V) relationship, with the intersection at the origin. Leakage currents from endolysosomes isolated from untransfected HEK293 cells are less than 50 pA at 100 mV; the underlying ion channels are likely potassium/sodium conductance channels and chloride channels.
In cases of voltage-activated ion channels, leakage current components in the measured current can be subtracted by recording a series of scaled voltage pulses before or after the stimulating voltage-gated endolysosomal currents. Leakage can also be subtracted offline. The amplitude of these scaled pulses is typically one-quarter or one-fifth of the experimental pulse amplitude, a process known as P/4 or P/5 leak subtraction11. Due to the very small magnitude of leakage currents, P/4 or P/5 subtraction is not typically used.
Voltage clamp
Repeatedly applying stepwise jumps in input voltage can record the current across the endolysosomal membrane as the voltage changes. This helps in determining whether the channel is a voltage-gated ion channel12. In addition to voltage clamp, current clamp is also feasible. Single-channel activity can be recorded using the available configurations in voltage clamp mode12. The typically low density of endolysosomal ion channels may hinder the process of obtaining patches containing a single channel. Thick-walled pipette glass is the best material for producing electrodes with small pipette tips and low capacitance. Coating the glass with Sylgard or wax can reduce the pipette capacitance.
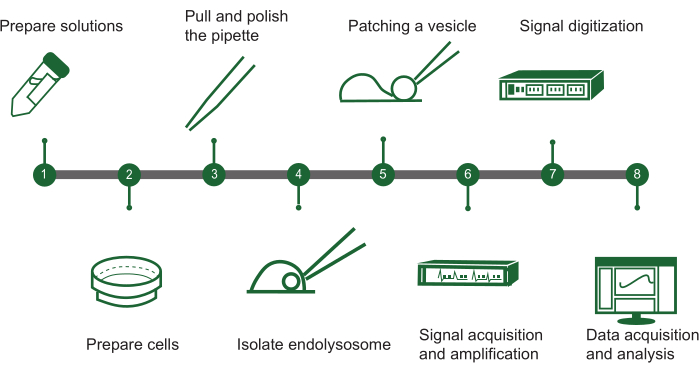
Figure 1: Overview of endolysosomal patch-clamp steps. The protocol for assessing ion channel activity in intracellular vesicles using a manual endolysosomal patch-clamp system can be outlined in a flowchart with the following key steps: (1) Solution preparation: Assemble all required chemical solutions. (2) Cell preparation: Grow and ready the cells for endolysosomal extraction. (3) Pipette fabrication: Create and polish the patch-clamp pipette to ensure precise handling. (4) Endolysosome isolation: Manually separate endolysosomes from the cultured cells. (5) Vesicle patching: Attach the pipette to a single endolysosomal vesicle. (6) Signal acquisition: Capture and amplify electrical signals from the vesicle. (7) Signal digitization: Convert the analog signals to digital form for analysis. (8) Data collection and analysis: Gather the data and interpret it to investigate ion channel function. Please click here to view a larger version of this figure.

Figure 2: Setup of electrophysiological hardware. During dual-electrode measurements, the measurement electrode contacts the target membrane, while the reference electrode is placed in the bath solution. The potential difference between the two is amplified by the amplifier, digitized by the digitizer, and then captured by the computer. Please click here to view a larger version of this figure.

Figure 3: Pharmacological tools used for endolysosomal patch-clamp analysis. Schematic showing the activity ranges of different pharmacological agents for patch-clamp. The combination of Wortmannin and Latrunculin B is highly specific for early endosomes, excluding recycling endosomes. YM201636 selectively enlarges late endosomes/lysosomes. Vacuolin enlarges early endosomes, recycling endosomes, as well as late endosomes/lysosomes13. Please click here to view a larger version of this figure.

Figure 4: Isolation of enlarged organelles. A schematic illustration of the manual isolation process of a target vesicle from a cell. The steps are as follows: (1) Coverslip preparation: The target cell containing the target vesicle is placed on a coverslip. (2) Cut apart the plasma membrane: The plasma membrane of the target cell is cut apart using an isolation pipette. (3) Isolation pipette: The isolation pipette is positioned to target the vesicle. (4) Squeeze the target vesicle outside the cell: The target vesicle is carefully squeezed outside the cell using the isolation pipette. Please click here to view a larger version of this figure.

Figure 5: Recommended relative position between patch pipette and vesicle for gigaseal formation. After applying positive pressure, approach the organelle from above with the patch pipette. Position the tip about one-third of the way from the top of the organelle, and slowly (mode 3-6) lower the patch pipette until the positive pressure causes the organelle membrane to move. Under the microscope, the organelle must roll or be pushed away from the tip. At that moment, release the positive pressure and wait for the gigaseal to form. Please click here to view a larger version of this figure.

Figure 6: Types of configurations. Depending on the contact method between the pipette and the organelle, there are four different measurement modes: organelle or vesicle-attached, whole-vesicle, inside-out, and outside-out. Please click here to view a larger version of this figure.
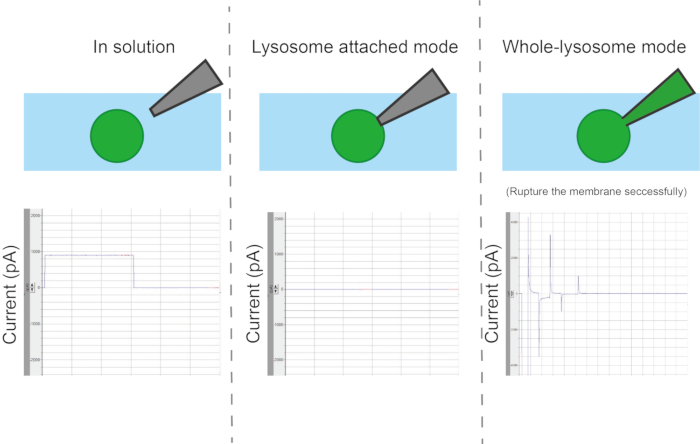
Figure 7: Currents for calculating pipette resistance, seal resistance, series resistance, and cell capacitance. The recording pipette is positioned in the bath solution, and a rectangular voltage pulse (5 ms duration, 5 mV) produces an almost rectangular current response. The resistance of the pipette can be determined by dividing the applied voltage by the measured current. As the gigaseal is formed during lysosome-attached mode, the current response quickly diminishes. The seal resistance can be calculated by dividing the voltage by the remaining, very small current. When a ZAP pulse is applied, the membrane rapidly ruptures, leading to an increase in capacitive currents, signaling the transition to the whole-endolysosomal configuration. For a spherical endolysosome, the resulting current follows a single exponential function of time. Series resistance is determined by dividing the capacitive current amplitude by the command voltage, and the capacitance of the endolysosome is calculated by dividing the time constant of the capacitive currents by the series resistance12. Please click here to view a larger version of this figure.
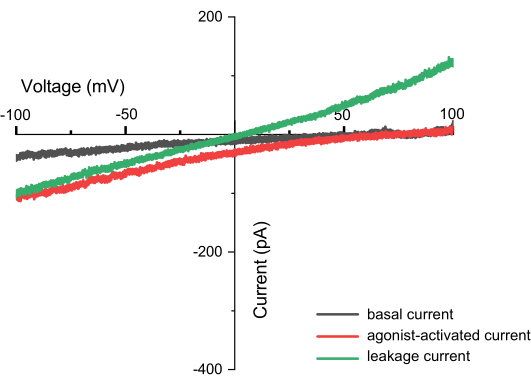
Figure 8: Currents observed during ramp recording (e.g., TPC2). This is a current-voltage (I-V) relationship plot, with the X-axis representing voltage ranging from -100 to +100 mV, and the Y-axis indicating the current generated at different voltages. The graph uses measurements from the TPC2 channel as an example. The black line shows the results obtained directly after applying the ZAP pulse, the red line represents the current generated when the TPC2 channel is activated by the agonist TPC2-A1N, and the green line indicates leak current, which may result from an incomplete seal. A successful measurement can be identified not only by capturing the shape of the channel but also by the absence of leak currents. If leak currents are present, the intersection of the current and the X-axis will be at 0, forming a straight line (with different ions inside and outside the membrane). Please click here to view a larger version of this figure.
| Endolysosomal patch pipette | Whole-cell patch pipette | |||||||
| Cycle | HEAT | PULL | VEL | TIME | HEAT | PULL | VEL | TIME |
| 1 | Value determined by ramp protocol +10 | Blank | 30 | 150 | Value determined by ramp protocol +10 | Blank | 40 | 150 |
| 2 | 30 | 150 | 40 | 150 | ||||
| 3 | 30 | 150 | 40 | 150 | ||||
| 4 | 30 | 150 | 40 | 150 | ||||
| 5 | Value determined by ramp protocol +25 | 18 | 150 | - | - | |||
| 6 | Value determined by ramp protocol +20 | 15 | 150 | - | - | |||
Table 1: Patch pipette pulling protocol. First, use the ramp function to find the appropriate HEAT temperature, then follow the table to adjust the six pulling cycles.
| Unspecific endolysosomes (Vacuolin-1) / specific LE/LY (YM201636) | Macrophage | 1 μM / 0.4 μM | 1-2 h / 1-3 h |
| COS-1, HEK293, Hela, fibroblast, etc | 1 μM / 0.4 μM | Overnight / Overnight | |
| Cardiomyocytes, skeletal muscle cells | 5 μM | 24-48 h |
Table 2: The concentration and treatment duration required to enlarge endolysosomes in different cell types using pharmacological agents. Different cell types exhibit significant differences in their efficiency of enlargement under drug treatment. Generally, cells with a more active endolysosomal system require shorter treatment times. However, the optimal treatment time and concentration still need to be determined experimentally13.
Discussion
Electrophysiological experimental setups have four main laboratory requirements: i) environment: methods to keep the sample healthy; ii) optics: methods to visualize the sample; iii) mechanics: methods to stably position the microelectrode; and iv) electronics: methods to amplify and record the signal.
To successfully perform endolysosomal patch-clamp experiments, several key steps are crucial. First, the condition of the cells-the cells must be tightly adhered to the coverslip so that when the isolation pipette is used to break the cells, the entire cell does not move. Ensuring that the cells are healthy and well-spread is important. If the cells cannot stay fixed in place, switching to an appropriate coating on the coverslip may help. Additionally, cell density needs to be monitored. When cell density is too high (greater than 90%), the effectiveness of drug-induced organelle enlargement diminishes, making experiments difficult to conduct. Second, the quality of the patch pipette-the patch pipette tip must be of appropriate size (5-8 MΩ), and the glass on both sides should be smooth and parallel.
Third, during the formation of a gigaseal, if it does not form immediately after releasing positive pressure, applying a moderate amount of negative pressure can help. However, once the gigaseal forms, the negative pressure must be released immediately to avoid the risk of the entire vesicle being sucked into the pipette. Additionally, when performing a whole-endolysosome patch-clamp, a small part of the membrane needs to be ruptured. Due to the lack of a cytoskeleton in endolysosomes, they are very fragile and often become unstable, causing parts to be sucked into the pipette. In this case, applying a slight positive pressure to push the sucked part out can help. If the capacitance of the remaining organelle at the pipette tip is less than 1 pF, it indicates that there is very little membrane left, likely not containing the channels of interest. In such cases, it is recommended to try a new vesicle. Lastly, when adding drugs, avoid directly hitting the pipette tip, as rapid fluid flow can disrupt the gigaseal. Additionally, when reaching into the Faraday cage, the experimenter should be adequately grounded to avoid generating excessive noise signals, which can hinder the observation of valuable results.
Current recordings with the whole endolysosomal structure present challenges because factors on both sides of the endolysosomal membrane may be lost, potentially leading to changes in gating characteristics during recordings. First, time-dependent changes in current characteristics may result from the dilution or loss of specific regulators (small molecules or other factors) by the pipette solution in the lysosomal lumen. Additionally, components of the pipette or cytosolic solution could modulate specific second messenger pathways that regulate endolysosomal ion channels. Combining the endolysosomal-attached mode with perforated patch techniques would be ideal for addressing specific issues arising from the luminal side of the endolysosomal membrane.
Second, in endolysosomal patch-clamp recordings, because the cytosolic side of the membrane is exposed to the recording solution (cytosolic solution), small molecules or factors associated with the cytosolic side of the endolysosomal membrane can be washed away. ATP has been shown to block TPC from the cytosolic side of the membrane14. Carefully designing the bath solution (e.g., ensuring no ATP is included in the cytosolic recording solution) can minimize this particular issue. Running time-dependent controls and initiating specific protocols at a consistent time after establishing whole-endolysosomal recording configurations can also help manage this problem. Another issue might be the loss of regulatory proteins associated with endolysosomal ion channels due to washing out. If these regulatory factors are part of a signaling cascade, the physiological regulation of endolysosomal channels could be lost.
Technically, although endolysosomal patch-clamp is currently the most commonly used electrophysiological technique for studying endolysosomal ion channels, it typically requires enlarging vesicles and manually isolating selected vesicles. This limitation is inherent to electrophysiological techniques based on capillary glass electrodes. The lack of a cytoskeleton to maintain organelle stability and interference from intracellular filaments remain the primary reasons for the low success rate of these experiments. Beyond the accumulation of experience by electrophysiologists, future advancements in advanced optical systems and high-precision fluorescent small-molecule tools may enable the measurement of ion channel activity in individual organelles within single cells.
Disclosures
The authors have no competing financial interests or other conflicts of interest.
Acknowledgements
National Science and Technology Council, Taiwan (MOST 110-2320-B-002-022), National Taiwan University (NTU-112L7818), and the National Health Research Institutes, Taiwan (NHRI-EX112-11119SC).
Materials
| Name | Company | Catalog Number | Comments |
| BOROSILICATE GLASS | SUTTER INSTRUMENT | BF150-75-10 | O.D.:1.5 mm, I.D. 0.75 mm 10 cm length, with filament |
| Digidata 1140A | Axon Instruments | ||
| Inverted microscope IX73 | OLYMPUS | ||
| MODEL P-97 micropipette puller | SUTTER INSTRUMENT | ||
| MPC-200 | SUTTER INSTRUMENT | ||
| MultiClamp 700B | Axon Instruments | ||
| POLISHER | |||
| Quick Release Chamber | Warner instruments | 641943 | QR-40LP, for 25 mm Coverslips |
References
- Cullen, P. J., Steinberg, F. To degrade or not to degrade: mechanisms and significance of endocytic recycling. Nat Rev Mol Cell Biol. 19 (11), 679-696 (2018).
- Sakmann, B., Neher, E. Patch clamp techniques for studying ionic channels in excitable membranes. Annu Rev Physiol. 46, 455-472 (1984).
- Kumar, P., Kumar, D., Jha, S. K., Jha, N. K., Ambasta, R. K. Ion channels in neurological disorders. Adv Protein Chem Struct Biol. 103, 97-136 (2016).
- Brailoiu, E., et al. An ancestral deuterostome family of two-pore channels mediates nicotinic acid adenine dinucleotide phosphate-dependent calcium release from acidic organelles. J Biol Chem. 285 (5), 2897-2901 (2010).
- Pitt, S. J., et al. TPC2 is a novel NAADP-sensitive Ca2+ release channel, operating as a dual sensor of luminal pH and Ca2+. J Biol Chem. 285 (45), 35039-35046 (2010).
- Gerndt, S., et al. Agonist-mediated switching of ion selectivity in TPC2 differentially promotes lysosomal function. Elife. 9, e54712 (2020).
- She, J., Guo, J., Jiang, Y., Wahl-Schott, C., Biel, M. Structure and function of plant and mammalian TPC channels. Endolysosomal voltage-dependent cation channels. , 155-180 (2023).
- Hu, M., et al. Parkinson's disease-risk protein TMEM175 is a proton-activated proton channel in lysosomes. Cell. 185 (13), 2292-2308.e20 (2022).
- Grimm, C., Bartel, K., Vollmar, A. M., Biel, M. Endolysosomal cation channels and cancer: A link with great potential. Pharmaceuticals (Basel). 11 (1), 4 (2018).
- Gasnier, B., Zhu, M. X. . Ion and molecule transport in lysosomes. , (2020).
- Hernández-Ochoa, E. O., Schneider, M. F. Voltage clamp methods for the study of membrane currents and SR Ca2+ release in adult skeletal muscle fibres. Prog Biophys Mol Biol. 108 (3), 98-118 (2012).
- Chen, C. C., et al. Patch-clamp technique to characterize ion channels in enlarged individual endolysosomes. Nat Protoc. 12 (8), 1639-1658 (2017).
- Chen, C. C., et al. Small molecules for early endosome-specific patch clamping. Cell Chem Biol. 24 (7), 907-916.e4 (2017).
- Cang, C., et al. mTOR regulates lysosomal ATP-sensitive two-pore Na+ channels to adapt to metabolic state. Cell. 152 (4), 778-790 (2013).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionExplore More Articles
This article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved