Degassing Liquids with Freeze-Pump-Thaw Cycling
Source: Laboratory of Dr. Neil Branda — Simon Fraser University
Degassing refers to the process by which dissolved gases are removed from a liquid. The presence of dissolved gases such as oxygen or carbon dioxide can impede chemical reactions that utilize sensitive reagents, interfere with spectroscopic measurements, or can induce unwanted bubble formation.
A number of different techniques are available for degassing liquids; some of these include heating, ultrasonic agitation, chemical removal of gases, substitution with inert gas by bubbling and freeze-pump-thaw cycling. Freeze-pump-thaw cycling is a common and effective method for small scale degassing, and will be demonstrated here in more detail.
Freeze-pump-thaw degassing is performed under reduced pressure using a high vacuum/inert gas double manifold. The process initially involves freezing the solvent using either liquid nitrogen or a dry ice/isopropanol slurry. A vacuum is then applied and the headspace above the frozen solvent is evacuated. The flask is sealed and then the solvent is thawed, allowing the release of dissolved gaseous species. The freeze-pump-thaw process is typically repeated for at least two additional cycles to decrease the percentage of dissolved gases.1,2
This method takes advantage of the pressure dependence of gas solubility in a liquid. In correspondence with Henry’s Law (Equation 1), the concentration of gas (Caq) dissolved in a liquid is directly proportional (k) to the partial pressure of the gas (Pgas) in the vapor phase above the liquid under constant temperature, volume and pressure.3
Caq = kPgas (Equation 1)
Lowering the pressure of the gas above the liquid causes the solubility of the dissolved gas in the liquid to decrease. Therefore, to re-establish the liquid-gas phase equilibrium, the dissolved gas is released from the liquid as a bubble.
In the following procedure, the freeze-pump-thaw cycling technique will be demonstrated with benzene using liquid nitrogen and warm tap water as external cooling and warming baths, respectively. The experimental setup required to perform this technique consists of a double manifold Schlenk line with connected vacuum and nitrogen sources. The Schlenk line is equipped with vacuum compatible tubing lines (Figure 1) for connection to appropriate glassware, such as a Schlenk flask.1,2
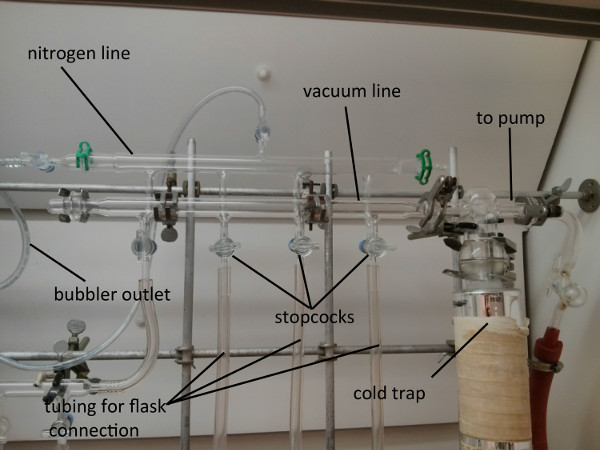
Figure 1. Photo of the Schlenk line equipped with vacuum and nitrogen sources.
- First, place the desired solvent or solution into a Schlenk flask and close the stopcock (Figure 2a). Seal all other openings on the Schlenk flask. Caution: do not use more than 50% of the volume of the flask and inspect the flask for cracks or fractures. An overfilled or broken flask may shatter during the process.
- Attach the flask to a Schlenk line and keep the corresponding valve on the Schlenk line closed. Freeze the liquid completely by submerging the flask into a Dewar containing liquid nitrogen or a dry ice slurry. (Figure 2b). Caution: before freezing, flush the Schlenk flask with nitrogen gas to ensure the environment is completely free of oxygen.
- When the solvent is frozen, open the stopcock on the Schlenk flask and the valve on the Schlenk line to vacuum (Figure 2c). Keep the flask under vacuum and inside the cooling bath for about 10 min. Seal the flask by closing the stopcock (Figure 2d).
- Thaw the solvent until it melts using a warm water bath. During this procedure gas bubbles visibly evolve from the solvent (Figures 2e, 2f). Allow the frozen solvent to thaw by itself slowly and do not to disturb the liquid.
- Once the solvent thawed, replace the warm water bath with the cooling bath and refreeze the solvent.
- Repeat steps 3–5 until you no longer see the evolution of gas as the solution thaws (Figure 2g). A minimum of three cycles is recommended to minimize the percentage of dissolved gas present.
- After the completion of three cycles, the Schlenk flask should be sealed under nitrogen before use (Figure 2h). Open the nitrogen gas valve on the Schlenk line and open the stopcock of the flask to expose the solvent to a nitrogen atmosphere. Once the Schlenk flask is filled with nitrogen, finally close the valve to the flask.
- The solvent is now degassed and ready for use.
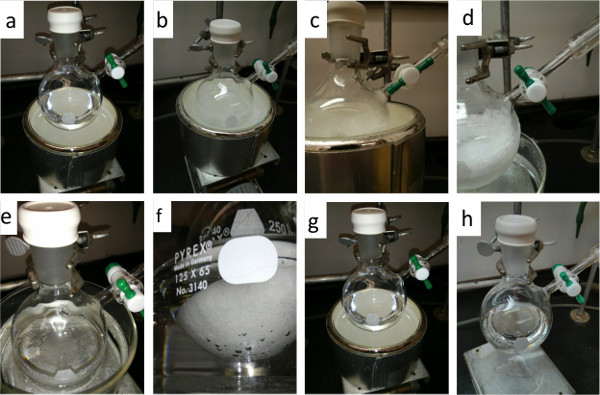
Figure 2. Detail photos of the free-pump-thaw steps: (a) step 1, place solvent in flask; (b) step 2, freeze the solvent in dry ice (or alternatively with liquid nitrogen); (c) step 3, introduce vacuum; (d) step 4, seal the flask under vacuum; (e), (f), step 5, thaw the solvent and observe evolution of gas bubbles; (g) step 7 repeat freeze thaw process (three cycles recommended); (h) step 8, seal the solvent under nitrogen.
The removal of dissolved gases is important in both academia and industry. It is often required for maintaining the quality of machinery and laboratory instruments, for protecting various chemical reactions, and obtaining accurate readings for chromatography and spectrophotometry.
Reactions that use or generate air sensitive reagents, for example, organometallic compounds, thiols, phosphines, and electron rich aromatics frequently require some level of degassing to maintain their integrity throughout the experiment. The yield or even the outcome of an air sensitive reaction could be altered if the appropriate precautions to remove dissolved gases are not taken. Dissolved oxygen affects photochemical studies by quenching excited states. For instance, aromatic triplet states can be quenched by small quantities of oxygen present in the solution, affecting the intensity and spectral distribution (Figure 3).
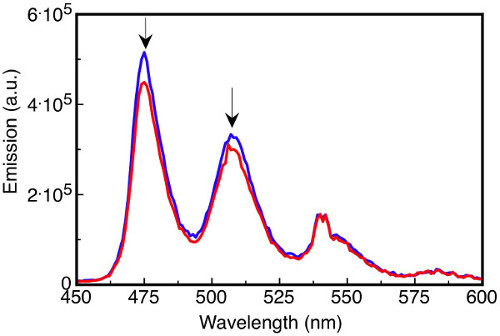
Figure 3. Fluorescence emission spectra of the solutions. Tetracene (16 µM) in degassed benzene (blue line) and oxygen-saturated benzene (red line) upon excitation at 410 nm where the emission intensity at 475 nm is reduced by 14% in the oxygen-saturated solution.
In industry, water is a commonly used fluid for heat exchange. The lifetime of metal pipes, boiler systems, and pumps is dependent on the quality of the running water. Water contains different levels of oxygen and carbon dioxide, can cause damage to metallic materials. Oxygen is an oxidizing reagent, and carbon dioxide is corrosive due to its conversion to carboxylic acid. Delivery of degassed water to the above mentioned systems will prolong equipment lifetime.
In addition, gases present in solvents can have negative consequences for laboratory instruments such as in high-performance liquid chromatography (HPLC) with respect to both performance and output. Many of the instruments have metal propellers or pumps that distribute solvent. When in contact with solvent that has dissolved gas, it can cause cavitation and corrosion leading to damage or degradation of metal components. The detector stability is also influenced by the presence of dissolved gases and the insufficient removal of oxygen can cause baseline drift.
Freeze-pump-thaw cycling is a relatively quick and efficient method appropriate for small to medium scale degassing of liquids. This process can help to overcome some of the issues discussed above associated with the presence dissolved gases in the solvent.
- Shriver, D. F., Drezdn, M. A. The Manipulation of Air Sensitive Compounds. 2nd ed. Wiley & Sons: New York, NY (1986).
- Girolami, G. S., Rauchfuss, T. B., Angelici, R. B. Synthesis and Technique in Inorganic Chemistry: A Laboratory Manual. 3rd ed. University Science Books: Sausalito, CA, (1999).
- Kotz, J., Treichel, P., Townsend, J. Chemistry and Chemical Reactivity. 8th ed. Brooks/Cole: Belmont, CA. (2012).
Atla...
Bu koleksiyondaki videolar:

Now Playing
Degassing Liquids with Freeze-Pump-Thaw Cycling
Organic Chemistry
55.9K Görüntüleme Sayısı

Katalize Giriş
Organic Chemistry
34.1K Görüntüleme Sayısı

Assembly of a Reflux System for Heated Chemical Reactions
Organic Chemistry
166.3K Görüntüleme Sayısı

Conducting Reactions Below Room Temperature
Organic Chemistry
70.3K Görüntüleme Sayısı

Schlenk Lines Transfer of Solvents
Organic Chemistry
41.5K Görüntüleme Sayısı

Preparing Anhydrous Reagents and Equipment
Organic Chemistry
79.1K Görüntüleme Sayısı

Purifying Compounds by Recrystallization
Organic Chemistry
705.3K Görüntüleme Sayısı

Separation of Mixtures via Precipitation
Organic Chemistry
157.2K Görüntüleme Sayısı

Solid-Liquid Extraction
Organic Chemistry
237.1K Görüntüleme Sayısı

Rotary Evaporation to Remove Solvent
Organic Chemistry
212.3K Görüntüleme Sayısı

Fractional Distillation
Organic Chemistry
332.8K Görüntüleme Sayısı

Growing Crystals for X-ray Diffraction Analysis
Organic Chemistry
32.3K Görüntüleme Sayısı

Performing 1D Thin Layer Chromatography
Organic Chemistry
288.4K Görüntüleme Sayısı

Column Chromatography
Organic Chemistry
358.4K Görüntüleme Sayısı

Nuclear Magnetic Resonance (NMR) Spectroscopy
Organic Chemistry
246.7K Görüntüleme Sayısı
JoVE Hakkında
Telif Hakkı © 2020 MyJove Corporation. Tüm hakları saklıdır