Solid-Liquid Extraction
Source: Laboratory of Dr. Jay Deiner — City University of New York
Extraction is a crucial step in most chemical analyses. It entails removing the analyte from its sample matrix and passing it into the phase required for spectroscopic or chromatographic identification and quantification. When the sample is a solid and the required phase for analysis is a liquid, the process is called solid-liquid extraction. A simple and broadly applicable form of solid-liquid extraction entails combining the solid with a solvent in which the analyte is soluble. Through agitation, the analyte partitions into the liquid phase, which may then be separated from the solid through filtration. The choice of solvent must be made based on the solubility of the target analyte, and on the balance of cost, safety, and environmental concerns.
Extraction uses the property of solubility to transfer a solute from one phase to another phase. In order to perform an extraction, the solute must have a higher solubility in the second phase than in the original phase. In liquid-liquid extraction, a solute is separated between two liquid phases, typically an aqueous and an organic phase. In the simplest case, three components are involved: the solute, the carrier liquid, and the solvent. The initial mixture, containing the solute dissolved in the carrier liquid, is mixed with the solvent. Upon mixing, the solute is transferred from the carrier liquid to the solvent. The denser solution settles to the bottom. The location of the solute will depend on the properties of both liquids and the solute.
Solid-liquid extraction is similar to liquid-liquid extraction, except that the solute is dispersed in a solid matrix, rather than in a carrier liquid. The solid phase, containing the solute, is dispersed in the solvent and mixed. The solute is extracted from the solid phase to the solvent, and the solid phase is then removed by filtration.
In this video, an example of the solid-liquid extraction technique will be illustrated by showing the extraction of organochlorine residue from soil. The illustrated solid-liquid extraction entails combination of the sample with n-hexanes followed by ultrasonic agitation, filtration, removal of residual water by drying over CaCl2, and pre-concentration under flowing nitrogen. The as-prepared sample is then ready for analysis by a range of spectroscopic and chromatographic methods.
1. Extraction of Adsorbed Organics from Soil
- Place 20 g of soil in a clean, dry wide-mouth Pyrex dish in a 50 °C oven and dry for a minimum of 12 h. After drying, remove the soil from the Pyrex dish and grind to a uniform powder using a mortar and pestle. Weigh 5.00 g of the soil and place it into a clean, dry round-bottom flask (100 mL in size). To the flask, add 15 mL of n-hexane. Place flask in an ultrasonic bath, and sonicate for 60 min.
2. Separation of Extract and Soil
- Prepare a Büchner funnel with analytical filter paper. Wet the filter paper with 1 mL of n-hexanes and begin vacuum filtration. Slowly pour the contents of the round-bottom flask over the filter paper. The Büchner flask now contains the n-hexanes with the organics extracted from the soil. The filter retains the stripped soil solids.
3. Clean up and Pre-concentration
- If the n-hexane solution is cloudy, there is residual water. To dry the n-hexane solution, add one small spatula of CaCl2. Swirl the solution and observe for a minimum of 15 min. If the solution is still cloudy and/or all of the CaCl2 is clumped, there is still water remaining, and step 3.1 should be repeated. If the solution is translucent and the CaCl2 is free flowing, then do not repeat step 3.1. Once a clear solution has been achieved, separate the hexanes from CaCl2 using gravity filtration. If the extract concentration is sufficient for detection, the filtered hexanes may be transferred to a clean, dry flask for storage and later analysis. If extract concentration is low relative to the limit of detection, transfer the filtered hexanes into a clean, dry three-necked round-bottom flask, 100 mL in size. Place a rubber stopper into the center neck of the flask, and a rubber septum over one of the other necks. Leave the third neck open. Pierce the rubber septum and introduce a nitrogen flow through the flask. The nitrogen should be flowing in the space above the solution, not bubbling through the solution. The extract can now be pre-concentrated by flowing nitrogen to evaporate excess solvent. The sample is now ready for analysis.
A soil sample was collected from a Brownfield site similar to one in Sewickley Pennsylvania, as shown in Figure 1. Brownfields, as defined by the United States Environmental Protection Agency (U. S. EPA), are real property, where the expansion, redevelopment, or reuse may be complicated due to the potential presence of hazardous contaminants. The soil was collected from the Brownfield site using a soil sampler, as shown in Figure 2.
The pollutant of interest in this experiment was atrazine (Figure 3); a common organochloride herbicide. Once the organic components of the soil were extracted and concentrated, they were analyzed by gas chromatography with a flame ionization detector (GC-FID). The GC analysis was carried out using a Shimadzu 14A GC (detector: FID) equipped with split/splitless injector and a CBP-10 capillary column (30 m × 0.22 mm i.d.). The column temperature was first set at 150 °C and then programmed from 150 to 230 °C at a rate of 5 °C per min. The injector temperature was 250 °C and the detector temperature was 260 °C. Injections were performed with splitless mode. Helium carrier gas was used at a constant flow rate of 1 mL/min. The atrazine concentration was calculated using atrazine standard concentrations, as shown in Figure 4. In this case, the approximate atrazine concentration in the Brownfield site studied was 2 mg of atrazine per kg of soil.

Figure 1. Brownfield site in Sewickley, PA.

Figure 2. Contaminated soil collected using a soil sampler.

Figure 3. Chemical structure of the organochloride atrazine.
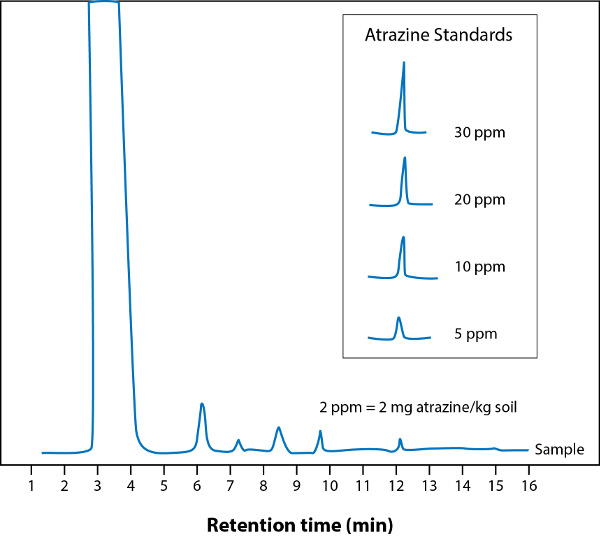
Figure 4. Gas chromatogram of soil sample with atrazine. Inset: atrazine standards.
The general solid-liquid extraction procedure is applicable to a range of fields from environmental monitoring (shown in this video) to cosmetics and food processing. The critical issue is to pick a solvent that effectively dissolves the analyte. With minimal changes in solvent, the sample preparation method in this video can be used to extract any of a broad range of semivolatile environmental contaminants that partition primarily on soils and sludges.
Examples of such semivolatiles include many harmful pollutants like pesticides, polycyclic aromatic hydrocarbons (PAHs), and polychlorinated biphenyls (PCBs). Because of the potential health effects of these molecules, identification and quantification of these species is of academic interest, and also widely practiced in the environmental consulting industry and in government agencies. The EPA maintains compendia of approved analytical and sampling methods to identify and quantify possible pollutants. The method shown in this video illustrates the basic principles contained in EPA method 3550C, which describes ultrasonic extraction of semivolatiles and nonvolatiles from solids.1 EPA method 3550C is one of the extraction methods referenced in EPA method 8081B, which describes GC analysis of organochlorine pesticides.2 Most of the EPA-approved method files are written with the assumption that the analyst has significant prior training. Thus, gaining familiarity with the basic characteristics of sample preparation aids in following the EPA methods.
The use of a Soxhlet apparatus can aid in the extraction of solutes that are poorly soluble in solvents. The setup consists of a round-bottom flask, a Soxhlet extractor, and a reflux condenser. This technique is demonstrated by the removal of PCBs from fish in order to examine the transfer of toxins between predator fish and prey fish.3 Additionally, this technique can be used to measure the wax content in fruit skins in order to understand the composition and degradation of native and engineered fruits.4 Finally, the extraction of carbohydrates from lignocellulose, or dry plant matter, can be accomplished using solid liquid extraction.5 When the carbohydrates are extracted, lignin is left behind. Both components can then be used for biofuel applications.
- US Environmental Protection Agency. Ultrasonic Extraction, Method 3550C. Washington: Government Printing Office (2007).
- US Environmental Protection Agency. Organochlorine pesticides by gas chromatography, Method 8081B. Washington: Government Printing Office (2007).
- Madenjian, C. P., Rediske, R. R., O'Keefe, J. P., David, S. R. Laboratory Estimation of Net Trophic Transfer Efficiencies of PCB Congeners to Lake Trout (Salvelinus namaycush) from Its Prey. J. Vis. Exp. (90), e51496, (2014).
- Chatterjee, S., Sarkar, S., Oktawiec, J., Mao, Z., Niitsoo, O., Stark, R. E. Isolation and Biophysical Study of Fruit Cuticles. J. Vis. Exp. (61), e3529, (2012).
- Mathews, S. L., Ayoub, A. S., Pawlak, J., Grunden, A. M. Methods for Facilitating Microbial Growth on Pulp Mill Waste Streams and Characterization of the Biodegradation Potential of Cultured Microbes. J. Vis. Exp. (82), e51373, (2013).
Atla...
Bu koleksiyondaki videolar:

Now Playing
Solid-Liquid Extraction
Organic Chemistry
237.1K Görüntüleme Sayısı

Katalize Giriş
Organic Chemistry
34.1K Görüntüleme Sayısı

Assembly of a Reflux System for Heated Chemical Reactions
Organic Chemistry
166.3K Görüntüleme Sayısı

Conducting Reactions Below Room Temperature
Organic Chemistry
70.3K Görüntüleme Sayısı

Schlenk Lines Transfer of Solvents
Organic Chemistry
41.5K Görüntüleme Sayısı

Degassing Liquids with Freeze-Pump-Thaw Cycling
Organic Chemistry
55.9K Görüntüleme Sayısı

Preparing Anhydrous Reagents and Equipment
Organic Chemistry
79.1K Görüntüleme Sayısı

Purifying Compounds by Recrystallization
Organic Chemistry
705.3K Görüntüleme Sayısı

Separation of Mixtures via Precipitation
Organic Chemistry
157.2K Görüntüleme Sayısı

Rotary Evaporation to Remove Solvent
Organic Chemistry
212.3K Görüntüleme Sayısı

Fractional Distillation
Organic Chemistry
332.8K Görüntüleme Sayısı

Growing Crystals for X-ray Diffraction Analysis
Organic Chemistry
32.3K Görüntüleme Sayısı

Performing 1D Thin Layer Chromatography
Organic Chemistry
288.4K Görüntüleme Sayısı

Column Chromatography
Organic Chemistry
358.4K Görüntüleme Sayısı

Nuclear Magnetic Resonance (NMR) Spectroscopy
Organic Chemistry
246.7K Görüntüleme Sayısı
JoVE Hakkında
Telif Hakkı © 2020 MyJove Corporation. Tüm hakları saklıdır