Preparing Anhydrous Reagents and Equipment
Source: Laboratory of Dr. Dana Lashley - College of William and Mary
Demonstrated by: Timothy Beck and Lucas Arney
Many reactions in organic chemistry are moisture-sensitive and must be carried out under careful exclusion of water. In these cases the reagents have a high affinity to react with water from the atmosphere and if left exposed the desired reaction will not take place or give poor yields, because the reactants are chemically altered.
In order to prevent undesired reactions with H2O these reactions have to be carried out under an inert atmosphere. An inert atmosphere is generated by running the reaction under nitrogen gas, or in more sensitive cases, under a noble gas such as argon.
Every component in such a reaction must be completely anhydrous, or free of water. This includes all reagents and solvents used as well as all glassware and equipment that will come into contact with the reagents. Extremely water-sensitive reactions must be carried out inside of a glovebox which provides a completely sealed off anhydrous environment to work under via a pair of gloves which protrudes out to one of the sides of the chamber.
Drying of Glassware
Glassware must be completely dry when running reactions with water-sensitive molecules. Glass, which consists of silicon dioxide (SiO2), has microscopic traces of water adsorbed to its surface, even when it looks dry to the eye. The Si-O bonds attract water and as a result a film of water molecules start coating the surface of glass and accumulate over time. In order to free glassware from water it can be dried over-night in an oven or alternatively flame-dried directly before conducting the reaction. Avoid washing glassware the same day as running a reaction inside of it. Note: for reactions that are not very water-sensitive it is possible to rinse the glassware out with acetone directly before use. This drying method is absolutely insufficient for reactions such as the Grignard reaction.
Advantages and disadvantages of the different methods:
Drying glassware in an oven is time-consuming but is also very convenient and works well for all types of glassware. Flame-drying glassware is much quicker but requires the set-up of a Bunsen burner (which is always an additional safety concern) and may not be used with conical vials. Due to the thickness of the base compared to the rest of the conical vial the tension created during heating can cause cracks in the glass. While drying glassware with acetone is a very quick fix for reactions that are not overly sensitive, one should always keep in mind the generated solvent waste and the cost and environmental burden associated with that.
Drying of Solvents
Many different techniques exist for the drying of solvents with varying degrees of effectiveness. Some laboratories use commercially available systems for the drying of solvents. These systems employ so called drying trains and can dry several different types of solvents simultaneously. This method is very safe and convenient yet rather expensive and not available in most laboratories. Residual water content values of 1-10 ppm can be achieved this way.
Another method of drying solvents is by use of highly reactive metals such as sodium in so-called solvent stills. This method poses several safety concerns due to the risk of fires and explosions and is not usually performed by students in undergraduate teaching labs. It is however frequently used in research labs by more advanced students and professionals. Solvent stills will deliver fairly dry solvents and should be employed for extensive drying of ethers (THF, diethylether, etc) or hydrocarbons. Note: this method should never be used for drying of chlorinated solvents because an explosive reaction may occur. When drying with sodium metal an indicator called benzophenone is used to monitor the drying progress. In the presence of water the solution will be clear or yellow, but when the solvent is dry the solution will be blue or purple. Benzophenone is a ketone and reacts with metallic sodium (Na0) into a ketyl radical, which has a blue/purple color. In the presence of water the radical is protonated to give a colorless product. Residual water content achieved by this method is typically around 10 ppm.
Water may also be removed from liquid reagents or solvents by the use of desiccants, or drying agents. These are highly hygroscopic solids, meaning that they readily absorb and thereby remove water from an organic liquid. In recent years a very effective method has been developed using molecular sieves for the drying of various solvents. This method is much more convenient than the use of active metal solvent stills and bypasses the safety concerns of that method. Molecular sieves are commonly used, and probably the most effective desiccant currently available. They are a microporous material made of sodium and calcium aluminosilicates. The pore-sizes of molecular sieves can vary typically between 2–5 Å (0.2–0.5 nm) and are used to trap or absorb small molecules while larger molecules do not fit inside the pores. The molecular sieves are available in powder or bead form and can be used to trap water (a small molecule) thereby removing it from another liquid with a larger molecular size. Molecular sieves are also common components of everyday life products, for example cat-litter. Molecular sieves are activated in an oven at temperatures above 300 °C at atmospheric pressure for a minimum of 3 h but better overnight. In a vacuum oven a temperature of around 200 °C will suffice. This activation process removes all water with which the pores are saturated even in a freshly purchased and freshly opened bottle of molecular sieves. After activation the molecular sieves should be stored in a conventional drying oven at temperatures above 120 °C or in a desiccator for several weeks before requiring reactivation. Note: whether molecular sieves are still active can easily be determined by placing a small amount of beads in a gloved-hand followed by two volume equivalents of water to the beads. If the sieves are still active they will become very hot to the touch.
Solvents are dried by removing the beads from the oven or desiccator and cooled to room temperature before adding them to a solvent of choice. The solvent is dried over the beads for at least 12 h-5 days before the solvent is considered anhydrous and can be used in a reaction.
The length of the storage time depends on the solvent as does the amount of molecular sieves required. This is typically reported as the % mass/ volume (m/v) loading and describes the amount of molecular sieves used per volume of solvent. For example a 5% m/v means that 5 g of molecular sieves are added per 100 mL of solvent.
For common solvents such as dichloromethane (DCM), acetonitrile, or toluene a storage time of 24 h over 3-Å molecular sieves with 10% m/v is sufficient to reach very low ppm values for residual water content (0.1–0.9 ppm). Tetrahydrofuran (THF) on the other hand should be dried for a duration of 3 days using 20% m/v of 3-Å molecular sieves to reach low residual amounts of water of about 4 ppm. Lower-mass alcohols such as methanol or ethanol should even be stored about 5 days over 3-Å molecular sieves and 20% m/v, which will yield residual water content of 8–10 ppm. Higher molecular weight alcohols should be dried using powdered 3-Å sieves rather than beads. Powdered molecular sieves adsorb at a much faster rate than beads. This results in a non selective adsorption of solvent molecules which are small enough in size to compete with water for entry into the sieve pore (e.g. small alcohol molecules, such as methanol). For large molecular weight alcohols it is safe to use the more active powdered form of the sieves because they are too large to compete with water for the pores.1Note: alcohols are typically very hygroscopic and very low residual water amounts cannot be reached. Table 1 summarizes the findings for the common solvents described above.
Note that slightly larger 4-Å beads are used for drying of amines, dimethylformamide (DMF), and hexamethylphosphoramide (HMPA) by storing them over the beads using 5% w/v for at least 24 h. Molecular sieves should not be used for drying acetone, because they are basic and induce an aldol reaction in acetone.
Another great advantage of molecular sieves is that they can be recycled by rinsing them thoroughly with a volatile organic solvent, followed by drying them at 100 °C for a few hours first (or alternatively air-drying) before reactivating them as usual at temperatures above 300 °C for at least 3 h. Acetone may auto-ignite at high temperatures of > 400 °C. So one must be sure that it has fully evaporated before moving the beads to the high temperature oven. Note: in undergraduate laboratories solvents are sometimes dried using the drying agents listed in Table 2 in the section below. This method is sufficient for reactions that are not very water sensitive but will not render sufficiently dry solvents to run sensitive reactions such as a Grignard reaction.
| Solvent | % m/v | Time of storing solvent over 3 Å molecular sieves | Residual water content (ppm) |
| DCM | 10% | 24 h | ~0.1 |
| Acetonitrile | 10% | 24 h | ~0.5 |
| Toluene | 10% | 24 h | ~0.9 |
| THF | 20% | 3 days | ~4.1 |
| Methanol | 20% | 5 days | ~10.5 |
| Ethanol | 20% | 5 days | ~8.2 |
Table 1. Desiccant amount, drying time and residual water content for various solvent dried over 3 Å molecular sieves.2
Drying of Reagents
Reagents in a chemical reaction can be solid or liquid (and in very rare cases gases). Different methods are employed to dry solids than are used to dry liquids.
Liquid reagents can generally be made anhydrous by similar methods as for solvents described above. Reagents that are freshly purchased are often sufficiently anhydrous. Reagents need to be dried if they are not fresh or if they were synthesized as part of a multi-step synthesis. In a multi-step synthesis the product of one reaction step is the reagent for the next step. The product formation of many reactions requires a quenching step, which means contact with a large quantity of water. Afterward the product, whether it is solid or liquid, should be dried in order to ensure anhydrous conditions for the following step. This is afforded first by extraction, a method by which the aqueous phase is separated from the organic phase thereby removing macroscopic amounts of water. After extraction the organic phase, which contains the product dissolved in an organic solvent, will still have microscopic traces of water present. Following extraction the organic phase must be dried over a highly hygroscopic drying agent that is usually an inorganic salt. There are many different drying agents, and some of the most common ones are listed in Table 2.
For drying purposes, the drying agent is added to the organic phase until freshly added drying agent no longer clumps together but rolls around freely and the solution is clear and not cloudy. The organic phase should be covered and stored over the drying agent for a short period of time (usually an hour) to ensure drying. Afterward the drying agent is filtered off and the solvent is removed under reduced pressure in a rotary evaporator.
For a product that is a liquid, further drying can be achieved by storing it over a drying agent and freshly distilling it before use. For a product that is solid, drying is achieved preferably by storage in a vacuum oven at a temperature below its melting point (mp). For example, if the solid's mp is below 100 °C the oven must be set to a temperature around 15–20 °C below its mp. Water will still evaporate over time and applied vacuum will accelerate the process. Alternatively the solid may be dried by storage inside a vacuum desiccator over an appropriate drying agent (typically P2O5). This may be indicated for cases where the solid's mp is extremely low (below ~50 °C) or when a vacuum oven is not available. After drying, the anhydrous reagent should be stored in a bottle under inert atmosphere (N2or Ar) and the bottle's lid should be tightly sealed with Parafilm. The bottle should be kept inside a desiccator until the reagent is needed. Note: some solid reagents, such as the magnesium metal for a Grignard reaction may be dried inside the apparatus during the flame-drying process.
Liquid reagents can alternatively be dried by molecular sieves as described in the previous section for solvents. This is indicated when large amounts of a reagent need to be dried. Typically reagents in small-scale syntheses are used in small amounts (a few mL or less). Drying of such small amounts with molecular sieves is impractical and drying with the above methods should suffice.
| Drying Agent | Capacity | Speed | Suitability |
| Na2SO4 | high | low | Generally useful |
| MgSO4 | high | high | Generally useful |
| CaCl2 | high | medium | Useful for hydrocarbons* |
| CaSO4 | low | high | Generally useful |
| * Organic liquids that are not hydrocarbons, such as alcohols, amines, and different carbonyl-containing compounds are also absorbed by CaCl2. It can’t be used to dry these liquids but it can help remove these types of impurities from a hydrocarbon. | |||
Table 2. The most commonly used drying agents in organic laboratories.
Drying of Glassware
1. Oven-Drying
- Remove all pieces that are not made of glass, such as the stopcock of an addition funnel.
- Place all glassware that are part of the apparatus in a drying-oven set to about 125 °C for at least 24 h before use.
- Put on heat protection gloves and remove glassware from the oven. Be very careful when handling hot glass while assembling the apparatus.
- For best results flush the apparatus with an inert gas such as N2 while assembling the apparatus.
2. Flame-Drying
- Set up the full apparatus but remove all components that are not made of glass (such as stopcocks of dropping funnels or rubber sleeves etc.). Do not attach any tubing to the apparatus at this point.
- Put on heat-protection gloves.
- Set up a Bunsen burner and carefully flame-dry the apparatus. Start from the bottom and move the flame upward, "accompanying" the water on its way out. Steam will develop and fog up the glassware. Continue flame-drying until fogging stops.
- Wait for the apparatus to cool down to about 60 °C. This takes a couple of minutes and the glass will still be warm to the touch but won't cause burns if contact is short.
- Carefully add the rest of the apparatus that is not made of glass, such as tubing and stopcocks. Do not remove heat-protection gloves when handling the apparatus to avoid burns.
- After everything is properly assembled, flush the apparatus with an inert gas such as N2to accelerate cooling of the glassware to a point where the reagents may be added.
Note: During the flame-drying process never have any solvent or the magnetic stir-bar inside the flask nor any of the reagents unless specifically demanded by the procedure. (However, when performing the Grignard reaction it is okay to leave the magnesium shavings in the flask during flame-drying).
3. Drying with Acetone
- Rinse glassware with acetone.
- Collect the acetone rinsings in a designated solvent waste container.
- Flush the glassware with N2 gas or dry compressed air to accelerate drying.
Drying of Solvents
1. Drying with Active Sodium Metal
- Carefully cut sodium metal under petroleum ether to minimize the risk of ignition as the metal reacts with air. The metal surface oxidizes quickly giving a white residue. Cut the metal into thin slices exposing as much metallic surface area as possible.
- Under N2 gas add the metal shavings to a distillation flask containing solvent.
- Next add benzophenone, an indicator that is used to monitor the reaction. In the presence of water the solution is clear or yellow, but when the solvent is dry the solution is blue or purple.
- Reflux the solution using a heating mantle until a change of coloration to blue/purple is observed.
- Distill the solvent, collect it in a dry flask, and use in a reaction immediately after cooling down.
Waste Disposal: After the drying process it is important to safely dispose of any remaining sodium in the distillation flask. - For this, carefully add ethanol to the sodium until hydrogen evolution has ceased.
- Stir the solution carefully, but well, and make sure that no sodium lumps remain that might have active sodium metal trapped inside.
- Carefully add methanol to the mixture and leave the mixture in the back of the hood for several hours to make sure the sodium is completely consumed.
- Finally, add the mixture very carefully to a large excess of water and dispose of the mixture in an appropriate waste disposal container.
2. Drying over Molecular Sieves
- Open a bottle containing molecular sieves and fill them into a thermo-stable glass beaker. Dry more than needed for the current experiment for future use.
- For activation place the beaker with the beads in a high temperature oven and store for 3–3.5 h at a temperature of 300–350 °C. Alternatively, if available, store for in a vacuum oven at 200 °C for the same amount of time.
- Using high heat-resistant gloves remove the beaker with the beads and place it in a conventional drying oven at temperatures above 120 °C. Alternatively store the beaker with the beads inside of a desiccator. The beads may be stored for weeks before use.
- Remove the beaker with the beads from the drying oven or the desiccator. Work fast from this point onward to minimize contact of the beads with atmospheric water.
- If removing the beads from an oven allow them to cool down to about room temperature. Cover the beaker with a dry towel to minimize air exposure during this cooling time.
- To test whether the beads are still active, put on disposable gloves and place a small amount of beads in your hand. Add about 2 volume equivalents of water. If the beads become very hot then it means they are activated.
- Weigh off the needed amount of active beads on a scale. For example, to achieve a 10% m/v of beads in a 500-mL bottle of solvent, 50 g of beads are required.
- Add the room-temperature beads to a freshly opened bottle of solvent. Be certain that the beads are cooled, especially if the solvent is volatile. Never add the beads to a solvent directly after removing them from the oven.
- For a volatile solvent, such as DCM, leave the lid on top of the bottle but wait a few minutes before fully screwing the lid on the bottle to avoid pressure build-up.
- Seal the area around the lid by wrapping it with Parafilm to keep moisture out.
- Store the solvent over the beads for the appropriate amount of time, for example 24 h.
Drying of Reagents
1. Drying of the Organic Phase after Extraction
- Upon separation of the phases in a separatory funnel, transfer the organic phase into a dry glass container, such as a beaker or Erlenmeyer flask (or a test tube for small scales).
- Add a drying agent from Table 2 (usually NaSO4or MgSO4) to the organic phase using a spatula. Initially the drying agent clumps together.
- Continue the addition until freshly added drying agent does not clump together and rolls about freely. Moreover, the solution should be clear and not cloudy.
- If available, cover the glass container with a stopper, otherwise with some aluminum foil.
- Allow the solution to stand for about 1 h over the drying agent. It is okay to allow it to stand for longer periods of time, for example over-night.
- Assemble a vacuum-filtration apparatus with Büchner funnel and side-arm flask.
- Add a filter paper to the Büchner funnel and turn on the vacuum.
- Slowly decant off the organic phase into the Büchner funnel. Initially, try not to transfer any of the drying agent or the filter will clog.
- When most of the liquid has been transferred onto the funnel and drained into the flask below, add the remainder of the organic phase together with the drying agent and allow to stand for a few minutes.
- Turn off the vacuum and transfer the organic phase into a dry round bottom flask.
- Connect the round-bottom flask to a rotary evaporator and remove all solvent under reduced pressure.
- If the product is a liquid, store it over a drying agent and freshly distill it before use.
2. Drying of Solid Reagents
- Place the solid reagent inside an open glass container and determine the combined weight.
- Place the container with the reagent into a drying oven set to a temperature below the melting point of the solid. When available, use a vacuum oven. Use heat protection gloves if necessary.
- Allow the reagent to dry for several hours inside the oven.
- Remove the container with the reagent from the oven.
- Allow the sample to cool to room temperature, ideally inside of a desiccator.
- Determine the weight and ensure that it is lower than before drying. If necessary repeat steps 2.2 – 2.5.
- Once the reagent is cooled to room temperature it is ready for use.
- If the reagent does not need to be used immediately, flush the container with an inert gas, such as N2 and seal it tightly with a lid and place Parafilm around the lid.
- Place the container inside a desiccator and store it there until the reagent is needed.
3. Drying of Heat-Sensitive Solid Reagents
- Prepare a desiccator by placing fresh anhydrous drying agent in the lower compartment (typically P2O5).
- Place the solid reagent inside an open glass container and determine its weight.
- Place the container with the reagent it inside a desiccator.
- Close the lid of the desiccator, sliding it on horizontally from the side to ensure proper sealing.
- Apply a vacuum to the desiccator using the stopcock connection.
- After a few minutes close the stopcock and turn off the vacuum.
- Allow the reagent to dry inside the desiccator for at least a few hours.
- Open the stopcock on the desiccator and allow air to flow in.
- Carefully slide off the lid of the desiccator horizontally. Do not attempt to pull the lid off vertically.
- Remove the reagent from the desiccator and weigh it. It should have a lower weight than before it was dried.
- If necessary repeat steps 3.3 – 3.10 until the weight remains stable and the reagent is fully dry.
- If the reagent does not need to be used immediately, flush the container with an inert gas such as N2 and seal it tightly with a lid and place Parafilm around the lid.
- Place the container inside a desiccator and store it there until the reagent is needed.
A classical example for a reaction that must be done under anhydrous conditions is the Grignard reaction. (Equation 1)
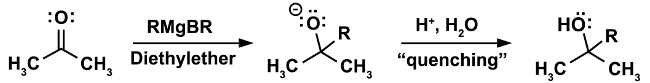
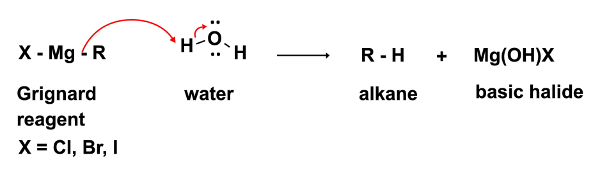
In the first step of the reaction, the nucleophilic attack of the Grignard reagent RMgX occurs on an electrophile (in this case a ketone). In this step it is imperative that not even the smallest traces of water be present. The Grignard reagent, while a strong nucleophile, is an even stronger base. In the presence of water it will preferentially act as a base and deprotonate water, resulting in the loss of the nucleophilic Grignard reagent and in the formation of an alkane, an undesired byproduct. (Equation 2)
- Burfield, D. R. and Smithers, R. H. Desiccant efficiency in solvent and reagent drying. 7. Alcohols. J. Org. Chem. 48 (14), 2420-2422 (1983).
- Williams, D. B. G. and Lawton, M. Drying of Organic Solvents: Quantitative Evaluation of the Efficiency of Several Desiccants. J. Org. Chem. 75 (24), 8351-8354 (2010).
Atla...
Bu koleksiyondaki videolar:

Now Playing
Preparing Anhydrous Reagents and Equipment
Organic Chemistry
79.1K Görüntüleme Sayısı

Katalize Giriş
Organic Chemistry
34.1K Görüntüleme Sayısı

Assembly of a Reflux System for Heated Chemical Reactions
Organic Chemistry
166.3K Görüntüleme Sayısı

Conducting Reactions Below Room Temperature
Organic Chemistry
70.3K Görüntüleme Sayısı

Schlenk Lines Transfer of Solvents
Organic Chemistry
41.5K Görüntüleme Sayısı

Degassing Liquids with Freeze-Pump-Thaw Cycling
Organic Chemistry
55.9K Görüntüleme Sayısı

Purifying Compounds by Recrystallization
Organic Chemistry
705.3K Görüntüleme Sayısı

Separation of Mixtures via Precipitation
Organic Chemistry
157.2K Görüntüleme Sayısı

Solid-Liquid Extraction
Organic Chemistry
237.1K Görüntüleme Sayısı

Rotary Evaporation to Remove Solvent
Organic Chemistry
212.3K Görüntüleme Sayısı

Fractional Distillation
Organic Chemistry
332.8K Görüntüleme Sayısı

Growing Crystals for X-ray Diffraction Analysis
Organic Chemistry
32.3K Görüntüleme Sayısı

Performing 1D Thin Layer Chromatography
Organic Chemistry
288.4K Görüntüleme Sayısı

Column Chromatography
Organic Chemistry
358.4K Görüntüleme Sayısı

Nuclear Magnetic Resonance (NMR) Spectroscopy
Organic Chemistry
246.7K Görüntüleme Sayısı
JoVE Hakkında
Telif Hakkı © 2020 MyJove Corporation. Tüm hakları saklıdır