Conducting Reactions Below Room Temperature
Source: Laboratory of Dr. Dana Lashley - College of William and Mary
Demonstration by: Matt Smith
When new bonds are formed in the course of a chemical reaction, it requires that the involved species (atoms or molecules) come in very close proximity and collide into one another. The collisions between these species are more frequent and effective the higher the speed at which these molecules are moving. A widely used rule of thumb, which has its roots in the Arrhenius equation1, states that raising the temperature by 10 K will approximately double the rate of a reaction, and raising the temperature by 20 K will quadruple the rate:
(1) 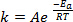
Equation (1) is often found in its logarithmic form:
(2) 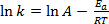
where k is the rate of the chemical reaction, A is the frequency factor (relating to frequency of molecular collisions), Ea is the activation energy required for the reaction, R is the ideal gas constant, and T is the temperature at which the reaction is taking place.
A higher temperature therefore means a reaction is completed much faster. Nonetheless, in some cases it is desirable to carry out reactions at low temperatures, in spite of the lowering effect on the rate of the reaction. A few scenarios in this regard are elaborated upon further below.
When it is useful to run a reaction below room temperature, chemists use cooling baths to maintain a certain temperature or temperature range. Reactions are cooled down to a desired temperature by placing the reaction flask inside an appropriate cooling bath. The reagents in the reaction never come in direct contact with the chemicals in the cooling bath. The cooling bath may consist of a single cryogenic (cooling) component (such as ice, dry ice, or liquid nitrogen) or may be a mixture of the cryogenic component with a certain solvent and/or an additive salt. The purpose of the solvent is to effectively transfer temperature of the cooling agent to the reaction flask, and the purpose of the additive is to lower (or depress) the freezing point of the mixture. (Note that it is possible for a substance to be both a solvent and an additive.)
Recall that when a solution freezes at a lower temperature than the pure liquid, this is caused by a colligative property known as freezing point depression. The lowering effect on the freezing point is proportional to the amount of solute (additive) that is added to a liquid solvent. This effect, is described by equation (3):
(3) ΔDTf = Tf (solvent) − Tf (solution) = Kf × m
ΔDTf is the freezing point depression and is described by the difference in freezing temperature of the solvent by itself, and that of the solution with additive/solute.
Kf is the freezing point depression constant for the system, and m is the molality of the solution. Chemists use this effect to their advantage to create a diversity of different temperatures with relative ease and cost-effectiveness.
The temperatures achieved by cooling baths can fluctuate. The bath must be monitored and adjustments made as necessary. For best results, the bath vessel itself should be well insulated. When available, a Dewar flask should be used for the cooling bath. In absence of a Dewar flask, it is possible to set up the bath in a glass or rubber vessel, with the vessel insulated as best as possible (using for example aluminum foil or a towel). The used vessel needs to be thermo-stable at the desired temperature and should not crack.
Many different bath variations exist for the relatively economical and simple achievement of different temperatures below room temperature in a chemical laboratory setting.
- For temperatures just slightly below zero (but if necessary down to - 55 °C) an ice-water bath with different addition of salts will serve.
- For temperatures down to -78 °C, dry-ice-baths in different solvents are employed.
- Temperatures below -78 °C down to -196 °C can be obtained by use of liquid nitrogen.
Setting up these cooling baths is relatively simple and the procedures are included at the end of this document.
Ice-water baths
This type of bath is very easy to set up and available in every undergraduate teaching lab. There is a lot of flexibility in the type of bath vessel to use, because ice-baths do not reach very low temperatures and there is no risk of cracking a vessel.
While ice-water itself has a temperature of 0 °C, a melting-point depression can be achieved by the addition of certain salts such as NaCl, MgCl2 or CaCl2. The achieved final temperatures vary and can be adjusted by the amount of additive used per 100 g of ice. A common ice-bath is one with NaCl as an additive where 33 g of NaCl are added per 100 g of ice. The final temperature achieved by this means is around -20 °C. The coldest temperature an ice-water bath can reach is around -55 °C, which is obtained by the addition of 143 g of CaCl2 hexahydrate per 100 g of ice.
Dry-Ice-baths
Dry ice is solid carbon dioxide and sublimes at a temperature of -78 °C. It is a fairly inexpensive cryogenic agent and readily available in many laboratories. For efficient heat-transfer of this temperature to a reaction vessel, a solvent is required that has a melting point of below -78 °C. Solvents with a higher melting point, or mp, (better referred to as freezing point in this case) can also be used and result in a higher bath temperature.
A solvent that is often used in a dry-ice-bath is acetone (mp = -95 °C), which is readily available and inexpensive. A dry-ice-bath in acetone maintains a temperature of -78 °C for a period of time, the length of which depends upon the degree of insulation. This is the most common dry-ice-bath system.
For higher temperature dry-ice-baths, solvents with higher freezing points are used. The acquired bath temperature does not always equal the freezing-point of the solvent. Please refer to Table 2 for temperatures obtained by different systems.
Due to the low temperatures reached by this type of bath, cryogenic protection gloves should always be worn when handling dry ice.
The bath vessel for a dry-ice-bath is ideally a Dewar. If a Dewar is not available, a glass, rubber, or stainless steel vessel is used, but be aware that insulation will not be very optimal and the bath will need to be adjusted more often.
| Dry-Ice Cooling Bath Temperatures | |
| Mixture | T (°C) |
| p-xylene/Dry ice | +13 |
| Cyclohexane/Dry ice | +6 |
| Benzene/Dry ice | +5 |
| Ethylene glycol/Dry ice | -15 |
| Carbon tetrachloride/Dry ice | -23 |
| 3-Heptanone/Dry ice | -38 |
| Acetonenitrile/Dry ice | -42 |
| Cylcohexanone/Dry ice | -46 |
| Diethyl carbitol/Dry ice | -52 |
| Chloroform/Dry ice | -61 |
| Carbitol acetate/Dry ice | -67 |
| Ethanol/Dry ice | -72 |
| Acetone/Dry ice | -78 |
| Isopropanol/Dry ice | -78 |
Table 2. List of different dry-ice-bath mixtures.
Liquid Nitrogen baths
Liquid nitrogen slush baths are used when very low temperatures, below that of a dry-ice-bath, are desired. Liquid nitrogen is a cryogenic agent with a melting point of -196 °C, which is the temperature of the bath when no additional solvent is used. Note, that in contrast to dry-ice, N2 is a liquid and the use of an additive solvent for uniform heat transfer is not necessary. If a higher temperature than -196 °C is desired, then a variety of different organic solvents is used for mixtures that will result in different temperatures, similar as was the case with dry-ice-baths. Please refer to Table 3 for temperatures obtained by different systems.
Due to the very low temperatures of liquid N2 baths, only a Dewar should be used as the bath vessel and always work with gloves when handling this cryogenic agent.

Table 3. List of liquid nitrogen baths with different solvents.2
Cooling Bath Setup
For a general set-up, prepare the cooling bath of choice as described below and immerse the reaction flask into the bath (Figure 1). Don't fill the bath vessel all the way, but leave enough room to allow for immersion of the reaction flask.
Note: if the reaction is moisture sensitive, be very careful when adding reagents to the flask or any other part of the apparatus (e.g. a dropping funnel). If an opening is generated while the glassware is immersed in the cooling bath, then room temperature air quickly flows inside and carries moisture in.
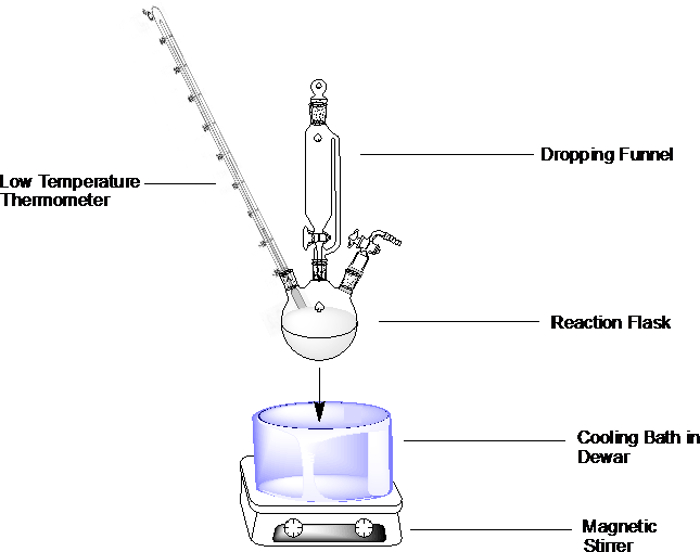
Figure 1. Example for a cooling bath set-up in a three-neck round-bottom flask with dropping funnel, thermometer under inert atmosphere.
1. Making an Ice-Water Bath
- For an ice-water bath with an additive, weigh and add the appropriate amount of ice to the bath vessel of choice. A Dewar is helpful but not necessary. The vessel can be plastic, rubber (e.g. a bucket), or glass (e.g. a large crystallization dish). For this experiment, add 500 g of ice to a 1 L vessel.
- Weigh the appropriate amount of additive consulting an ice-bath (Table 1), and add the additive to the ice. For this experiment, weigh and add 165 g (=5x 33 g) of NaCl to the bath vessel.
- Add a small amount of deionized water to the bath vessel and stir thoroughly using a stir rod. Add just enough water until all the ice is covered.
- Check with a thermometer to ensure that the desired temperature has been reached. Adjust the amount of additive if necessary. The bath will not maintain its temperature for very long and adjustments need to be made in frequent intervals of about every 20–30 min. For this it may be necessary to pipette off some of the water in the bath and add more ice and additive.
| Substance | g/100 g H2O | Final Temperature (°C) |
| Na2CO3 | 20 | -2.0 |
| NH4NO3 | 106 | -4.0 |
| NaC2H3O2 | 85 | -4.7 |
| NH4Cl | 30 | -5.1 |
| NaNO3 | 75 | -5.3 |
| Na2S2O3 ● 5H2O | 110 | -8.0 |
| CaCl2 ● 6H2O | 41 | -9.0 |
| KCl | 30 | -10.9 |
| KI | 140 | -11.7 |
| NH4NO3 | 60 | -13.6 |
| NH4Cl | 25 | -15.4 |
| NH4NO3 | 45 | -16.8 |
| NH4SCN | 133 | -18.0 |
| NaCl | 33 | -21.3 |
| CaCl2 ● 6H2O | 81 | -21.5 |
| H2SO4 (66.2%) | 23 | -25 |
| NaBr | 66 | -28 |
| H2SO4 (66.2%) | 40 | -30 |
| C2H5OH (4°) | 105 | -30 |
| MgCl2 | 85 | -34 |
| H2SO4 (66.2%) | 91 | -37 |
| CaCl2 ● 6H2O | 123 | -40.3 |
| CaCl2 ● 6H2O | 143 | -55 |
Table 1. Salt/ice cooling mixtures that are obtained by mixing the salts with water or ice at the specified temperatures and in the specified amounts.1
2. Making a Dry-Ice Bath
- Put on cryogenic protection gloves and safety goggles. Always practice this when handling dry ice and never touch it with bare hands as it can rapidly burn skin and cause frostbites.
- For a bath vessel with a volume of about 1 L, take about 1/3 of a block of dry ice (usually available in ~2 lb blocks) and break it up into a few smaller pieces.
- Add the pieces of dry-ice to the bath vessel.
- Slowly add the organic solvent (e.g. acetone) to the dry ice while stirring with a glass rod. There is a vigorous fizzing as a result of CO2 gas development.
- Continue to slowly add solvent and stir until a homogenous slurry forms and most of the dry ice dissolves. This is to ensure that the heat transfer to the reaction flask is as uniform as possible.
- Insert a cold-temperature thermometer into the bath to ensure that the desired temperature is reached.
- Check on the dry-ice-bath in regular intervals and add more chunks of dry ice when a rise in the bath temperature is noticed. The interval time depends on the degree of insulation, but is usually around every 45–60 min.
3. Making a Liquid Nitrogen Bath
- Put on cryogenic protection gloves and safety goggles. Always practice this when handling liquid nitrogen as it can rapidly burn skin tissues and eye fluids, causing frostbites or permanent eye damage.
- For a bath without additives, add the appropriate amount of N2 to a Dewar to obtain a temperature of -196 °C. Move to step 3.3 if this is the desired temperature.
- For a bath with additives, add an organic solvent of choice (consult Table 3 to find the right solvent for the right temperature) to the Dewar first, then slowly add liquid N2 to the solvent.
- Insert a cold-temperature thermometer into the bath to ensure that the desired temperature has been reached. In contrast to the other baths, the liquid N2 bath inside of a Dewar maintains its temperature for hours at a time.
- Check on the bath in appropriate intervals (a few hours) to see if more N2 is needed.
When is it useful to run a reaction at a low temperature?
In order to answer this question let us investigate four different applications:
Application 1. Sometimes reactions are too vigorous and exothermic and the reaction mixture must be cooled in order to prevent spilling and pressure build-up due to gas development. A highly exothermic reaction can also become a safety hazard as the reaction mixture can rapidly boil over (many organic solvents usually have low boiling points) and spurt out. A very common application for this is the quenching or work-up step where a reaction initially carried out under anhydrous conditions is reacted with water and acid at the end in order to protonate the final product and to react off any remaining reactive intermediates and reactants. For example, in the Grignard reaction, a very common reaction in organic chemistry, the quenching step at the end will require cooling, even though an ice-water bath at 0 °C will suffice:
(4) 
Application 2. Cooling can also be required for addition steps at the beginning of a reaction, when an exothermic reaction would otherwise result in the boiling off of the organic solvent. This is undesirable, because reactions are best carried out in solvents. Having to add more solvent to compensate for the loss of solvent is not only wasteful and uneconomical but also tedious as solvents in many reactions require a prior drying step to make them anhydrous. Moreover, it is possible for certain reagents to thermally decompose at higher temperatures.
To avoid these occurrences in an exothermic reaction, a reagent is often added dropwise by syringe or dropping-funnel to a flask containing another reagent in solvent, while stirring and cooling. This way, the addition can be stopped anytime if the reaction becomes too vigorous. Often, the reaction must be cooled well below 0 °C and an ice-water bath does not suffice.
An example for a reaction where this is necessary is the addition of the strong base n-butyllithium (n-BuLi) to diisopropylamine to form lithium diisopropylamide (LDA).
(5) 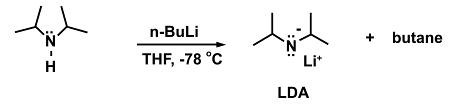
In the absence of a cooling bath the n-BuLi may decompose as higher temperatures are reached:
(6) 
Application 3. In some chemical reactions there is more than one possible product resulting from a competing chemical pathway. One product may be the result of the pathway with a more stable transition state, requiring less activation Energy (Ea1), while the other product may require more activation energy (Ea2) but is overall more stable. The former is called the kinetic product while the latter is called the thermodynamic (TD) product (see energy diagram in Figure 2).
By controlling the reaction temperature we can control which one of these products is formed. Because the kinetic product requires less activation energy it is the product that is formed at low temperatures. Conducting a reaction at low temperatures often ensures the formation of the kinetic product over the thermodynamic product.
A classical example in the realm of enolate chemistry is the reaction of 2-methylcyclohexanone with different bases under different reaction conditions. The reactant is an unsymmetrical ketone and therefore possesses two different types of α- hydrogens. Small bases, such as NaOH deprotonate the ketone at the more-highly substituted side, which results in the more stable, thermodynamic enolate (7). Bases, which are more sterically demanding, deprotonate the ketone on the less hindered side, resulting in the kinetic enolate (8). The formation of the kinetic enolate will have a much higher yield when the reaction is carried out at -78 °C as compared to room temperature. The two forms of the enolate can then be reacted with an appropriate electrophile, such as methyiodide, to form the α-alkylated products shown below.
(7)(8)
The sterically demanding base used to obtain the kinetic enolate is often LDA, the preparation of which was shown earlier in scheme (5). It is important to control the temperature to -78 °C to prevent the kinetic enolate to equilibrate back into the thermodynamic enolate. (Note: there is no significance to the temperature of -78 °C other than that it is easily obtained by a dry-ice-bath in acetone.)
Aside from temperature control, the addition order and manner of addition of reagents is crucial. For best results favoring the kinetic enolate, a solution of the ketone reactant is added dropwise to the LDA base in solvent. The anhydrous solvent used for the reaction with LDA is often THF. An example reaction is shown in scheme (9).
(9) 
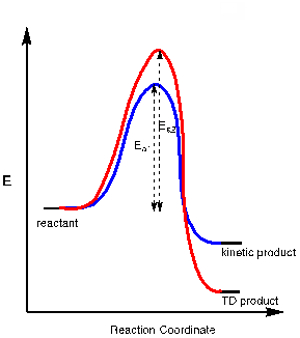
Figure 2. Energy diagram for a reaction that has a kinetic and a thermodynamic product.
Application 4. In some cases it is possible to regulate the reactivities of reagents with temperature. Consider for example the reduction of an ester. Reactions with the strong hydride reducing agent lithium aluminum hydride (LAH) result in the reduction of the ester all the way down to the respective primary alcohol (10). However, use of the bulky hydride reducing agent diisobutylaluminum hydride (DIBAL) allows for selective reduction of an ester to the respective aldehyde. Over-reduction to the primary alcohol can be avoided, so long as the reaction temperature is kept at below -78 °C (yet better down to -90 °C ) and only one stoichiometric equivalent of DIBAL is used (12). At temperatures above -70 °C, DIBAL becomes too reactive and will reduce the ester to the primary alcohol (11).
(10)-(12)
- Gordon, A. J., Ford, R. A. The Chemist's Companion - A Handbook of Practical Data, Techniques, and References. Chapter 11 (1973) ISBN: 978-0-471-31590-2.
- Rondeau, R. E. Slush baths. J. Chem. Eng. Data. 11, 124 (1966)
Atla...
Bu koleksiyondaki videolar:

Now Playing
Conducting Reactions Below Room Temperature
Organic Chemistry
70.3K Görüntüleme Sayısı

Katalize Giriş
Organic Chemistry
34.1K Görüntüleme Sayısı

Assembly of a Reflux System for Heated Chemical Reactions
Organic Chemistry
166.3K Görüntüleme Sayısı

Schlenk Lines Transfer of Solvents
Organic Chemistry
41.5K Görüntüleme Sayısı

Degassing Liquids with Freeze-Pump-Thaw Cycling
Organic Chemistry
55.9K Görüntüleme Sayısı

Preparing Anhydrous Reagents and Equipment
Organic Chemistry
79.1K Görüntüleme Sayısı

Purifying Compounds by Recrystallization
Organic Chemistry
705.3K Görüntüleme Sayısı

Separation of Mixtures via Precipitation
Organic Chemistry
157.2K Görüntüleme Sayısı

Solid-Liquid Extraction
Organic Chemistry
237.1K Görüntüleme Sayısı

Rotary Evaporation to Remove Solvent
Organic Chemistry
212.3K Görüntüleme Sayısı

Fractional Distillation
Organic Chemistry
332.8K Görüntüleme Sayısı

Growing Crystals for X-ray Diffraction Analysis
Organic Chemistry
32.3K Görüntüleme Sayısı

Performing 1D Thin Layer Chromatography
Organic Chemistry
288.4K Görüntüleme Sayısı

Column Chromatography
Organic Chemistry
358.4K Görüntüleme Sayısı

Nuclear Magnetic Resonance (NMR) Spectroscopy
Organic Chemistry
246.7K Görüntüleme Sayısı
JoVE Hakkında
Telif Hakkı © 2020 MyJove Corporation. Tüm hakları saklıdır