Method Article
Production of Arbuscular Mycorrhizal (AM) Fungal Inoculum and Phenotypic Evaluation of Rice and AM Symbiosis Under Saline Conditions
* These authors contributed equally
In This Article
Summary
This article describes a protocol for generating arbuscular mycorrhizal (AM) fungi inoculum to investigate AM-enhanced salt stress tolerance in rice.
Abstract
Rice (Oryza sativa L.) is a vital food crop for more than half of the global population. However, its growth is severely impacted by saline soils, which present a significant challenge to crop production worldwide. Arbuscular mycorrhizal (AM) fungi, which form mutualistic symbiotic relationships with over 90% of agricultural plants and 80% of terrestrial plant species, have been shown to enhance the salt tolerance of rice plants. AM fungi are obligate symbionts that cannot complete their life cycle without a host root. Therefore, effectively utilizing plants to produce AM fungal inoculum is crucial for advancing research in this field. In this study, we present a series of robust methods that begin with generating sand inoculum containing spores of Rhizophagus irregularis using Allium tuberosum L. These methods include inoculating rice seedlings with the sand inoculum, analyzing the growth phenotype of mycorrhizal rice, and quantifying fungal colonization levels using trypan blue staining under salt stress. These approaches can efficiently generate AM fungal inoculum for further investigation into how AM symbiosis enhances the salinity tolerance of rice.
Introduction
Saline soil is a significant obstacle to crop production worldwide1,2,3. Recent studies indicate that up to 50% of cultivated land will be degraded by 2050 due to salinization4. Salt-affected soils primarily cause toxicity in plants due to the accumulation of sodium (Na+) and chloride (Cl−) ions in plant tissues. These ions, which dominate saline soils, are also the most harmful to plants5,6,7. For example, sodium inhibits many cytosolic enzyme activities8. Salt stress also affects photosynthetic efficiency and induces changes in ionic toxicity, osmotic pressure, and cell wall structure, collectively leading to the accumulation of reactive oxygen species (ROS)9,10,11,12,13.
Arbuscular mycorrhizal (AM) symbiosis is an endosymbiotic association between fungi of the phylum Glomeromycota and plant roots, which evolved approximately 400-450 million years ago with the emergence of early land plants14,15. Over 80% of vascular plants can be colonized by AM fungi16. This mutualistic relationship enhances plant nutrient uptake from the soil, thereby improving growth and stress tolerance17,18,19,20. For example, during salt stress, AM fungi can maintain ion balance and help enhance water and nutrient availability, antioxidant activity, photosynthetic efficiency, and secondary metabolite production for plants2,21,22,23. Additionally, AM symbiosis prevents excessive Na+ uptake and transport from roots to shoots, promoting the absorption of essential cations such as K+, Mg2+, and Ca2+. This process increases the Mg2+/Na+ or K+/Na+ ratio in plants under saline conditions23,24,25,26,27,28,29.
Rice (Oryza sativa L.), a crucial food crop for over half of the global population, belongs to the family Gramineae (Poaceae) and is highly susceptible to salt stress30. Studies have also highlighted the role of AM fungi in enhancing salt stress tolerance in rice31,32,33. For instance, the AM fungus Claroideoglomus etunicatum improves the CO2 fixation efficiency of rice (Oryza sativa L. cv. Puntal) under salt stress31. Moreover, the expression of key rice transporter genes associated with vacuolar sodium sequestration and Na+ recirculation from shoots to roots is enhanced in AM-colonized plants under salt stress32. Additionally, upland rice plants inoculated with Glomus etunicatum display enhanced photosynthetic capacity, elevated osmolyte production, improved osmotic potential, and greater grain yield under saline conditions33. Our previous research also demonstrated that mycorrhizal rice (Oryza sativaL. cv. Nipponbare) exhibited better shoot and reproductive growth, a notably higher K+/Na+ ratio in the shoot, and improved reactive oxygen species (ROS) scavenging capacity due to AM symbiosis34. These findings all demonstrate the positive impact of AM symbiosis on salt stress tolerance in rice through phenomic approaches. However, the experimental methods have not been published in video format.
AM fungi are obligate symbionts that require a host root to complete their life cycle, making the use of plants to produce AM fungal inoculum crucial for research progress35. A substrate-based production system, where AM fungi are grown in substrates like vermiculite or sand and spores are collected for inoculum36, offers a cost-effective solution for large-scale AM fungal inoculum production. The efficiency of spore production depends on plant compatibility and growth, which affect fungal colonization and propagation37,38. However, this method is often time-consuming, with traditional approaches taking up to 120 days and yielding low spore production. Recent improvements have reduced the production period to 90 days using maize as the host plant under LED light conditions39. However, a robust method is presented for generating sand inoculum containing spores of Rhizophagus irregularis using Allium tuberosum L. within 10 weeks. This sand inoculum can be used to analyze the growth phenotype of mycorrhizal rice and quantify fungal colonization levels using trypan blue staining under salt stress. These approaches efficiently generate AM fungal inoculum for further investigation into how AM symbiosis enhances the salinity tolerance of rice.
Protocol
The details of the reagents and the equipment used in this study are listed in the Table of Materials.
1. Generation of sand inoculum containing spores of Rhizophagus irregularis using Allium tuberosum L.
- Wash sand with tap water and autoclave it.
- Add 2/3 of the sand to a pot (top diameter 14.7 cm, bottom diameter 11.5 cm, height 13 cm). Add 1,000 spores of AM fungi Rhizophagus irregularis. Cover with a thin layer of sand. Add 30 seeds of garlic chives (Allium tuberosum L.) and cover the seeds with sand.
- Grow the garlic chives in the chamber with a 16-h/8-h day/night cycle at 23.5 °C (55% relative humidity). During the first week (1-week post-inoculation, wpi), cover the garlic chives with alumina paper to block light and water them three times a week.
- Starting from 2 wpi, fertilize the garlic chives twice a week with 80 mL half-strength Hoagland solution containing 25 µM KH2PO4. Fertilize once a week with 80 mL water.
- After 10 weeks, harvest the roots of garlic chives for trypan blue staining to assess the level of fungal colonization. If the colonization level exceeds 70%, stop watering the garlic chives until the sand is dry (about 5 weeks). Put all sand inoculum into a plastic bag and store it in a fridge at 4 °C.
2. Trypan blue staining to check fungal colonization level
- Incubate root pieces for 30 min at >90 °C in 10% KOH. Remove the KOH.
- Rinse the root pieces with double-distilled water (ddH2O) three times.
- Incubate the root pieces with 0.3 M HCl for 15 min to 2 h. Remove the HCl.
- Add 1 mL of 0.1% trypan blue and incubate the samples for 8 min at >90 °C.
- Wash the root pieces with 50% acidic glycerol. Transfer 10 root pieces onto slides and add a drop of 50% acidic glycerol.
- Seal the coverslips and slide with nail polish.
- Examine 10 fields of view of each root under a microscope to record the presence of fungal structures. Calculate the fungal colonization level as a percentage.
NOTE: 50% acidic glycerol: Prepare by mixing glycerol and 0.3 M HCl in a 1:1 ratio. 0.1% trypan blue: Dissolve 100 mg of trypan blue in a mixture of 2:1:1 lactic acid, glycerol, and ddH2O.
3. Inoculation of rice seedlings with sand inoculum and salt stress treatment
- Remove the hull (husk) from rice seeds.
- Sterilize the seeds with 70% ethanol (EtOH) for 4 min and 30 s.
- Place the rice seeds into a centrifuge tube. Add 3% bleach (prepared with sterile dH2O) and shake for 30 min.
- Remove the bleach and wash the seeds with sterile dH2O 3-4 times inside the laminar flow hood.
- Grow the seeds in half-strength Murashige-Skoog (1/2 MS) medium containing 0.8% agar at 30 °C in the dark for 5 days.
- Grow the rice seedlings with a 12-h day/night cycle at 30/28 °C and 70% air humidity for 2 days.
- Transfer the rice seedlings into plastic tubes containing sterilized sand. Add either no inoculum (mock) or 5 mL of sand inoculum containing spores of Rhizophagus irregularis (Ri).
- Water the rice plants with dH2O 7 days a week for the first week after inoculation. Fertilize the plants every second day with a half-strength Hoagland solution containing 25 µM of KH2PO4.
- At 5 weeks post-inoculation (wpi), treat one batch with 150 mM of NaCl (saline condition) and leave the other batch without NaCl (non-saline condition).
- For the non-saline condition, water the plants with half-strength Hoagland solution containing 25 µM of KH2PO4 on Tuesday and with water for the rest of the week.
- For the saline condition, water the plants with half-strength Hoagland solution containing 25 µM of KH2PO4 on Tuesday, with 150 mM of NaCl on Monday, Wednesday, and Friday, and with water for the rest of the week.
- At 8 wpi, harvest the plants to measure their fresh weight. Place the plants in a 70 °C oven for 2 days to measure the dry weight. Analyze the fungal colonization level by trypan blue staining.
Results
The step-by-step workflow is shown in Figure 1.At 10 weeks post-inoculation (wpi), fungal structures such as vesicles and spores, which are characteristic of the late stage and AM symbiosis, were clearly observed inside the roots of garlic chives (Figure 2A). The levels of intraradical hyphae, arbuscule, vesicle, extraradical hyphae, and spore were 80%, 47%, 63%, 4%, and 1%, respectively, indicating the progression of fungal development inside the roots of garlic chives. Therefore, the total colonization level reached 80% (Figure 2C). These results indicated that the symbiotic relationship between garlic chives and AM fungi was successfully established and that the AM fungi were able to complete their life cycle and generate more spores. Using the sand inoculum generated from the symbiosis between garlic chives and AM fungi, the rice plants were successfully colonized by AM fungi. At 8 wpi, vesicles and spores were observed inside rice roots (Figure 2D), and the levels of intraradical hyphae, arbuscule, vesicle, extraradical hyphae, spore, and total fungal structures were 91%, 82%, 95%, 46%, 2%, and 93%, respectively (Figure 2E). Then rice plants were grown without (mock) or with this sand inoculum for 5 weeks and then treated without or with salt solution (150 mM of NaCl) for 3 weeks. Mycorrhizal plants exhibited fewer wilted blade tips than mock plants under salt stress (Figure 2F). Under non-saline conditions, mycorrhizal rice plants showed higher shoot biomass than mock ones (Figure 2G). Under salt stress, the shoot biomass of mock plants was severely reduced, whereas mycorrhizal plants maintained their shoot biomass, which was 1.4 times higher than that of the mock plants (Figure 2G). AM symbiosis did not significantly affect root biomass under either condition (Figure 2G). These results suggest that AM symbiosis helps rice plants sustain better shoot growth under salt stress. Fungal colonization levels reached 84% and 83% under non-saline and saline conditions, respectively, indicating successful colonization of rice roots by AM fungi. In addition, extraradical hyphae levels were higher under salt stress. No significant differences were observed in other fungal structures between non-saline and saline conditions, suggesting that salt stress had a mild impact on AM symbiosis (Figure 2H).
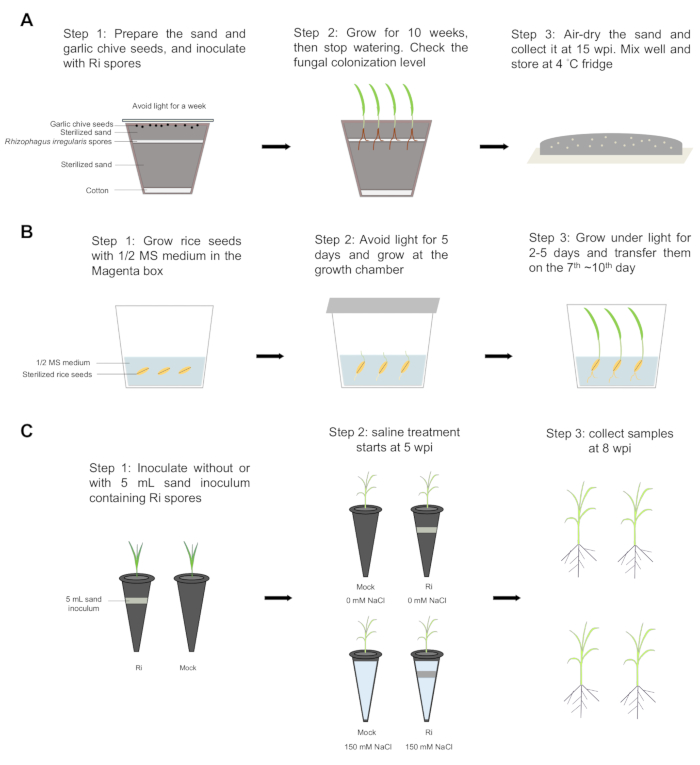
Figure 1: Step-by-step workflow. (A) The sand inoculum was prepared using the following steps: Step 1: A layer of cotton was put at the bottom of the pot, and 2/3 of sterilized sand was added to the pot. About 1000-2000 Rhizophagus irregularis spores were evenly spread using cut tips, then covered with the remaining 1/3 of sterilized sand. Thirty Allium tuberosum L. seeds were evenly spread over the sand surface, and covered with alumina paper to block light. Plants were incubated at 23 °C for a week. Step 2: The seeds were grown for 10 weeks at 23 °C with a 16/8 light/dark cycle, then watering was stopped. The colonization level of roots was checked. Step 3: The sands were air-dried, collected at 15 weeks post-inoculation (wpi), mixed well, and stored at 4 °C fridge. (B) Rice seeds were germinated in the Magenta box. Steps 1 and 2: Rice seeds were sterilized with 70% ethanol for 4 min 30 s, and ethanol was replaced with 3% of sodium perchlorate. The seeds were shaken for 30 min and then rinsed with sterilized water five times. Seeds were germinated on 1/2 Murashige and Skoog (MS) medium with 0.8% agar for 5 days in the dark at 30 °C and 2 days under light (12/12 h day/night cycle at 30/28 °C). Step 3: The rice seedlings were transferred on the 7th ~10th day. (C) Inoculation and saline treatment. Step 1: The rice seedlings were transplanted into plastic tubes containing sterilized sand without (mock) or with 5 mL sand inoculum containing Rhizophagus irregularis (Ri) spores. The plants were grown in a growth chamber with a 12-h day/night cycle at 30/28°C, and the plants were grown twice a week with one-half Hoagland solution having 25 µM of phosphate (Pi). Step 2: At 5 wpi, the mycorrhizal and mock plants were divided into two groups. One group was treated with 150 mM of sodium chloride (NaCl) (saline condition), and the other group was grown under non-saline conditions. Step 3: At 8 wpi, all the plants were collected, and then the shoots and roots were separated to assess the fresh weight and dry weight. Please click here to view a larger version of this figure.

Figure 2: The phenotype of Allium tuberosum L. (garlic chive), O. sativa L. japonica cv. Nipponbare (rice) and Rhizophagus irregularis (AM fungi). (A) Photograph of mycorrhizal garlic chive roots at 10 wpi. (B) Photograph of mycorrhizal garlic chive plants at 10 wpi. (C) Fungal colonization level of garlic chive roots. (D) Photograph of mycorrhizal rice roots at 8 wpi. (E) Fungal colonization level of rice roots. (F) The phenotype of mock and mycorrhizal rice plants under salt stress. (G) The dry weight of mock and mycorrhizal rice plants under non-saline and salt stress, and (H) fungal colonization level of mycorrhizal rice roots under salt stress at 8 wpi. In (A-C), garlic chives were inoculated with 200 spores of AM fungi (R. irregularis, Ri), grown under 25 µM phosphate conditions, and harvested at 10 weeks post-inoculation (wpi). In panels (D) and (E), rice plants were inoculated with 5 mL of sand inoculum derived from the symbiosis between garlic chive and AM fungi (Rhizophagus irregularis, Ri), grown under 25 µM phosphate conditions, and harvested at 8 weeks post-inoculation (wpi). In panel (F), rice plants were grown either without (mock) or with sand inoculum containing AM fungi (R. irregularis, Ri) for 5 weeks, followed by treatment with a salt solution (150 mM of NaCl) for 3 weeks. Roots were stained with trypan blue in panels (A) and (D). In these panels, spores are indicated by white arrowheads, vesicles by white arrows, and extraradical hyphae by black arrows. Scale bars: 100 µm in panels (A) and (D); 1 cm in panel (B); and 10 cm in panel (F). Abbreviations: int hyphae, intraradical hyphae; ext hyphae, extraradical hyphae. The standard error was calculated from 3-4 biological replicates. Different letters indicate significant differences between treatments (p < 0.05, two-way ANOVA followed by a least significant differences post hoc test). Please click here to view a larger version of this figure.
Discussion
There are a few tips regarding the preparation and usage of sand inoculum. First, from our experience, the colonization level of garlic chive should be higher than 70% (Figure 2C). Otherwise, the following inoculation on other plants, such as tomato and rice, will not successfully reach over 50% at 7 weeks post-inoculation (wpi) (Figure 2E). Second, the sand inoculum should be air-dried thoroughly before storage and kept inside a clean plastic bag in the fridge to prevent it from getting wet again (step 1.5). Otherwise, the quality of the sand inoculum will deteriorate. Third, the sand inoculum can be stored in the fridge for about 10 months without any problems. Fourth, the sand inoculum must be mixed thoroughly by shaking the storage bag before adding it to the pot to inoculate other plants (step 3.7).
Regarding trypan blue staining, the roots should be cut into pieces about 1-1.5 cm in length for staining so the fungal structure can be stained homogeneously with trypan blue (step 2.1). To accurately represent the mycorrhizal colonization level of a root, one must observe ten fields of view at nearly equal intervals from one end of the root to the other (step 2.7).
Regarding salt stress treatment on mock and mycorrhizal rice, the time for sterilizing rice seeds with alcohol must be precise; otherwise, it will affect the germination rate of the rice (step 3.2). Since sand is used as the cultivation medium, it is important to ensure that the rice receives enough water throughout the entire growth process. Otherwise, the rice may experience drought and high salt stress simultaneously, making it difficult to accurately assess the growth results (steps 3.8-3.9).
By following this protocol, AM-enhanced salt stress tolerance can be observed in the rice cultivar Nipponbare. However, whether this salt stress treatment can also be used to observe AM-enhanced salt stress tolerance in other rice cultivars is not known. If not, some steps can be modified, such as using more sand inoculum, starting the salt stress treatment after 5 wpi, increasing the time period for salt stress, or re-watering the plants after salt stress.
AM fungi are obligate symbionts that need host roots to complete their life cycle, making plant-based inoculum production essential for research35. Substrate-based systems, where fungi grow in materials like vermiculite or sand, offer a cost-effective way to produce large-scale inoculum. However, traditional methods can take up to 120 days and yield low spore numbers36,37,38. Recent improvements have reduced this to 90 days using maize under LED light39. Here, a method is presented for generating Rhizophagus irregularis spores in sand using Allium tuberosum L. in just 10 weeks. This inoculum can be used to study rice growth, fungal colonization, and salinity tolerance, providing an efficient tool for AM symbiosis research.
Disclosures
The authors declare that they have no conflicts of interest.
Acknowledgements
We acknowledge Yun-Hsin Chen establishing the system for investigating AM-enhanced salt stress tolerance in rice, and Kai-Chieh Chang establishing the system to generate sand inoculum. This work was supported by grants from the National Science and Technology Council, Taiwan (NSTC 113-2326-B-002 -008 -MY3).
Materials
| Name | Company | Catalog Number | Comments |
| (NH4)6Mo7O24.4H2O | FERAK | 12054-85-2 | half-strength Hoagland solution |
| Bleach | Gaulix | Gaulix-2108 | rice sterilization |
| Ca(NO3)2.4H2O | Sigma | 13477-34-4 | half-strength Hoagland solution |
| CuSO4.5H2O | Sigma | 7758-99-8 | half-strength Hoagland solution |
| EtOH | Honeywell | 67-63-0 | rice sterilization |
| Fe-citrate | Sigma | 3522-50-7 | half-strength Hoagland solution |
| Garlic chives seeds | KNOWN-YOU SEED Co., LTD. | V-015 | Allium tuberosum L. seeds |
| Glycerol | J.T.Baker | 56-81-5 | Trypan blue staining |
| HCl | Sigma | 7647-01-0 | Trypan blue staining |
| KCl | Merck | 7447-40-7 | half-strength Hoagland solution |
| KH2PO4 | Merck | 7646-93-7 | half-strength Hoagland solution |
| KNO3 | Avantor | 7757-79-1 | half-strength Hoagland solution |
| KOH | Honeywell | 1310-58-3 | Trypan blue staining |
| Lactic acid | Sigma | 50-81-7 | Trypan blue staining |
| MgSO4.7H2O | Sigma | 10034-99-8 | half-strength Hoagland solution |
| MnSO4.H2O | Honeywell | 10034-96-5 | half-strength Hoagland solution |
| MS salts | PhytoTech | M404 | half-strength Murashige–Skoog (1/2 MS) medium |
| Na2B4O7.10H2O | Sigma | 1330-43-4 | half-strength Hoagland solution |
| NaCl | Bioshop | 7647-14-5 | salt stress treatment |
| NaOH | J.T.Baker | 1310-73-2 | half-strength Murashige–Skoog (1/2 MS) medium |
| Rhizophagus irregularis spore | Premier Tech | L-ASP-A | AM fungal spore (MycoriseASP, Premier Tech, Rivière-du-Loup, Québec, Canada ) |
| Sucrose | Bioshop | 57-50-1 | half-strength Murashige–Skoog (1/2 MS) medium |
| Trypan blue | Sigma | 72-57-1 | Trypan blue staining |
| ZnSO4.7H2O | Avantor | 7446-20-0 | half-strength Hoagland solution |
References
- Flowers, T., Yeo, 6. Breeding for salinity resistance in crop plants: Where next. Funct Plant Biol. 22 (6), 875-884 (1995).
- Porcel, R., Aroca, R., Ruiz-Lozano, J. M. Salinity stress alleviation using arbuscular mycorrhizal fungi: A review. Agron Sustain Dev. 32, 181-200 (2012).
- Mukhopadhyay, R., Sarkar, B., Jat, H. S., Sharma, P. C., Bolan, N. S. Soil salinity under climate change: Challenges for sustainable agriculture and food security. J Environ Manage. 15 (280), 111736 (2021).
- Hossain, M. S. Present scenario of global salt-affected soils, its management and importance of salinity research. Int. Res J Biol Sci. 1, 1-3 (2019).
- Hualpa-Ramirez, E., et al. Stress salinity in plants: New strategies to cope with in the foreseeable scenario. Plant Physiol Biochem. 208, 108507 (2024).
- Hussain, S., et al. Effects of salt stress on rice growth, development characteristics, and the regulating ways: A review. J Integr Agric. 16, 2357-2374 (2017).
- Tavakkoli, E., Fatehi, F., Coventry, S., Rengasamy, P., Mcdonald, G. K. Additive effects of Na+ and Cl- ions on barley growth under salinity stress. J Exp Biol. 62 (6), 2189-2203 (2011).
- Flowers, T., Troke, P., Yeo, A. The mechanism of salt tolerance in halophytes. Annu Rev Plant Physiol. 28 (1), 89-121 (1977).
- Shomer, I., Novacky, A. J., Pike, S. M., Yermiyahu, U., Kinraide, T. B. Electrical potentials of plant cell walls in response to the ionic environment. Plant Physiol. 133 (1), 411-422 (2003).
- Sudhir, P., Murthy, S. Effects of salt stress on basic processes of photosynthesis. Photosynthetica. 42 (2), 481-486 (2004).
- Sharma, P., Jha, A. B., Dubey, R. S., Pessarakli, M. Reactive oxygen species, oxidative damage, and antioxidative defense mechanism in plants under stressful conditions. J Bot. 2012, 217037 (2012).
- Singh, M., Kumar, J., Singh, V., Prasad, S. Proline and salinity tolerance in plants. Biochem. Pharmacol. 3, e170 (2014).
- Atta, K., et al. Impacts of salinity stress on crop plants: Improving salt tolerance through genetic and molecular dissection. Front. Plant Sci. 14, 1241736 (2023).
- Remy, W., Taylor, T. N., Hass, H., Kerp, H. Four hundred-million-year-old vesicular arbuscular mycorrhizae. Proc Natl Acad Sci USA. 91. 91, 11841-11843 (1994).
- Redecker, D., Kodner, R., Graham, L. E. Glomalean fungi from the Ordovician. Science. 289 (5486), 1920-1921 (2000).
- Harley, J., Smith, S. . Mycorrhizal symbiosis. , (1983).
- Porras-Soriano, A., Soriano-Martin, M. L., Porras-Piedra, A., Azcon, R. Arbuscular mycorrhizal fungi increased growth, nutrient uptake and tolerance to salinity in olive trees under nursery conditions. J Plant Physiol. 166, 1350-1359 (2009).
- Kapoor, R., Evelin, H., Mathur, P., Giri, B. . Plant acclimation to environmental stress. , 359-401 (2013).
- Rivero, J., Ñlvarez, D., Flors, V., Azcón-Aguilar, C., Pozo, M. J. Root metabolic plasticity underlies functional diversity in mycorrhiza-enhanced stress tolerance in tomato. New Phytol. 220 (4), 1322-1336 (2018).
- Begum, N., et al. Role of arbuscular mycorrhizal fungi in plant growth regulation: Implications in abiotic stress tolerance. Front. Plant Sci. 19 (10), 1068 (2019).
- Evelin, H., Kapoor, R. Arbuscular mycorrhizal symbiosis modulates antioxidant response in salt-stressed Trigonella foenum-graecum plants. Mycorrhiza. 24 (3), 197-208 (2014).
- Sarwat, M., et al. Mitigation of NaCl stress by arbuscular mycorrhizal fungi through the modulation of osmolytes, antioxidants and secondary metabolites in mustard (Brassica juncea L.) plants. Front Plant Sci. 7, 869 (2016).
- Evelin, H., Devi, T. S., Gupta, S. R. K. Mitigation of salinity stress in plants by arbuscular mycorrhizal symbiosis: Current understanding and new challenges. Front Plant Sci. 12 (10), 470 (2019).
- Kapoor Giri, R., Mukerji, K. Influence of arbuscular mycorrhizal fungi and salinity on growth, biomass, and mineral nutrition of Acacia auriculiformis. Biol Fertility Soils. 38 (3), 170-175 (2003).
- Giri Mukerji, K. G. Mycorrhizal inoculant alleviates salt stress in Sesbania aegyptiaca and Sesbania grandiflora under field conditions: Evidence for reduced sodium and improved magnesium uptake. Mycorrhiza. 14 (5), 307-312 (2004).
- Colla, G., et al. Alleviation of salt stress by arbuscular mycorrhizal in zucchini plants grown at low and high phosphorus concentrations. Biol Fertility Soils. 44 (3), 501-509 (2008).
- Hammer, E. C., Nasr, H., Pallon, J., Olsson, P. A., Wallander, H. Elemental composition of arbuscular mycorrhizal fungi at high salinity. Mycorrhiza. 21, 117-129 (2011).
- Estrada, B., Aroca, R., Maathuis, F. J., Barea, J. M., Ruiz-Lozano, J. M. Arbuscular mycorrhizal fungi native from a Mediterranean saline area enhance maize tolerance to salinity through improved ion homeostasis. Plant, Cell Environ. 36, 1771-1782 (2013).
- Talaat, N. B., Shawky, B. T. Influence of arbuscular mycorrhizae on yield, nutrients, organic solutes, and antioxidant enzymes of two wheat cultivars under salt stress. J Plant Nutr Soil Sci. 174, 283-291 (2011).
- Chinnusamy, V., Jagendorf, A., Zhu, J. K. Understanding and improving salt tolerance in plants. Crop Sci. 45, 437-448 (2005).
- Porcel, R., et al. Arbuscular mycorrhizal symbiosis ameliorates the optimum quantum yield of photosystem ii and reduces non-photochemical quenching in rice plants subjected to salt stress. J. Plant Physiol. 1 (185), 75-83 (2015).
- Porcel, R., Aroca, R., Azcon, R., Ruiz-Lozano, J. Regulation of cation transporter genes by the arbuscular mycorrhizal symbiosis in rice plants subjected to salinity suggests improved salt tolerance due to reduced Na(+) root-to-shoot distribution. Mycorrhiza. 26 (7), 673-684 (2016).
- Tisarum, R., et al. Alleviation of salt stress in upland rice (Oryza sativa L. ssp. Indica cv. Leum pua) using arbuscular mycorrhizal fungi inoculation. Front Plant Sci. 11, 348 (2020).
- Hsieh, C., Chen, Y., Chang, K., Yang, S. Transcriptome analysis reveals the mechanisms for mycorrhiza-enhanced salt tolerance in rice. Front Plant Sci. 13, 1072171 (2022).
- Roth, R., Paszkowski, U. Plant carbon nourishment of arbuscular mycorrhizal fungi. Curr Opin Plant Biol. 39, 50-56 (2017).
- Ijdo, M., Cranenbrouck, S., Declerck, S. Methods for large-scale production of am fungi: Past, present, and future. Mycorrhiza. 21, 1-16 (2011).
- Genre, A., Bonfante, P. Building a mycorrhizal cell: How to reach compatibility between plants and arbuscular mycorrhizal fungi. J Plant Interact. 1, 3-13 (2005).
- Zuccaro, A., Lahrmann, U., Langen, G. Broad compatibility in fungal root symbioses. Curr Opin Plant Biol. 20, 135-145 (2014).
- Kiddee, S., et al. Improving inoculum production of arbuscular mycorrhizal fungi in Zea mays L. Using light-emitting diode (led) technology. Agronomy. 14 (10), 2342 (2024).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved