Method Article
Rapid Formation and Testing of Self-expanding NiTi Frames with a Small Form Factor Suitable for Minimally Invasive Implants
In This Article
Summary
This work illustrates a low-cost fabrication technique for shape-setting nitinol wires/frames with a small form factor using sacrificial fixtures. The technique is demonstrated for the fabrication of self-expanding frames designed for minimally invasive implants with complex shapes.
Abstract
NiTiNOL (commonly referred to as nitinol or NiTi) wires feature exceptional shape memory and super-elastic characteristics, while shape-setting is often a costly process. Among the steps in this process, heat treatment requires exposure to high temperatures for shape-setting. Traditionally, metal fixtures are used for this purpose. However, their manufacturing costs can be significant, which is unideal for iterating prototypes. This work demonstrates a recently introduced approach using sacrificial fixtures made of copper tubes, which eliminates the need for expensive fixtures. These copper tubes allow for the formation of complex geometries, and they offer a scaffold for various phases of the fabrication process. Moreover, ammonium persulfate is used for selective copper etching, which simplifies the production of NiTi frames. This work's findings confirm the effectiveness of this technique and demonstrate the successful shape-setting of NiTi wires for self-expanding frames. This methodology paves the way for future research, allowing for rapid prototyping of NiTi wireframes for various applications, especially those in medical devices.
Introduction
NiTi wires are widely used in medical implants but require an initial shape-setting process during device fabrication1. Various devices are made from NiTi, including catheter tubes, guidewires, stone retrieval baskets, filters, needles, dental files, as well as other surgical instruments2. NiTi's biocompatibility, superelasticity, and fatigue resistance make it suitable for these applications. Additionally, it has applications in the automotive and aerospace industries3.
Usage of NiTi is limited due to its high cost and complex processes needed for shape-setting. In the shape-setting process, NiTi structures are traditionally exposed to high temperatures (about 500 °C) while confined in a fixture4. This elevated temperature, as well as the stresses during the shape-setting process, requires a fixture with high mechanical strength. This is why typical fixtures are usually made from metals1. As such, the use of metal fixtures that are typically machined increases costs and poses challenges for the rapid prototyping and testing of NiTi structures. One alternative approach involves the use of reconfigurable fixtures constructed from pins and plates1, which simplifies the process; however, this process has limitations in the shaping of complex geometries. Accordingly, a low-cost, shape-setting process using low-cost materials and manufacturing is highly desirable for research that requires shape-setting NiTi frames.
To address the need for rapid prototyping of NiTi, we recently introduced a protocol utilizing low-cost 3D printed parts and crafted manufacturing for shape-setting NiTi wires5. This method incorporates sacrificial fixtures with a minimal mass. The fixture is shown to be beneficial in securing the NiTi wire during wire forming and shape-setting (heat treatment) processes. Copper tubes were employed as an accessible and low-cost material. It acts as a reinforcing sacrificial fixture and the standard wire bending techniques can be used for shape-setting complex structures. It was observed that the brass tubes could be used as an alternative. Ammonium persulfate was utilized in the final stage for the selective etching of copper, after the annealing process. This step finally released the shape-set NiTi wires. This approach illustrates the innovative use of sacrificial structures as spacers. When this approach is combined with additive manufacturing, the fabrication of complex shapes can be achieved.
In vitro deployment test is among the basic tests for assessing the feasibility of a self-expanding prototype implant, designed to be deployed through a catheter. These tests involve assessing if a self-expanding implant can successfully pass through a sheath/catheter with the required dimension. Such tests have been used in various transcatheter devices or implant prototypes; some examples include left atrial appendage occluders6,7, soft-stents8, NiTi flow diverter9, and NiTi stents10. These works highlight the need for a methodology for rapidly fabricating NiTi frames with complex topologies, which could self-expand through catheters thereby satisfying the preliminary requirements for a transcatheter implant.
The aim of this paper is to outline cost-efficient and well-crafted manufacturing methods, providing a detailed, step-by-step guide through each process. It focuses on demonstrating a variety of self-expanding NiTi wire frames suitable for implants and analyzes key aspects of the method needed to produce complex topologies using affordable and efficient techniques. This paper includes testing these frames and deploying them through a Fr-12 catheter in a benchtop setup that simulates transeptal implant delivery to the atrial septum. This test is similar to basic tests, employed by prior work6,8. This method demonstrated the capability of deployment of a prototype self-expanding frame after passing through a catheter. Ultimately, this methodology can help determine if a certain topology/design for a NiTi frame can meet the preliminary mechanical requirements for deployment through a specific catheter.
While this work focuses on the fabrication of prototypes for NiTi frames and the basic characterization of their topology and conformality, various other characterizations11 and regulatory safety tests12,13 are necessary for the development of implants. Some characterizations include characterization of surface properties/chemistry14, corrosion14, fatigue analysis13, hemocompatibility13, and biocompatibility15.
Protocol
NOTE: See the Table of Materials for details related to all materials used in this protocol. Figure 1A shows an example of the copper/NiTi frame. Use safety gloves.
1. Iteration of a design of a NiTi frame/prototype
- Align NiTi wire inside copper tubes (or brass tubes; Figure 2A).
- Select NiTi wire (0.008 in) and a copper tube (OD 1.00 mm x 400 mm).
- Turn on the stereoscope and visually look at the NiTi on the monitor and copper while manipulating them. Align the wire inside the tube. Push the wire fully into the tube.
- Prepare 3D printed fixtures (Figure 2B-D).
- Download one .STL file for the fixture/template (https://osf.io/54rm3/files/osfstorage).
NOTE: For some .STL file examples, see this repository (https://osf.io/54rm3/files/osfstorage). - If any adjustment is needed, download the .SDLRD file from the same repository, make design adjustments in the propriety CAD software, and then export it as a .STL file. Alternatively, create a model in open-source CAD software and export a .STL file.
NOTE: For some .SDLRD or .FCSTD design examples, see this repository (https://osf.io/54rm3/files/osfstorage). - Open the slicing software (e.g., Elegoo Cura) and import the .STL file. Select the object to be 3D printed and click on the bottom of the slice pane. Save the file. gcode and save it on a micro-sd card. Pull out the micro-SD card.
- Turn on the FDM 3d printer. Put in the micro-sd card. From the screen, select prepare | preheat | PLA. Select back | print. Select the .gcode file and then tap on print.
- Leave the machine to 3D print the part.
- After completion of the 3D printing, remove the printed part and cut any support part using pliers.
- File the part, where there are coarse edges, and mark the areas to be drilled with a marker.
- Drill holes in the 3D-printed geometry using a hand drill (Figure 2B).
CAUTION: Use safety gloves and safety glasses. - Pass screws through the holes of the 3D printed part using a screwdriver (Figure 2C).
- Download one .STL file for the fixture/template (https://osf.io/54rm3/files/osfstorage).
- Form the 3D structure of the Cu/NiTi using the fixture and hand tools. Pass the wire through the holes and bend it over the screws step by step. If needed, bend the wire using hand tools (Figure 2D,E).
- Hold NiTi/Cu and pass it through the central hole. Next, fold/bend the Cu tube using tweezers or pliers around all the screws to form the desired shape (Figure 2E).
- Unscrew the screws. Heat up to soften the 3D printed fixture (from step 1.2) using a soldering gun.
- Use scissors to cut the 3D-printed part. Remove the unwanted 3D part using tweezers or pliers (Figure 2F).
- Heat treat the NiTi/Cu structure/frame (Figure 2G).
- Turn on the furnace tube and monitor the temperature using a thermocouple. When the temperature reaches 500 °C, place the Cu/NiTi frame in the furnace for 3 min.
NOTE: Use high-temperature gloves, lab coat, and safety face shield. - Monitor the temperature using K-type thermocouples by placing the thermometer in the tube furnace.
- Take out the NiTi/Cu frame using a hook after 3 min (Figure 2H) and quench it in distilled water.
- Turn on the furnace tube and monitor the temperature using a thermocouple. When the temperature reaches 500 °C, place the Cu/NiTi frame in the furnace for 3 min.
- Etch the sacrificial copper tubes (Figure 2I).
- Weigh ammonium persulfate on a scale. Weigh water in a glass container as well. Mix them such that the weight of ammonium persulfate is 23% of that of water.
NOTE: Do this process inside the fume hood and use a lab coat, safety glass, and safety gloves. - Add ammonium persulfate to achieve a 23% weight ratio (ammonium persulfate to water). Stir the solution using a glass stirrer until the ammonium persulfate is dissolved.
NOTE: Do this process inside the fume hood and use a lab coat, safety glass, and safety gloves. - Submerge the NiTi/Cu frames from step 1.3 into the solution for ~8 h to etch the copper (Figure 2I).
NOTE: Do this process inside the fume hood and wear a lab coat, safety glass, and safety gloves. - Visually monitor the copper etching. If they are not fully etched, dispose of the etchant, produce the fresh etchant (see steps 1.5.1 and 1.5.2), and pour the fresh one into the container.
- If the copper is fully etched, take it out using tweezers (Figure 2J) and wash the released NiTi frame in distilled water by triple-rinsing it. See Figure 1B for an example of a released NiTi frame after these steps.
- Turn on the microscope. Place the NiTi wire under the microscope; look for any undesirable curvature or dimensions.
- Weigh ammonium persulfate on a scale. Weigh water in a glass container as well. Mix them such that the weight of ammonium persulfate is 23% of that of water.
2. Covering the sides of the frame with films or fabric
- Spin coat the aromatic polyurethane elastomer (polycarbonate urethane is an alternative, see the Table of Materials; the complete protocol is provided elsewhere6).
- Place a 4 inch silicon wafer in the oxygen plasma machine and plasma treat it for 2 min. Next, remove the wafer.
- Open the vacuum desiccator and pour a few drops of the silane (C8H4Cl3F13Si; see Table of Materials) into a plastic container in the desiccator.
- Place the wafer in the desiccator, close the lid, and apply vacuum to the desiccator.
- Close the desiccator valve and turn off the vacuum pump.
- Leave the desiccator for 2 h, afterward take out the silicon wafer.
- Place the wafer on the spin coater, center it, and pour some aromatic or aliphatic polyurethane elastomer dissolved in DMAc (see the Table of Materials) on its center.
- Spin-coat the wafer; then, remove the wafer and place it in the 80 °C oven for 2 h, under a fume hood.
- After 2 h, take out the wafer and peel off the cured film using tweezers (use fingers if needed).
- Cut the peeled film into smaller pieces using scissors.
- Heat press the aromatic polyurethane elastomer films on the NiTi frames.
- Design a spacer for the heat processing procedure.
- Use the proprietary or open-source CAD software to design the spacer, export the .STL file in the environment, and slice the object to create gcode files (see step 1.2.3). Alternatively, download and use the spacer design provided (https://osf.io/54rm3/files/osfstorage).
- Start 3D printing the spacer by opening the .STL file in slicing the software (e.g., CHITUBOX), Alternatively, follow the 3D printing steps in 1.2.4, 1.2.5 instead of 2.2.4, 2.2.5, and 2.2.6.
NOTE: The spacer fabricated through the latter steps will have longer durability. - Choose the object to be 3D printed, hit the slice pane and save the file in a .CBT format on a USB stick.
- Put the USB stick into the SLA 3D printer, pour photopolymer resin into the container of the 3D printer, turn on the 3D printer, choose print, and hit the triangle sign to start the 3D printing process.
- After the 3D printing process is finished remove the spacer from the print bed, place it for 10 minutes inside the UV LED system, then wash it in water, and store it for the next steps.
- Open the heat press.
- Laminate the polyurethane elastomer film on the spacer (Figure 2K), and place NiTi wire/frame around the spacer and on top of the film. Laminate a second film layer on the wire. Set the temperature at 240 °F (If desired, add additional layers of elastomers between the two layers or between polyurethane and the spacer to avoid adhesion).
- Close the top of the press and lock it; wait for 60 s.
- Repeat the same process of heat pressing for the other side of the NiTi wire frame and the spacer.
- Cut the extra parts of the bonded film with scissors (Figure 2L).
- As an alternative to bonding thermoplastic materials, cover the NiTi wire frame by sewing PET fabrics.
NOTE: Figure 3 shows the frame covered by layers of a hemocompatible polymer. Here, the layers include an additional layer of micropatterned polydimethylsiloxane that is sandwiched between them.
3. Testing frame deployment
- Hold a FR 12 catheter by hand (Figure 4A) and pass it through a dilator and a needle (Figure 4B,C).
- Secure a silicone piece on the holder (Figure 4E).
- Using the needle and dilator, create a hole in the silicone piece (Figure 4E).
- Pass the catheter through the hole gradually (Figure 4F) and retract the dilator and needle.
- Fold the NiTi frame and push it through the proximal end of the catheter (Figure 4G).
- Push the frame toward the distal end of the catheter using the polytetrafluoroethylene (PTFE) rod (Figure 4D).
- Dislodge the first side of the NiTi frame (Figure 4I).
- Retract the catcher (Figure 4H) and dislodge the second side of the NiTi frame on the other side of the silicone rubber (Figure 5).
- Examine the frame under the microscope to check for any type of failure or undesired deformations.
Results
NiTi frames were shape-set into various topologies using low-cost plastic fixtures and hand tools (Figure 1). In protocol steps 1.1 to 1.4 (Figure 1A), NiTi/Cu frames were formed into complex topologies. Following protocol step 1.5, Cu was etched to release the NiTi frames (Figure 1B). Here, the Cu fixture was completely etched away, allowing the NiTi frame to be released using low-cost jigs/fixtures that were 3D printed (step 1.2). After confirming the successful formation of the NiTi, its utility is tested by following protocol sections 2 and 3. Subsequently, a polyurethane film, which is hemocompatible16 (Figure 3), was applied to both sides of the frame following protocol section 2 (Figure 3). The NiTi frame, now covered with polyurethane films, was evaluated as an occluder capable of anchoring on the atrial septum. This functionality was tested by following protocol section 3, assessing the mechanical performance of the NiTi frame's topology upon deployment (Figure 3).
To test the NiTi frame/occluder, protocol section 3 was followed, which simulates deployment through a FR-12 catheter (Figure 4). The NiTi frames maintained their shape post deployment. In protocol section 3, the NiTi frames/occluders were passed through a FR-12 catheter (Figure 1 and Figure 4A) and deployed onto a silicone part representing the interatrial septum. During this process, a dilator (Figure 4B) and a needle (Figure 4C) punctured the silicone membrane (Figure 4E). The catheter was then inserted through the septum (Figure 4F). Using a PTFE rod (Figure 4D), the occluder (Figure 4G) was passed through the catheter (Figure 4H) and eventually dislodged with the rod (Figure 4I). Successful completion of this test allows the device to deploy onto the silicone septum (Figure 5A-C). The most significant aspects of these results are the complex and flexible NiTi frames, combined with their low manufacturing costs and the brief fabrication time they require. Complex shapes (Figure 3) can be formed using copper tubes and low-cost manufacturing techniques. Furthermore, the fabrication time for a functional NiTi frame is notably short, typically taking just about 10 h.
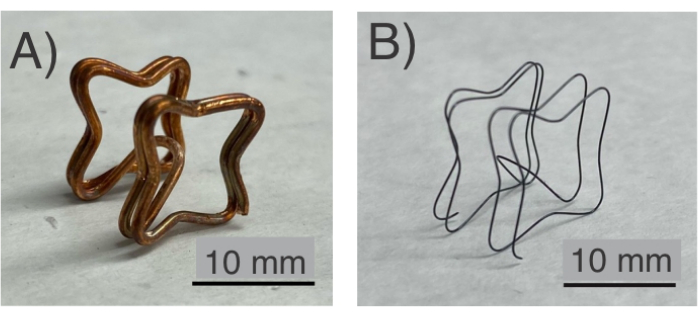
Figure 1: NiTi frame. (A) Before the etching process, the NiTi frame is confined in a Cu fixture. (B) The final sample of the NiTi frame after heat treatment and etching. Please click here to view a larger version of this figure.
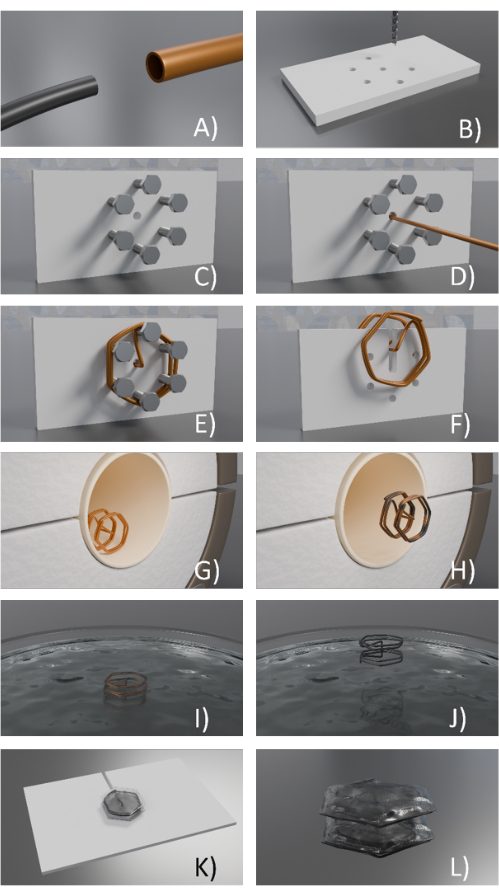
Figure 2: Design of a NiTi frame/prototype. (A) NiTi wire is aligned in a cooper tube, (B) 3D printed/fixture is used to form holes being drilled on the 3D printed fixture (holes could be also designed in the part in advance), (C) Screws are placed in the holes, (D) the NiTi /Cu wire is passed through the central hole, (E) The NiTi /Cu wire bent around the screws, (F) The NiTi /Cu frame is removed form the fixture, (G) The frame is placed in a furnace, (H) The copper/ NiTi frame is removed after the heat treatment process, (I) the copper/NiTi frame is immersed in the etchant, (J) the NiTi frame is released after copper was etched, (K) Thermoplastic polyurethane covers the top loop of NiTi wire, while placed on a spacer before heat pressing, and (L) Thermoplastic polyurethane is heat pressed and bounded on both sides of the frame and the extra parts are cut. Please click here to view a larger version of this figure.
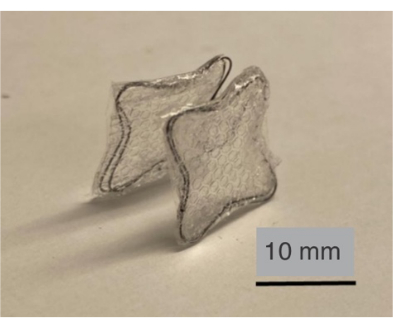
Figure 3: NiTi frame covered by a hemocompatible elastomer. Here, polyurethane film serves as an occluder/film to be anchored on a septum (an additional polymeric film is sandwiched between the polyurethane films). Please click here to view a larger version of this figure.
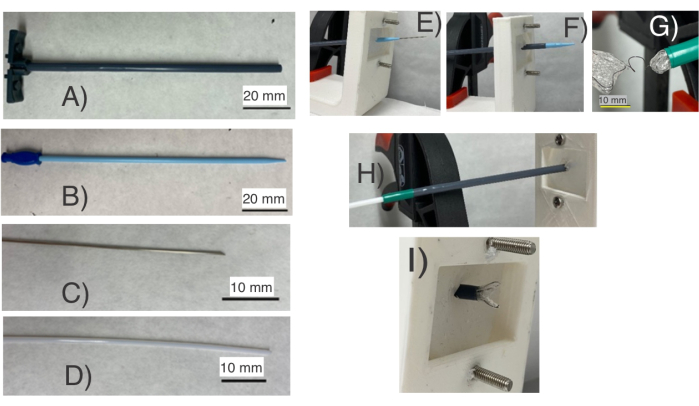
Figure 4: Testing of NiTi frames/occluder for septal defect occlusion using a catheter setup for a primary deployment test. It consists of (A) a FR-12 catheter, (B) a dilator, (C) a needle, and (D) a PTFE rod. (E) The dilator and needle are used to puncture a silicone membrane representing the septum, and the catheter is inserted through the (F) septal membrane (Silicone). Next, (G) the occluder is passed through the catheter and (H) dislodged using the PTFE rod. This setup assesses if the frame has the correct shape for compression and (I) self-expansion necessary for deployment through a Fr-12 catheter. The test assists in finding the NiTi wires topologies suitable for deployment. Please click here to view a larger version of this figure.
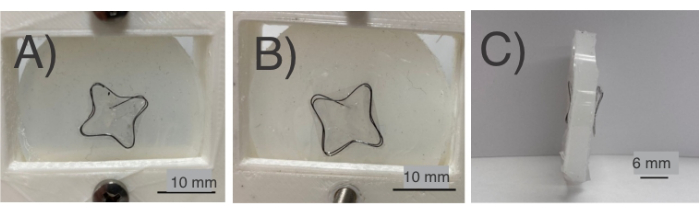
Figure 5: NiTi frames after placing in the silicone (mimicking the interatrial septum). Various parts are shown such as (A) front view of the NiTi frame placed in the silicone sample; (B) back view of the NiTi frame placed in the silicone sample; (C) side-view of the NiTi frame placed in the silicone septum. The frame and the film successfully covered both sides. Please click here to view a larger version of this figure.
Discussion
In this protocol, multiple steps require meticulous attention such as the heat treatment (annealing), etching, and design of 3D-printed fixtures. Large variations in temperature from 500 °C 17 or the annealing time of NiTi can be detrimental to the superelasticity of the NiTi wire and to achieving the desired shapes18. Heat treatment with inaccurate conditions (temperature and time) can also lead to a loss of the superelastic property19. The etching process requires a sufficient amount of ammonium persulfate to release the frames with the correct shapes20. Pulling out the NiTi frame before the etching process is complete may result in undesirable deformations of the NiTi frame. Finally, 3D printed fixtures can assist in bending the NiTi/wire frames and improve the performance of frames in the deployments. They often require multiple iterations to adjust the flexibility of the frames.
One important variable for the frame fabrication process is the heat treatment's temperature, which can vary depending on the type of furnace used. In the current experiments, the Applied Test Systems Series 3210 Split Furnace was used. Here, the furnace temperature reaches 500 °C and then heat treat samples for 3 min. However, using different furnaces or NiTi wire types may require adjustments in the temperature and heating duration. Additionally, one can explore modifications in the use of copper tubes with various diameters based on the thickness of the NiTi wires. Overly thick copper tubes require excessive force. In such instances, employing hand-held wire-bending tools can assist in shaping NiTi wires.
Another challenge is maintaining the ideal temperature during the heat treatment. A small variation can lead to undesirable mechanical performance; however, in a traditional method, a metal fixture can improve the uniformity of temperature distribution. Achieving tight tolerances in the NiTi wires can be challenging in comparison with a traditional metal fixture. If one works with larger samples/frames, larger furnaces will be needed, which can be costly. Further, if a large amount of copper exists for the etching process, etching may need larger amounts of etchants with additional cost20. Finally, this method, unlike the traditional method, requires a chemical etching process with additional processes and costs. Achieving very small curvature radii can be challenging due to the use of copper tubes.
Following protocol section 1 enabled the fabrication of complex shapes at a minimal cost. This adaptability is advantageous for prototyping and customizing medical implant fabrication. The proposed technique eliminates the need for machined metal fixtures commonly obtained with existing methods, which is cost-prohibitive. The method expedites the prototyping of NiTi structures. Incorporating low-cost 3D-printed polymers and eliminating the need for fixtures makes the process more time-efficient compared to traditional methods21. This method is especially suitable for a small laboratory setting for research on new structures.
Protocol sections 2 and 3 can be used to rapidly eliminate or verify frames fabricated using protocol 1. As shown in Figure 4 and Figure 5, these protocols can be used to assess, upon addition of other parts to a frame, if the implant can be deployed through a catheter or not. While these steps (as shown in Figure 5) would justify thorough studies for regulatory/safety tests12,13, a failure (broken or deformed parts) can eliminate the unsatisfactory frame designs. For instance, in Figure 5, it is observed that all basic components of the prototype/implant or the delivery system (films, NiTi frame, and the catheter) did not show visual signs of failure. This information can be used as feedback for the iteration of different frames through protocol section 1, which in turn allows for the rapid development of complex topologies/designs for NiTi frames.
This method paves the way for future research, including prototyping and studying the mechanics of NiTi wires/frames to be used in self-expanding medical devices. Natural protection of copper during annealing enables the use of techniques like salt bath annealing without concerns about chemical contamination of NiTi. The demonstrated method can integrate with other manufacturing technologies such as computerized wire-forming processes. This integration will significantly reduce the cost of prototyping NiTi frames/structures for medical devices.
Disclosures
The authors declare no conflicts of interest.
Acknowledgements
Research reported in this publication was supported by the National Institute of Biomedical Imaging and Bioengineering of the National Institutes of Health under Award Number R21EB030654. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. S. Alaie and J. Mata also thank the Department of Mechanical and Aerospace Engineering and the College of Engineering at New Mexico State University for their support. The authors thank Oscar Lara and Angel de Jesus Zuniga Ramirez for their contributions in generating Figure 2 and editing the references. The authors also thank Andrea Gonzalez Martinez and Jesus Armando Gil Parra for their contributions to the video demonstrations.
Materials
| Name | Company | Catalog Number | Comments |
| 304 SS Hypotubes Generic Name: Needle | Tegra Medical | ||
| 3D printed frame for testing Generic Name: PLA filament | R3D | ||
| 3D printed polymeric part for heat press Generic Name: PLA filament | R3D | ||
| Ammonium Persulfate Generic Name: Ammonium Persulfate | Sigma-Aldrich | ||
| Chronoflex AR 22% Generic Name: Polyurethane | AdvanSource biomaterials | aromatic polycarbonate urethane elastomer | |
| Copper Web Type Electrodes (1.00 mm x 400 mm) Generic Name: Copper Tube | Holepop edm supplies &electrodes | ||
| Dilator Generic Name: Dilator | QOSINA | ||
| Ecoflex 00-30 Generic Name: Ecoflex 00-30 | Smooth-on | silicone | |
| Fr 12 or 13 Catheter Generic Name: Sheath | QOSINA | ||
| Nickel Titanium Wire (0.008) Generic Name: NiTi Wire | Malin Co. | ||
| PTFE Teflon rod 1/8" Diameter x 36" Generic Name: Polytetrafluoroethylene | Sterling Seal & Supply, Inc. (STCC) | ||
| Tecoflex Generic Name: Thermoplastic Polyurethane | Lubrizol | aliphatic polyurethane elastomer | |
| Trichloro(1H,1H,2H,2H-tridecafluoro-n-octyl)silane Generic Name: C8H4Cl3F13Si | Sigma-Aldrich | ||
| Dimethylacetamide (DMAC) Generic Name: Dimethylacetamide | Sigma-Aldrich | ||
| SOLIDWORKS Generic Name: Proprietary CAD software | Dassault Systèmes | ||
| FreeCAD Generic Name: Open Source CAD software | freecad.org | ||
| ABS Like Photopolymer Resin Generic Name: Photopolymer Resin | ELEGOO |
References
- Smith, S., Hodgson, E. Shape setting nitinol. Proc of the Mater Process Med Devices Conf. , 266-270 (2004).
- Kapoor, D. Nitinol for medical applications: A brief introduction to the properties and processing of nickel titanium shape memory alloys and their use in stents. Johnson Matthey Tech Rev. 61 (1), 66-76 (2017).
- Viscuso, S., Gualandris, S., De Ceglia, G., Visentin, V. Shape memory alloys for space applications. Shape Mem Alloy Eng. , 609-623 (2021).
- Liu, X., Wang, Y., Yang, D., Qi, M. The effect of ageing treatment on shape-setting and superelasticity of a nitinol stent. Mater Charact. 59 (4), 402-406 (2008).
- Dulal, H., Swan, T., Al'aref, S. J., Alaie, S. Low-cost prototyping of nitinol wires/frames using polymeric cores and sacrificial fixtures with application in individualized frames anchoring through the atrial septum. Sci Rep. 13, 21853 (2023).
- Alaie, S., Robinson, S. S., Amiri Moghadam, A. A., Auge, J. Advanced manufacturing of patient specific occluders for the left atrial appendage with minimally invasive delivery. Adv Eng Mate. 22, 1901074 (2020).
- Robinson, S. S., et al. Patient-specific design of a soft occluder for the left atrial appendage. Nat Biomed Eng. 2, 8-16 (2018).
- Amiri Moghadam, A. A., et al. Toward development of inflatable stents with application in endovascular treatments. Adv Funct Mater. 28 (51), 9 (2018).
- Chen, Y. . Design, parameter optimization and in vitro evaluation of implantable medical devices. , (2018).
- Bernini, M., et al. Oversizing of self-expanding nitinol vascular stents-a biomechanical investigation in the superficial femoral artery. Journal of the Mechanical Behavior of Biomedical Materials. 132, 105259 (2022).
- Bernini, M., et al. Surface finishing of n itinol for implantable medical devices: A review. J Biomed Mater Res Part B Appl Biomater. 110 (12), 2763-2778 (2022).
- Funk, K. A., Hampshire, V. A., Schuh, J. C. Nonclinical safety evaluation of medical devices. Toxicol Pathol. , 95-152 (2018).
- Non-clinical engineering tests and recommended labeling for intravascular stents and associated delivery systems - Guidance for industry and FDA staff. U.S. Food and Drug Administration Available from: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/non-clinical-engineering-tests-and-recommended-labeling-intravascular-stents-and-associated-delivery (2018)
- Rokicki, R., Hryniewicz, T., Pulletikurthi, C., Rokosz, K., Munroe, N. Towards a better corrosion resistance and biocompatibility improvement of nitinol medical devices. J Mat Eng Perform. 24, 1634-1640 (2015).
- Hryniewicz, T., Rokicki, R. Modification of nitinol biomaterial for medical applications. World Scientific News. (96), 35-58 (2018).
- Handa, H., et al. Hemocompatibility comparison of biomedical grade polymers using rabbit thrombogenicity model for preparing nonthrombogenic nitric oxide releasing surfaces. J Mater Chem B. 2 (8), 1059-1067 (2014).
- Li, P., Wang, Y., Meng, F., Cao, L., He, Z. Effect of heat treatment temperature on martensitic transformation and superelasticity of the Ti49Ni51 shape memory alloy. Materials. 12 (19), 2539 (2019).
- Duerig, T., Pelton, A., Stöckel, D. An overview of nitinol medical applications. Mat Sci and Eng: A. 273, 149-160 (1999).
- Kwok, D., Schulz, M., Hu, T., Chu, C., Chu, P. Surface treatments of nearly equiatomic niti alloy (nitinol) for surgical implants, biomedical engineering. Trends in Mater Sci. , 269-282 (2011).
- Williams, K. R., Gupta, K., Wasilik, M. Etch rates for micromachining processing-part ii. II. J of Microelectromech Syst. 12 (6), 761-778 (2003).
- Yip, M. C., et al. Low-cost and rapid shaping of nitinol for medical device prototyping. ASME Open J of Eng. 2, 021027 (2023).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved