Method Article
An Ex Vivo Tissue Culture Model of Cartilage Remodeling in Bovine Knee Explants
In This Article
Summary
Here, we present a protocol describing isolation and culturing of cartilage explants from bovine knees. This method provides an easy and accessible tool to describe tissue changes in response to biological stimuli or novel therapeutics targeting the joint.
Abstract
Ex vivo culture systems cover a broad range of experiments dedicated to studying tissue and cellular function in a native setting. Cartilage is a unique tissue important for proper function of the synovial joint and is constituted by a dense extracellular matrix (ECM), rich in proteoglycan and type II collagen. Chondrocytes are the only cell type present within cartilage and are widespread and relatively low in number. Altered external stimuli and cellular signalling can lead to changes in ECM composition and deterioration, which are important pathological hallmarks in diseases such as osteoarthritis (OA) and rheumatoid arthritis.
Ex vivo cartilage models allow 1) profiling of chondrocyte mediated alterations of cartilage tissue turnover, 2) visualizing the cartilage ECM composition, and 3) chondrocyte rearrangement directly in the tissue. Profiling these alterations in response to stimuli or treatments are of high importance in various aspects of cartilage biology, and complement in vitro experiments in isolated chondrocytes, or more complex models in live animals where experimental conditions are more difficult to control.
Cartilage explants present a translational and easily accessible method for assessing tissue remodeling in the cartilage ECM in controllable settings. Here, we describe a protocol for isolating and culturing live bovine cartilage explants. The method uses tissue from the bovine knee, which is easily accessible from the local butchery. Both explants and conditioned culture medium can be analyzed to investigate tissue turnover, ECM composition, and chondrocyte function, thus profiling ECM modulation.
Introduction
Chondrocytes produce and maintain the cartilage matrix. In order to study the biology of chondrocytes and how they and the surrounding ECM react to external stimuli, it is crucial to interrogate them in their native environment1,2. Studying cartilage tissue turnover is important to augment the understanding of the underlying mechanisms in joint diseases such as OA, a disease for which there is currently no disease modifying treatment. Consequently, there is a significant need for better translation models2.
Ex vivo characterization of cell and tissue effects is essential to complement other preclinical models, both in vitro, such as chondrocyte monolayer cultures, and in vivo, such as surgery-induced OA models or the autoimmune collagen-induced arthritis model (CIA). Numerous studies have highlighted the differences between how cells behave in 2D monolayer cultures and 3D structures or in their native tissue3,4. Many cells in 2D layers adopt unnatural morphologies, including differences in cell polarity and tissue attachment, resulting in both visual and functional differences in cells within native tissues5. The differences are also apparent in the functionality of the cells, which may shift protein expression, leading to profoundly altered differentiation patterns, regulatory machinery, and cell functionality5,6,7,8.
The cartilage ECM consists mainly of type II collagen providing a matrix framework, and aggrecan, a proteoglycan that helps retain fluid within the tissue. Other matrix molecules such as collagen type IV, VI, IX, X, XI, XII, fibronectin, cartilage oligomeric protein (COMP), biglycan, decorin, and perlecan are also present9.
While the aetiology of OA remains unclear10,11, the onset of the disease is believed to be caused by imbalances in tissue turnover and repair processes12,13. The degradation of the articular cartilage is a hallmark of OA. Cartilage-resident chondrocytes or cells in the surrounding tissues increase their release of cytokines, stimulating elevated production of proteinases such as matrix metalloproteinases (MMPs) and aggrecanases, which increase degradation of cartilage ECM14. This degradation results in the release of small unique protein fragments called neo-epitopes, which can be quantified in serum, urine, or culture medium15. Upon formation and maturation of collagen, so-called profragments are also released; these can be quantified as a measure of matrix production16.
The aim of this protocol is to establish an ex vivo cartilage model to compare the effect of stimulation and/or drug treatment on ECM tissue turnover. Cartilage turnover is profiled by measuring matrix-derived neo-epitope biomarkers directly in the conditioned culture medium using ELISA: AGNx1 (reflecting aggrecanase activity), C2M (reflecting matrix MMP activity), and ProC2 (reflecting type II collagen formation). The findings can be verified by histological staining of the ECM, which also visualizes the organization of chondrocytes in the individual explants. The described protocol can be used to test the effect of novel treatments on chondrocyte function and cartilage ECM turnover. A number of studies have used cartilage explants to describe biological processes or the effect of intervention on cytokine-challenged explants using quantitative histological or immunohistochemical approaches, mRNA, protein expression, or proteomics2,17,18. However, these protocols are outside the scope of the current manuscript.
Protocol
1. Tissue isolation
- Tissue sourcing
- Perform the entire tissue sourcing section outside a laminar flow hood in an aseptic environment.
- From the local slaughterhouse, obtain an entire fresh bovine tibiofemoral knee joint from calves between 1.5 and 2 years of age.
- Gently dissect the calf knee by first removing the excess flesh, uncovering the condyles, meniscus, tendons, and synovial membrane. Cut the tendons and synovial membrane, allowing the joint to dismember. Remove the meniscus to expose the femoral condyles.
- Isolate explants from the load-bearing area of the femoral condyles using a 3 mm biopsy puncher and release them from the articular surface by cutting with a scalpel parallel to and as close to the subchondral bone as possible. The hard structure of the subchondral bone should ensure that explants do not contain calcified matrix. Strive for explants with uniform height.
- Immediately store and mix the explants in DMEM/F12- GlutaMAX + 1% P/S culture medium in a 50 mL tube or Petri dish. Do not mix explants from different cow knees but keep separate for each study.
- Tissue culturing
- Transfer the explants to a sterile 96-well plate in a laminar flow hood.
- Wash the explants 3 times in culture medium or PBS and culture them in 200 μL of culture medium per well until the start of the experiment. Use a washout period of 1 day to synchronize biopsy cellular activity and passive biomarker release.
- Culture the explants up to 10 weeks in a 37 °C incubator with 5% CO2. Place all replicates within each group diagonally in the culture plate to minimize the variation induced by evaporation. To further avoid evaporation of the supernatant, add PBS to the outer wells of the culture plate.
2. Bovine cartilage explant treatment and assessment of metabolic activity
- Culture medium change and treatment
- Change the culture medium every 2-3 days in a laminar flow hood.
- If applying any treatments, prepare these prior to changing the medium. Prepare the treatments to the wanted concentration in the explant wells by dilution in the culture medium.
- Gently remove the supernatant from each well and transfer it to a new 96 well plate. Store the supernatant with sealing tape at −20 °C for biomarker analysis of tissue turnover and protein expression.
- Immediately add 200 µL of fresh culture medium or treatment per well. Do not let the explants dry out during the medium change and ensure that all the explants are completely submerged in the new medium.
- Resazurin staining
- Measure metabolic activity once weekly as an indirect measurement of cell viability. The resazurin test is an easy way to assess if the metabolic activity of the explants deteriorates for an individual explant due to cell death or cellular changes. Explants in culture medium alone have a relatively stable resazurin reading throughout the experiment period.
- Make a solution of culture medium with 10% resazurin.
- Harvest the supernatant as described in step 2.1.3.
- Immerse the explants in 10% resazurin solution for 3 h at 37 °C or until the supernatants turn purple. Include 4 wells without explants as background controls.
- Transfer the conditioned and background control resazurin solution to a black microtiter plate and measure fluorescence at 540 nm excitation/590 nm emission.
- Wash thoroughly 3 times in culture medium or PBS and submerge the explants in wash medium for 5-10 min to allow the resazurin to completely diffuse out. Add new culture medium or treatments if used.
3. Termination, fixation, and sample storage
- Termination of culturing period
- Measure the metabolic activity as described in step 2.2. Add 200 µL of PBS per well.
- Fixation and storage
- Remove the PBS, add 200 µL of formaldehyde per well, and leave for 2 h at room temperature.
- Dispose of the formaldehyde and add 200 µL of PBS per well. Cover the plate with sealing tape, and store at 4 °C for histochemical analysis. We recommend performing histochemical analysis within 3 months.
4. Tissue turnover biomarkers (ELISA)
- Indirect competitive ELISAs
- Coat a streptavidin-plate with the specific biotinylated assay target-peptide diluted 1:100 in assay buffer (100 µL per well) for 30 min at 20 °C.
- Wash 5 times with standard washing buffer and add sample-supernatant (20 µL per well) together with primary monoclonal antibody against the assay target-peptide diluted 1:93.3 for ProC2 and 1:100 in assay buffer for AGNx1 (100 µL per well) and incubate for 2 h at 20 °C with shaking for ProC2 and 3 h at 20 °C for AGNx1.
NOTE: The sample volume is directly taken from the stored supernatant plates. If the measured concentration is out of the assay measuring range, dilute the supernatant in a v-bottomed dilution plate in PBS or assay buffer. - Wash 5 times with standard washing buffer and incubate with peroxidase-labeled secondary antibody diluted 1:100 in assay buffer (100 µL per well) for 1 h at 20 °C.
- Wash 5 times with standard washing buffer and incubate with shaking for 15 min in the dark at 20 °C with tetramethylbenzidine (TMB) as a peroxidase substrate (100 µL per well).
- End the reaction with standard stop solution, 0.1 M H2SO4 (100 µL per well).
- Read the colorimetric reaction at 450 nm absorbance using a reference absorbance at 650 nm on a standard laboratory plate reader.
- Direct Competitive ELISAs for measurement of the cartilage tissue turnover in the supernatant
NOTE: This quantifies C2M.- Coat a streptavidin-plate with specific biotinylated assay target-peptide diluted 1:100 in assay buffer (100 µL per well) for 30 min at 20 °C.
- Wash 5 times with washing buffer and add sample-supernatant together with 100 µL of peroxidase-labeled monoclonal antibody against the assay target-peptide diluted 1:100 in assay buffer (20 µL per well). Incubate for 20 h at 2–8 °C with shaking.
NOTE: The sample volume is directly taken from the stored supernatant plates. If the measured concentration is out of the assay measuring range, dilute the supernatant in a v-bottomed dilution plate in PBS or assay buffer. - Wash 5 times with standard washing buffer and incubate with shaking for 15 min in the dark at 20 °C with TMB as a peroxidase substrate (100 µL per well).
- End the reaction with standard stop solution, 0.1 M H2SO4 (100 µL per well).
- Read the colorimetric reaction at 450 nm absorbance with a reference absorbance at 650 nm on a standard laboratory plate reader.
- AGNx1
- Quantify aggrecan degradation by measuring the release of the AGNx1 neo-epitope. This indirect competitive ELISA assay targets the aggrecan C-terminal peptide (NITEGE373) generated by ADAMTS-4 and 5 cleavage. The monoclonal antibody recognizes all fragments with an exposed NITEGE epitope. The experimental details of the assay have been published elsewhere19.
- ProC2
- Quantify type II collagen formation by measuring the release of the profragment of type II collagen. This indirect competitive ELISA assay targets the epitope of the PIIBNP propeptide (QDVRQPG) generated by N-propeptidases during trimming of newly synthesized type II collagen. The experimental details of the assay have been published elsewhere16.
- C2M
- Quantify type II collagen degradation by measuring the release of the C2M neo-epitope fragment. This direct competitive ELISA recognizes the MMP-cleaved C-terminal peptide (KPPGRDGAAG1053). This assay differs from AGNx1 and ProC2 as it is the primary antibody that is peroxidase-labeled and thus, used as detector. The experimental details of the assay have been published elsewhere20.
5. Histological analysis
- Infiltration, embedding, and cutting
- Place the fixated explants (see step 3.2) into individually labeled cassettes. Include both a label within the cassette and label cassettes to ensure identification.
- Transfer the cassettes containing explants to a tissue processor machine. Then infiltrate the explants with paraffin in a series of dehydration and paraffin infiltration steps.
- Dehydrate with 96% ethanol for 90 min with no temperature adjustment. Repeat this step 3 times.
- Clear the ethanol with toluene for 90 min with no temperature adjustment. Repeat this step 2 times.
- Clear the ethanol with toluene for 90 min at 60 °C.
- Infiltrate with paraffin wax for 30 min at 60 °C.
- Infiltrate with paraffin wax for 60 min at 60 °C.
- Infiltrate with paraffin wax for 90 min at 60 °C.
- For each step, add the solutions into the sample chamber with slow pump-out and pump-in flows under 33–34 kPa. Run the infiltration process in a pressure/vacuum cycle with a maximum vacuum of −65 to −70 kPa.
- Following infiltration, place the cassettes on a heating block to allow careful removal of the explants from the cassette. Gently embed the infiltrated explants into individual paraffin blocks. With heated forceps, place the explants with the superficial articular cartilage and subchondral bone sides perpendicular to the cutting surface, ensuring visualization of the different cartilage layers within each specimen section.
- Cut 5 µm sections of cooled paraffin-blocks with embedded explants on a microtome. Transfer the cut sections to a cold-water bath. If necessary, sections can be separated using either a scalpel or a cover glass.
- Using an uncoated glass slide carefully, transfer the sections to a warm water bath (50 °C), where the sections unfold. Lift each section onto a labeled cover slide and place on a hot plate for 30 min.
- Place the slides in a basket and incubate at 60 °C for 1 h and then keep them overnight at 37 °C. Hereafter, store slides in closed containers at 4 °C until staining.
- Safranin O/Fast Green staining and visualization
- Place the slides to be stained in a basket and incubate the slides at 60 °C for 1 h.
- Prepare and filter all reagents with a 0.45 mm filter.
- In preparation for staining, pour the filtered reagents in beakers to a volume that allows the solutions to completely cover the slides when submerging the basket. The beakers used required a volume of 250 mL to cover the slides.
- Deparaffinize the melted slides by submerging the basket in toluene for 10 min twice, 99% ethanol for 2 min twice, 96% ethanol for 2 min twice, and 70% ethanol for 2 min twice. Then, hydrate the slides in water for 2 min.
- Stain the deparaffinized and hydrated slides by submerging the basket in Weigert’s Iron Hematoxylin solution (pH 1.5) for 10 min, dip in 1% HCl once, and rinse with running tap water for approximately 5 min or until excess color has washed away.
- Next, stain in 0.05% Fast Green solution (pH: 5.75) for 5 min, dip in 1% CH3COOH once, and stain in 0.1% Safranin O (pH: 6.5) for 20 min.
- Dehydrate and clear the slides by dipping twice in 70% ethanol, 96% ethanol for 2 min twice, 99% ethanol for 2 min twice, and toluene 2 min twice.
- Mount the uncoated glass slides with resinous medium covering the histology slides.
Results
Bovine full-depth explants were isolated, cultured, and treated for 3 weeks (Figure 1). The culture medium was changed with the addition of treatment 3 times per week. Once weekly, metabolic activity was measured by the resazurin assay. Biomarkers of ECM turnover were measured in the supernatant harvested from the culture plate 3 times per week. Explants were divided into 4 groups for treatment: 1) Oncostatin M and TNFα (O+T); 2) O+T + GM6001 (GM6001); 3) Insulin like Growth Factor-1 (IGF-1); and 4) a control without treatment (w/o).
Metabolic activity.
For all four groups, the metabolic activity was relatively stable throughout the 3 weeks (Figure 2A). There was a tendency for IGF-1 to increase the metabolic activity slightly above the w/o group and for the O+T groups to decrease it. The resazurin assay was used to easily assess the activity of the chondrocytes in each explant and to indirectly assess cell viability without extracting explants from the experiment. If an explant shows a substantial drop in metabolic activity during the experiment, the explant can be excluded from further analysis.
Catabolic treatment.
O+T was applied 3 times weekly to the culture wells to investigate O+T-mediated cartilage degradation (Figure 3, Figure 4). MMP-mediated type II collagen degradation and aggrecanase-mediated aggrecan degradation were assessed by C2M and AGNx1 ELISAs. O+T increased type II collagen degradation from days 7-21 (Figure 3A) and aggrecan degradation from days 3-14 (Figure 4A) compared to the w/o group. When adding GM6001 (a broad-spectrum MMP-inhibitor) in combination with O+T treatment, the O+T-mediated C2M release was blocked (Figure 3A,B). A decreased AGNx1 release was observed when adding GM6001 on days 3-7, but the AGNx1 release peaks on day 10 at similar levels to the O+T group (Figure 4A), indicating the GM6001 only decreases aggrecan degradation to a limited extent. This pattern in AGNx1 and C2M release is the general picture observed in the bovine cartilage model stimulated with O+T. First, AGNx1 is released from approximately day 3 and peaks at days 10-14, representing an early degradation of aggrecan. Next, after 2 weeks of culturing with O+T, type II collagen degradation is observed as measured by the C2M biomarker.
Anabolic treatment.
To investigate how anabolic stimulation modulates the cartilage ECM turnover, Insulin like Growth Factor-1 (IGF-1) was applied 3 times weekly to bovine full-depth explants. The effect of IGF-1 on the cartilage explants was mainly observed on measurements of type II collagen formation, assessed by ProC2, as expected for anabolic stimuli (Figure 5). Day 0 in this model always shows high ProC2 measurements, perhaps as a reaction to the extraction of samples. These high levels decrease substantially and level out from days 7-21. When treating with IGF-1, the ProC2 levels decrease less than those observed in the w/o group, indicating that IGF-1 stimulates type II collagen formation from day 7 (Figure 5B). The ProC2 graphs also show the biological variation of cows. Explants from two cows were used in these experiments with 6 explants per cow per group. The first cow had thicker cartilage and generated larger explants, resulting in higher ProC2 levels at baseline, whereas the second cow was smaller with thinner cartilage, resulting in lower ProC2 levels at baseline. For the w/o, IGF-1, and O+T groups, the ProC2 levels depicted represent the mean of the explants from both cows, but GM6001 was measured only in the second cow with thinner explants. Thus, the GM6001 group started with lower ProC2 levels at day 0, which is evident in the ProC2 area under the curve (AUC) (Figure 5C). Normalization of the ProC2 values to the day 0 levels takes the biological variance into account, thus showing the effectiveness of the treatment (Figure 5B,D).
Safranin O and Fast Green histological stainings were performed to visualize the proteoglycan content and cartilage structure of the explant throughout the experiment (Figure 6). On days 0, 7, 14, and 21, explants from the w/o, IGF-1, and O+T group were fixated for histological staining (Figure 6). The w/o and IGF-1 group appeared to have similar Safranin O staining intensity to the day 0 explant throughout the experiment, which correlates with biomarker results showing that neither of the two groups increased AGNx1 release (Figure 4). Treatment with O+T resulted in substantial proteoglycan content loss on day 7 and complete loss on day 21. Furthermore, the Fast Green staining intensity decreases from days 14-21, indicating a collagen loss in alignment with the C2M results.
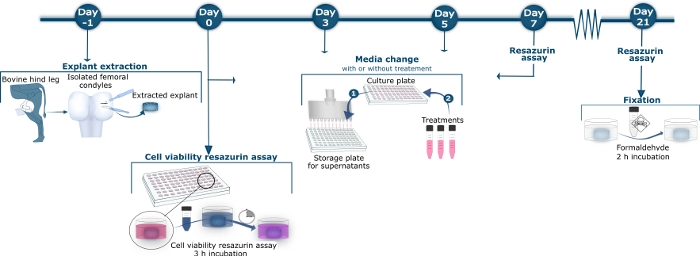
Figure 1: Schematic overview of bovine cartilage method.
On day −1, bovine femoral condyles were isolated from the hind tibiofemoral joint. Full-depth cartilage explants were released from the condyles with a scalpel and biopsy puncher. The extracted explants were washed and transferred to a sterile 96 well culture plate. On day 0, 3, 5, 7, 10, 12, 14, 17, 19, and 21, the supernatant was harvested from the culture plate, transferred to a storage plate, and kept at −20 °C, as illustrated in Medium Change Step 1. The stored supernatant was later thawed for measurement of the tissue turnover biomarkers by specific ELISA assays. In Medium Change Step 2, after removing the supernatant, new culture medium containing the different treatments or no treatment for control explants was applied. On day 0, 7, 14, and 21, the explants were incubated with 10% resazurin solution for 3 h after harvesting the supernatant. The 10% resazurin supernatant was transferred to a black 96-well plate where the colorimetric reaction was measured. The culture wells were washed 3 times before new culture medium with or without treatment was added to the explants as shown in Medium Change Step 2. On Day 21, after harvest of supernatant and resazurin measurement, the explants were fixated by incubation with formaldehyde for 2 h. Please click here to view a larger version of this figure.
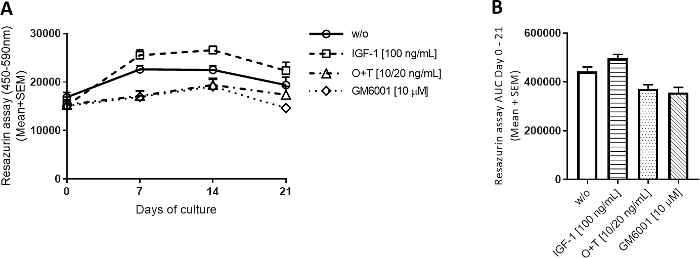
Figure 2: Metabolic activity measured by resazurin.
Bovine full-depth cartilage explants were isolated and cultured for 3 weeks. Culture medium was changed with the addition of new treatment 3 times per week (n = 12 explants from 2 cows). Treatment consisted of IGF-1 [100 ng/mL], OSM + TNFα (O+T) [10/20 ng/mL], and O+T [10/20 ng/mL] + GM6001 (GM6001) [10 µM]. A control group without treatment (w/o) was included. For the w/o, IGF-1, and O+T group, the mean and standard error of the mean (SEM) of 12 replicates from 2 cows (6 replicates per cow) are shown. For the GM6001 group, the mean and SEM of 6 replicates from 1 cow are shown. (A) Metabolic activity measured by resazurin. (B) Area under the curve (AUC) for days 0-21 for metabolic activity graphs shown in (A). ****p > 0.0001. Please click here to view a larger version of this figure.
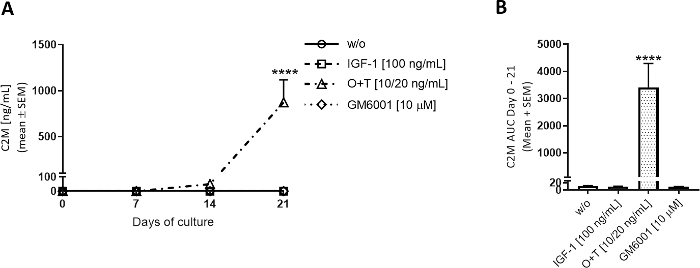
Figure 3: Type II collagen degradation measured by C2M.
Bovine full-depth cartilage explants were isolated and cultured for 3 weeks. Culture medium was changed with the addition of new treatment 3 times per week. Treatment consisted of IGF-1 [100 ng/mL], OSM + TNFα (O+T) [10/20 ng/mL], and O+T [10/20 ng/mL] + GM6001 (GM6001) [10 µM]. A control group without treatment (w/o) was included. For the w/o, IGF-1, and O+T groups, the mean and SEM of 12 replicates from 2 cows (6 replicates per cow) are shown. For the GM6001 group, the mean and SEM of 6 replicates from 1 cow are shown. (A) C2M measurements. Statistical significance level of w/o was calculated by repeated measures (RM) two-way ANOVA with Sidak’s multiple comparison test. (B) AUC for days 0-21 for C2M graphs shown in (A). Statistical significance was calculated by the Kruskal-Wallis test with Dunn’s multiple comparison test. ****p > 0.0001. Please click here to view a larger version of this figure.

Figure 4: Aggrecan degradation measured by AGNx1.
Bovine full-depth cartilage explants were isolated and cultured for 3 weeks. Culture medium was changed with addition of new treatment 3 times per week. Treatment consisted of IGF-1 [100 ng/mL], OSM + TNFα (O+T) [10/20 ng/mL], and O+T [10/20 ng/mL] + GM6001 (GM6001) [10 µM]. A control group without treatment (w/o) was included. For the w/o, IGF-1, and O+T group, the mean and SEM of 12 replicates from 2 cows (6 replicates per cow) are shown. For the GM6001 group, the mean and SEM of 6 replicates from 1 cow are shown. (A) AGNx1 measurements. Statistical significance level of w/o was calculated by RM two-way ANOVA with Sidak’s multiple comparison test. (B) AUC for days 0-21 for AGNx1 graphs shown in (A). Statistical significance was calculated by the Kruskal-Wallis test with Dunn’s multiple comparison test. **p > 0.01, ***p > 0.001, ****p > 0.0001. Please click here to view a larger version of this figure.
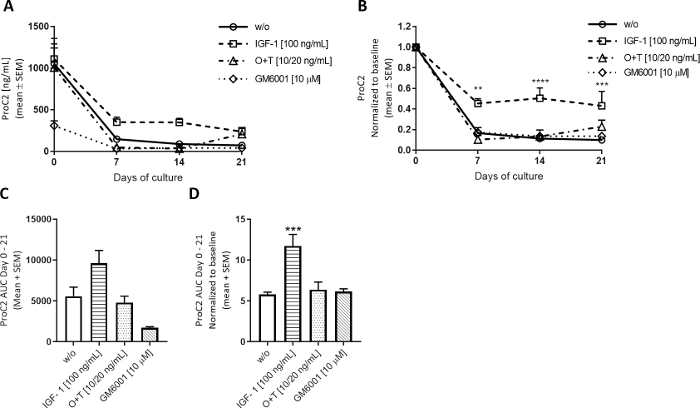
Figure 5: Type II collagen formation measured by ProC2.
Bovine full-depth cartilage explants were isolated and cultured for 3 weeks. Culture medium was changed with the addition of new treatment 3 times per week. Treatment consisted of IGF-1 [100 ng/mL], OSM + TNFα (O+T) [10/20 ng/mL], and O+T [10/20 ng/mL] + GM6001 (GM6001) [10 µM]. A control group without treatment (w/o) was included. For the w/o, IGF-1, and O+T group, the mean and SEM of 12 replicates from 2 cows (6 replicates per cow) are shown. For the GM6001 group, the mean and SEM of 6 replicates from 1 cow re shown. (A) ProC2 measurements from days 0-21. (B) ProC2 values normalized to day 0 measurements for each individual explant. The ProC2 results often benefit from day 0 normalization to uncover the treatment effect that may be disguised by the high biomarker levels on day 0. In A and B, the statistical significance level was calculated by RM two-way ANOVA with Sidak’s multiple comparison test. (C) AUC for days 0-21 for ProC2 graphs shown in (A). (D) AUC for days 0-21 for day 0 normalized ProC2 graphs shown in (B). In C and D, the statistical significance was calculated by the Kruskal-Wallis test with Dunn’s multiple comparison test. **p > 0.01, ***p > 0.001, ****p > 0.0001. Please click here to view a larger version of this figure.
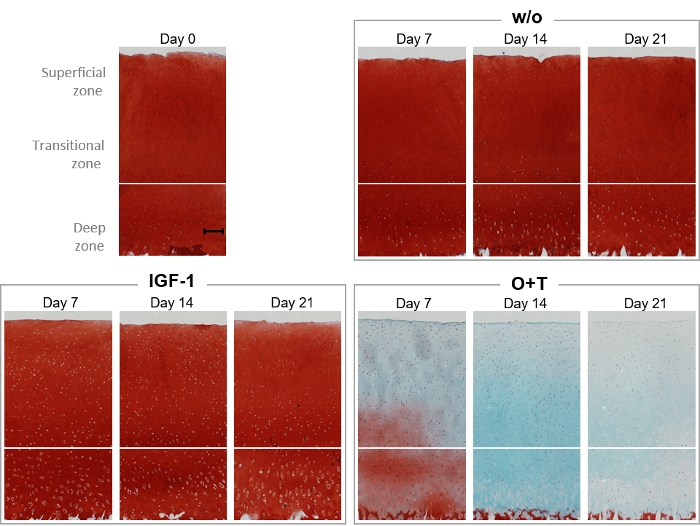
Figure 6: Histological visualization of proteoglycan content by Safranin O/Fast Green staining.
Bovine full-depth cartilage explants were isolated and cultured for 3 weeks. Culture medium was changed with the addition of new treatment 3 times per week. Treatment consisted of IGF-1 [100 ng/mL] and OSM + TNFα (O+T) [10/20 ng/mL]. A control group without treatment (w/o) was included. On day 0, 7, 14, and 21, explants were fixated, infiltrated with paraffin, embedded in paraffin, sliced, placed onto cover slides, and stained with hematoxylin, Safranin O, and Fast Green. For each treatment group and each timepoint, a representative explant is shown. The scalebar shown in the baseline sample (day 0) represents 200 µm. Please click here to view a larger version of this figure.
Discussion
The protocol presented here for the profiling of cartilage tissue turnover in bovine cartilage explants can be used for characterizing treatment effects of many types of drugs, including inhibitors of inflammatory intracellular pathways, inhibitors of proteolytic enzymes, or anabolic growth factors.
Two different setups were described in this protocol: an anabolic setup where explants were stimulated with insulin-like growth factor 1 (IGF-1), and a catabolic setup comprising stimulation with TNF-alpha and Oncostatin M, in which tissue turnover can be inhibited using a broad-spectrum MMP inhibitor. The main output in this method is the quantification of neo-epitope biomarkers directly in the conditioned medium, which is harvested throughout the culture period. Several biomarkers can be measured in the supernatant, allowing for simultaneous profiling of different catabolic and anabolic processes in the same sample. Histological staining with Safranin O/Fast Green was used to validate the findings from the biomarker analysis. Oncostatin M, TNF-alpha, and IGF-1 were used to describe the protocol; however, the method is not limited to specific cytokine stimulators and these can easily be exchanged for others depending on the hypothesis or test treatment.
Interpretation of biomarker output is a temporal exercise due to the dynamic changes in chondrocyte function and expression profiles with anabolic or catabolic stimulation over time. In untreated explants, type II collagen formation measured by the biomarker ProC2 rapidly decreases within the first 7-10 days. Stimulation with IGF-1 or similar growth factors maintains ProC2 release in the conditioned medium at a level comparable to baseline; thus, the decline is more gradual, and the release is increased relative to untreated explants. In a catabolic setup, pro-inflammatory cytokines induce increased expression of proteases by the chondrocyte in days 0-14; this consists mainly of aggrecanases. This causes an initial large increase in aggrecanase-derived protein fragments, including AGNx1. At the later stages of culture, chondrocytes express more MMPs, which drives the release of MMP-generated markers, such as C2M, around day 14 and onwards. Thus, in order to profile the effect of a treatment, it is important to measure biomarkers in the right time interval.
As described, treatment with inflammatory cytokines such as the O+T cocktail will cause cartilage tissue degradation over time. The total pool of ECM is limited by the explant size and should be considered when analyzing the biomarker profile. Consequently, after the initial increase in biomarker release, the levels may decrease with time simply due to the reduction in the remaining amount of explant ECM.
Previously, OA was primarily considered a disease of the articular cartilage. However, recent studies suggest that OA should be viewed as a disease of the entire joint, where early disease-related changes in the individual joint compartments, synovium, bone, and cartilage, occur in parallel, and over time result in joint failure12,21. It is therefore important to recognize that in this model system, the cartilage is isolated from the rest of the joint (and organism), limiting the influence of tissue interaction effects and systemic factors that may regulate homeostasis of the tissue. Instead, it is a simplified single tissue culture where experimentally controlled conditions can be modulated to detect pathological or interventional changes to the tissue using biochemical techniques, biomarkers, or histological visualization. Due to the architecture of cartilage, variation in cell number, matrix composition, and amount variation is expected both between explants and between tissue sources. Because the relative magnitude of the biomarker output may be different between experiments, it is recommended to normalize data sets for better comparison.
To ensure the least possible variation and best results, it is important to use cartilage from knees that are as fresh as possible, preferably between 1 and 24 h after butchering. Isolation of cartilage tissue should be done in a homogenous way with explants being roughly the same thickness. Explants should be isolated from areas of thick cartilage, avoiding the areas closest to the middle. The tissue should always be moist to avoid cell death and matrix decomposition. The length of the experiment3, the time between medium changes, timing of cytokine stimulation, and treatment intervals can be adjusted to fit the hypothesized mode of action of the individual compound or mechanism.
Disclosures
CST, ACBJ and MK are employees of Nordic Bioscience. ACBJ and MK holds shares in Nordic Bioscience. The remaining authors have nothing to disclose.
Acknowledgements
The authors thank the technical staff at Nordic Bioscience for laboratory support, as well as the Danish Research Foundation for general support of our research.
Materials
| Name | Company | Catalog Number | Comments |
| 45% Iron(III) chloride solution | Sigma-Aldrich | 12322 | |
| Acetic acid | Merck | 1.00056.2500 | |
| Alamar Blue | Life tech Invitrogen | DAL1100 | |
| Biopsy processing cassettes – green | IHCWORLD | BC-0109G | |
| Biopsy punch W/Plunger (3 mm) | Scandidat | MTP-33-32 | |
| Bovine cartilage (Bovine knees) | Local slaughterhouse | ||
| C2M | Nordic Bioscience | Fee for service | |
| Corning 96-well plate | Sigma-Aldrich | CLS7007 | |
| Cover Glass Ø 13 mm | VWR | 631-0150P | |
| DMEM/F12-GlutaMAX Dulbecco's Modified Eagle Medium/Nutrient Mixture F-12) without HEPES | Gibco | 31331-028 | |
| Ethanol ≥96% | VWR | 83804.36 | |
| Ethanol absolute ≥99.5% | VWR | 83813.36 | |
| exAGNx1 | Nordic Bioscience | Fee for service | |
| exPRO-C2 | Nordic Bioscience | Fee for service | |
| Fast green | Sigma-Aldrich | F7252 | |
| Formaldehyde solution 4% | Merck | 1004965000 | |
| GM6001 | Sigma-Aldrich | M5939-5MG | |
| Hematoxylin | Sigma-Aldrich | H3136 | |
| Hydrochloric acid | Merck | 30721-M | |
| IGF-1 | Sigma-Aldrich | I3769-50UG | |
| Oncostatin M | Sigma-Aldrich | O9635-10UG | |
| Penicillin-streptomycin (P/S) | Sigma-Aldrich | P4333 | |
| Pertex (mounting medium for light microscopy) | HistoLab | 811 | |
| Phosphate Buffered Saline (PBS) | Sigma-Aldrich | D8537 | |
| Safranin O | Sigma-Aldrich | S2255 | |
| Sterile Standard Scalpels | Integra Miltex | 12-460-451 | |
| Sulfuric acid | Sigma-Aldrich | 30743 | |
| SUPERFROST PLUS Adhesion Microscope Slides | Thermo scientific | J1800AMNT | |
| TNF-alpha | R&D Systems | 210-TA-100 | |
| Toluene | Merck | 1.08327.2500 | |
| Vacuum Filtration "rapid"-Filtermax | TPP | 99955 |
References
- Cope, P. J., Ourradi, K., Li, Y., Sharif, M. Models of osteoarthritis: the good, the bad and the promising. Osteoarthritis and Cartilage. 27 (2), 230-239 (2018).
- Thysen, S., Luyten, F. P., Lories, R. J. U. Targets, models and challenges in osteoarthritis research. Disease Models & Mechanisms. 8 (1), 17-30 (2015).
- Reker, D., et al. Articular cartilage from osteoarthritis patients shows extracellular matrix remodeling over the course of treatment with sprifermin (recombinant human fibroblast growth factor 18). Osteoarthritis and Cartilage. 26, S43 (2018).
- Kjelgaard-Petersen, C., et al. Synovitis biomarkers: ex vivo characterization of three biomarkers for identification of inflammatory osteoarthritis. Biomarkers. 20 (8), 547-556 (2015).
- Henriksen, K., et al. A specific subtype of osteoclasts secretes factors inducing nodule formation by osteoblasts. Bone. 51 (3), 353-361 (2012).
- Gigout, A., et al. Sprifermin (rhFGF18) enables proliferation of chondrocytes producing a hyaline cartilage matrix. Osteoarthritis and Cartilage. 25 (11), 1858-1867 (2017).
- Reker, D., et al. Sprifermin (rhFGF18) modulates extracellular matrix turnover in cartilage explants ex vivo. Journal of Translational Medicine. 15 (1), 3560 (2017).
- Karsdal, M. A. Introduction. Biochemistry of Collagens, Laminins and Elastin. , (2016).
- Heinegård, D., Saxne, T. The role of the cartilage matrix in osteoarthritis. Nature Reviews Rheumatology. 7 (1), 50-56 (2011).
- Karsdal, M. A., et al. Osteoarthritis– a case for personalized health care?. Osteoarthritis and Cartilage. 22 (1), 7-16 (2014).
- Karsdal, M. A., Bay-Jensen, A. C., Henriksen, K., Christiansen, C. The pathogenesis of osteoarthritis involves bone, cartilage and synovial inflammation: may estrogen be a magic bullet?. Menopause International. 18 (4), 139-146 (2012).
- Loeser, R. F., Goldring, S. R., Scanzello, C. R., Goldring, M. B. Osteoarthritis: a disease of the joint as an organ. Arthritis and Rheumatism. 64 (6), 1697-1707 (2012).
- Goldring, M. B., Goldring, S. R. Osteoarthritis. Journal of Cellular Physiology. 213 (3), 626-634 (2007).
- Karsdal, M. A., et al. The coupling of bone and cartilage turnover in osteoarthritis: opportunities for bone antiresorptives and anabolics as potential treatments?. Annals of the Rheumatic Diseases. 73 (2), 336-348 (2014).
- Genovese, F., Karsdal, M. A. Protein degradation fragments as diagnostic and prognostic biomarkers of connective tissue diseases: understanding the extracellular matrix message and implication for current and future serological biomarkers. Expert Review of Proteomics. 13 (2), 213-225 (2016).
- Gudmann, N. S., et al. Cartilage turnover reflected by metabolic processing of type II collagen: a novel marker of anabolic function in chondrocytes. International Journal of Molecular Sciences. 15 (10), 18789-18803 (2014).
- Madej, W., van Caam, A., Davidson, E. B., Buma, P., van der Kraan, P. M. Unloading results in rapid loss of TGFβ signaling in articular cartilage: role of loading-induced TGFβ signaling in maintenance of articular chondrocyte phenotype?. Osteoarthritis and Cartilage. 24 (10), 1807-1815 (2016).
- Kjelgaard-Petersen, C. F., et al. Translational biomarkers and ex vivo models of joint tissues as a tool for drug development in rheumatoid arthritis. Arthritis & Rheumatology. 70 (9), 1419-1428 (2018).
- Wang, B., et al. Suppression of MMP activity in bovine cartilage explants cultures has little if any effect on the release of aggrecanase-derived aggrecan fragments. BMC Research Notes. 2 (4), 259 (2009).
- Bay-Jensen, A. C., et al. Enzyme-linked immunosorbent assay (ELISAs) for metalloproteinase derived type II collagen neoepitope, CIIM—Increased serum CIIM in subjects with severe radiographic osteoarthritis. Clinical Biochemistry. 44 (5-6), 423-429 (2011).
- Lories, R. J., Luyten, F. P. The bone-cartilage unit in osteoarthritis. Nature Reviews Rheumatology. 7 (1), 43-49 (2011).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved