Method Article
Recombineering Homologous Recombination Constructs in Drosophila
In This Article
Summary
Homologous recombination techniques greatly advance Drosophila genetics by enabling the creation of molecularly precise mutations. The recent adoption of recombineering allows one to manipulate large pieces of DNA and transform them into Drosophila6. The methods presented here combine these techniques to rapidly generate large homologous recombination vectors.
Abstract
The continued development of techniques for fast, large-scale manipulation of endogenous gene loci will broaden the use of Drosophila melanogaster as a genetic model organism for human-disease related research. Recent years have seen technical advancements like homologous recombination and recombineering. However, generating unequivocal null mutations or tagging endogenous proteins remains a substantial effort for most genes. Here, we describe and demonstrate techniques for using recombineering-based cloning methods to generate vectors that can be used to target and manipulate endogenous loci in vivo. Specifically, we have established a combination of three technologies: (1) BAC transgenesis/recombineering, (2) ends-out homologous recombination and (3) Gateway technology to provide a robust, efficient and flexible method for manipulating endogenous genomic loci. In this protocol, we provide step-by-step details about how to (1) design individual vectors, (2) how to clone large fragments of genomic DNA into the homologous recombination vector using gap repair, and (3) how to replace or tag genes of interest within these vectors using a second round of recombineering. Finally, we will also provide a protocol for how to mobilize these cassettes in vivo to generate a knockout, or a tagged gene via knock-in. These methods can easily be adopted for multiple targets in parallel and provide a means for manipulating the Drosophila genome in a timely and efficient manner.
Introduction
Clean molecularly-defined manipulations of single genes at their endogenous loci offer an invaluable tool to study a myriad of questions relevant to eukaryotic biology. Drosophila reverse genetic techniques for generating loss-of-function alleles had proven to be challenging until Golic and colleagues introduced in vivo gene targeting using homologous recombination to Drosophila 1-3. They demonstrated that specific genomic loci could be targeted using a linear fragment of DNA from an integrated transgenic construct. This linear "donor" DNA is generated in vivo through FRT-mediated recombination (to excise the DNA from the chromosome as a circular molecule) followed by linearization with the meganuclease I-SceI. Although this methodology has been successfully used to generate a variety of defined lesions, the technique has not been easily scalable for the manipulation of numerous genes in parallel because each individual knockout construct requires distinct and custom design. For example, difficulties in seamlessly manipulating large fragments of DNA (>5 kb) in vitro using classical restriction enzyme/ligation cloning or PCR, as well as the size limitations of traditional in vivo transformation vectors often interfere with the rapid creation of homologous recombination targeting vectors. To overcome these limitations, we combined the recombineering/transgenesis P[acman] system, which allows the sub-cloning and transgenesis of up to 100 kb of DNA, with the ends-out gene targeting methodology to establish an efficient and relatively rapid platform that facilitates Drosophila gene targeting.
Recombination-mediated genetic engineering (recombineering) is a powerful homologous recombination-based cloning technology 4,5. In contrast to conventional restriction enzyme/ligase cloning, recombineering is not limited by the sequence or size of the manipulated DNA. Recombineering uses a special E. coli strain that harbors recombination machinery provided by a defective λ prophage 4. This technique has recently been adopted for use in Drosophila 6,7. Recombineering in Drosophila relies on a modified conditionally amplifiable bacterial artificial chromosome (BAC) vector called P[acman] 6,7. This vector carries two origins of replication: OriV, which produces high-copy number upon chemical induction for the purification of large quantities of DNA required for sequencing and embryo injection and OriS, which maintains low-copy number under basal conditions. Additionally, the P[acman] vector is equipped with a bacterial attachment (attB) site. The attB site serves as a substrate for ΦC31 integrase-mediated transgenesis that allows incorporation of large DNA fragments into a predetermined landing site within the Drosophila genome 8,9.
We have generated a P[acman] vector (referred to as P[acman]-KO 1.0) that can be used as a targeting vector for ends-out homologous recombination 10,11. To incorporate ends-out gene targeting technology into the system, we added two FRT and two I-SceI sites. We have also included a Gateway cassette within this modified vector to streamline the process of incorporating the homology arms into P[acman]-KO 1.0. This provides a rapid and simple way to introduce virtually any genomic region of interest into the targeting vector. In this protocol we will describe how to engineer a targeting vector using P[acman]-KO 1.0, and how to mobilize this vector in vivo to target the endogenous locus. For the purpose of this protocol we will use the RFP/Kan cassette to replace a gene of interest, but a variety of cassettes that contain an antibiotic selection marker can be used with this protocol. We have designed and successfully used a set of cassettes for gene replacement and tagging 10,11.
Protocol
1. Selection of BAC and Region to Target
- To acquire a BAC with the gene of interest (GOI) (here CG32095 is used as an example), search for CG32095 at www.flybase.org. Under the section stocks and reagents, check the section entitled, "Genomic Clones" for BACs containing CG32095. Make sure that the BAC includes at least 10 kb upstream and 5 kb downstream the gene of interest. Alternatively, clones in the P[acman] vector7 can also be found at http://www.pacmanfly.org/libraries.html.
- The BAC clones come in DH10B cells and the P[acman] clones come in EPI300 cells. The genomic clones need to be transformed into SW102 cells, which are amenable for recombineering. "Dirty minipreps" are performed (a Qiagen miniprep without using a column and precipitating the DNA using isopropanol) starting from a 5 ml O/N culture inoculated with cells containing the BAC. After re-suspension of the freshly made DNA pellet in 20 μl of dH2O, dilute 1 μl into 100 μl of dH2O and use 1 μl to electroporate electrocompetent SW102. To make electrocompetent SW102 use the following protocol:
-
- Inoculate SW102 cells into 4 ml of LB. Grow culture at 32 °C O/N shaking at 250 rpm. Important: SW102 cells should NEVER be exposed to a temperature higher than 32 °C unless the induction of recombination machinery is desired.
- Inoculate 1 ml of the saturated SW102 culture into 44 ml LB and grow on a 32 °C shaker to OD600 between 0.2-0.3 (usually about 1-1.5 hr). NOTE: Other recombineering protocols recommend growing the culture in a smaller volume to OD600 0.6-0.7, however growing the bacteria to a lower density in a larger volume significantly increases transformation efficiency 12. In addition, this modification reduces the waiting time.
- Cool the culture in ice-water slurry for 5 min. Important: During the following steps cells MUST be kept at low temperatures. This is crucial for obtaining good-quality electrocompetent cells.
- Centrifuge culture at 4 °C at 1,500 x g for 10 min. Discard supernatant and wash pellet in 25 ml of cold (4 °C) 10% glycerol 13. First, resuspend cells in 5 ml by tapping and swirling the tube in ice-water slurry (DO NOT PIPETTE UP AND DOWN) and then add the remaining 10% glycerol (20 ml).
- Centrifuge at 4 °C at 1,500 x g for 10 min, discard supernatant and wash the pellet a second time with 25 ml ice cold 10% glycerol.
- Centrifuge at 4 °C at 1,500 x g for 10 min, discard supernatant and wash the pellet a third time with 25 ml ice cold 10% glycerol.
- Centrifuge at 4 °C at 1500 x g for 10 min and discard supernatant. The cell pellet may be very loose at this point and care should be taken when discarding the supernatant. Resuspend the pellet in 1 ml of cold 10% glycerol. Transfer the cells to a 1.5 ml tube and centrifuge for 30 sec at 12,000 x g in a tabletop microcentrifuge at 4 °C. Discard most of the supernatant leaving ≈90 μl of glycerol/cells. The cells are now ready to be electroporated. NOTE: At this point cells can be stored at -80 °C for later use. However, this may decrease competence.
- Electroporate DNA into cells using a 1 mm cuvette at 1,800 V, 25 μF, 200 Ω. After electroporation, add 300 μl of S.O.C. and let the cells recover for 2 hr at 32 °C (shaking is not required). Plate cells on LB agar plus 25 μg/ml chloramphenicol (or other appropriate antibiotics depending on the transformed BAC or PAC) and incubate at 32 °C for 24-30 hr.
- Homology arms for gene targeting are designed by selecting 10 kb of genomic DNA upstream and 5 kb downstream relative to the GOI. Select 500 bp at the end of the 10 kb and 5 kb homology arms to amplify (See Section 2) (Figure 1). These 500 bp fragments serve as the homology arms used to recombine the genomic region surrounding the GOI into P[acman] KO 1.0. These fragments are referred to as left arm (LA) for the region that corresponds to the upstream 5' homology arm and right arm (RA) for the region that corresponds to the downstream 3' homology arm. Important: Homology arms should not contain BamHI restriction sites, as the linearization of the plasmid (in step 3.2) is accomplished via a BamHI restriction digest.
2. Insert the Homology Arms into P[acman]-KO 1.0
- Set up two individual PCR reactions; one to amplify the LA and another to amplify the RA. For the LA, use primers attB1-I-SceI-LA-F and BamHI-LA-R and use the primers BamHI-RA-F and attB2-I-SceI-RA-R to amplify the RA (Table 1). Use 1 μl of BAC DNA from step 1.2 or 0.5 μl of boiled overnight bacterial culture containing BAC of interest as a template. Make sure to use a DNA polymerase with proofreading capability. This applies to all subsequent PCRs -with the exception of the PCR check in steps 2.8 and 3.13. NOTE: Extension time for a proofreading DNA polymerase may be different from regular Taq DNA polymerase depending on the manufacturer. PfuUltra II Fusion HotStart, which polymerizes at a rate of 15 sec/kb, can be used in these steps.
LA/RA PCR
PCR Conditions1 μl DNA from dirty miniprep from step 1.2 of Bac 0.25 μl Each 20 μM Primer 2.5 μl 10X PfuUltra II Buffer 0.75 μl 10mM(each) dNTP 0.5 μl PfuUltra II Fusion HotStart DNA Polymerase 19.75 μl Water 25 μl Total Volume Step1 95 °C 2 min to activate enzyme Step2 95 °C 20 sec denature Step3 60 °C 20 sec annealing Step4 72 °C 20 sec extension Step5 Go To step 2 and repeat 29 cycles Step6 4 °C Hold - Run samples on a 1% agarose gel and extract products using a Zymoclean DNA recovery kit. Elute the DNA from the Zymoclean column with 10 μl of sterile water.
- Perform splicing by overlapping extension (PCR SOE) with the two fragments using 10-30 ng of total DNA from each amplified product purified in 2.2 (Figure 2). This is usually around 1/20th of each purified PCR product:
LA/RA PCR-Soe
PCR Conditions0.5 μl each LA and RA purified PCR product approximately 10-30 ng 0.25 μl each 20 μM Primer 2.5 μl 10X PfuUltra II Buffer 0.75 μl 10mM(each) dNTP 0.5 μl PfuUltra II Fusion HotStart DNA Polymerase 19.25 μl Water 25 μl Total Volume Step1 95 °C 2 min to activate enzyme Step2 95 °C 20 sec denature Step3 55 °C 20 sec annealing (Lower temp. to allow for arms to anneal) Step4 72 °C 20 sec extension Step5 Go To step 2 and repeat 1 cycle Step6 95 °C 20 sec denature Step7 60 °C 20 sec annealing Step8 72 °C 20 sec extension Step9 Go To step 2 and repeat 27 cycles Step10 4 °C Hold - Run the PCR sample on an agarose gel and recover the 1.0 kb band by gel extraction. This is the attB1-LA-BamHI-RA-attB2 cassette.
- Use Gateway BP ClonaseII Enzyme kit to set up a BP reaction following the protocol provided by the manufacturer. The cassette generated in 2.4 will act as the donor DNA and the P[acman]-KO 1.0 as the destination vector.
- Dilute 50 μl of TransforMax EPI300 electrocompetent cells by adding 30 μl of cold 10% glycerol or cold dH2O. Transform 1 μl of the BP reaction into electrocompetent cells following the protocol provided by the manufacturer. The addition of water dilutes the fairly high salt concentration of the BP reaction to prevent electrical discharge (arcing) during electroporation. Electroporate DNA into cells using a 1 mm cuvette at 1,800 V, 25 μF, 200 Ω.
- Add 300 μl of S.O.C. and let cells recover for 1 hr at 37 °C (shaking is not required). Plate cells on LB-agar + 50 μg/ml of Amp (P[acman]-KO 1.0 contains an Amp resistance gene). Incubate plates at 37 °C for 18-24 hr.
- PCR check individual colonies by conventional colony PCR using T3 (T3 sequence is downstream of the Gateway cassette in the P[acman]-KO 1.0 (Figure 1) and attB1-I-SceI-LA-F primers. Positive clones will show a band that runs at 1.2 kb.
- Grow an overnight culture of a positive clone in LB with 50 μg/ml ampicillin.
- Amplify the P[acman]-KO 1.0 vector containing the LA and RA in a 10 ml LB with 50 μg/ml ampicillin culture using copy control solution following the manufacturer's protocol (Epicentre).
- Perform a Qiagen miniprep following the manufacturer's protocol.
3. Recombining the Genomic Region of Interest into P[acman]-KO 1.0
Day 1
- Inoculate SW102 cells containing the BAC of interest into 4 ml of LB plus 25 μg/ml chloramphenicol (Chl) or the appropriate selection antibiotic. Grow culture at 32 °C O/N shaking at 250 rpm. Important: Besides during induction in step 3.5, SW102 cells should not be exposed to a temperature higher than 32 °C, as high temperature activates the recombination machinery of these cells.
Day 2
- Digest 0.4 μg of the P[acman]-KO-1.0 containing the LA and RA arms generated in section 2 with ≈20 units of BamHI in a 25 μl reaction. Incubate at 37 °C for at least 3 hr. Important: Long digestion is critical to ensure digestion of all DNA. This will decrease the number of false positives clones later on.
- Inoculate 1.0 ml of the saturated SW102/BAC culture into 44 ml LB-Chl(25 μg/ml) and grow on a 32 °C shaker to OD600 between 0.2-0.3 (usually about 1-1.5 hr). Include an additional identical sample for non-HS control. This sample will be treated just like the experimental one in every single aspect with the exception of the HS. This non-heat-shocked culture serves as a negative control. The appearance of many colonies on the non-HS control plate (step 3.13) usually indicates transformation with undigested P[acman] KO 1.0 LA/RA plasmid.
- While the culture is growing, run the BamHI-restricted P[acman]-KO on a gel. Then gel-extract and elute the DNA in 10 μl of water warmed up to 55 °C. NOTE: Do not elute DNA in any buffer. Salts present in the buffer may cause electrical discharge during electroporation.
- Heat-shock the culture at 42 °C for exactly 15 min in the water bath. This will activate the recombineering machinery of SW102 cells. Do not heat shock the control culture.
- Immediately after heat-shock, transfer heat-shocked and non-HS control cultures to chilled 50 ml canonical tubes and let the cultures chill in an ice-water slurry for 5 min. Important: During the following steps cells MUST be kept at low temperatures. This is crucial to obtain good-quality electrocompetent cells.
- Centrifuge culture at 1,500 x g for 10 min. Discard supernatant and wash pellet in 25 ml of cold (4 °C) 10% glycerol 13. First, resuspend cells in 5 ml by tapping and swirling the tube in ice-water slurry (DO NOT PIPETTE UP AND DOWN) and then add the remaining 10% glycerol (20 ml).
- Centrifuge at 4 °C at 1,500 x g for 10 min, discard supernatant and wash the pellet with 25 ml ice cold 10% glycerol a second time.
- Centrifuge at 4 °C at 1,500 x g for 10 min, discard supernatant and wash the pellet with 25 ml ice cold 10% glycerol a third time.
- Centrifuge at 4 °C at 1,500 x g for 10 min and discard supernatant. The cell pellet may be very loose at this point and care should be taken when discarding the supernatant. Resuspend the pellet in 1 ml of cold 10% glycerol. Transfer the cells to a 1.5 ml tube and centrifuge for 30 sec at 12,000 x g in a tabletop microcentrifuge at 4 °C. Discard most of the supernatant leaving ≈90 μl of water/cells. NOTE: At this point cells can be stored at -80 °C for later use. However, this may decrease competence.
- Add 2 μl of the BamHI-digested P[acman] (aim for 50 ng) to the cells and mix gently using a pipette tip. Transfer cells into a 1 mm electroporation cuvette and electroporate at 1,800 V, 25 μF, 200 Ω.
- Add 300 μl of S.O.C. and let the cells recover for 2 hr at 32 °C (shaking is not required). Plate cells on LB agar plus 50 μg/ml ampicillin and incubate at 32 °C for 24-30 hr.
Day 3
- Screen for proper gap-repair using colony PCR. Use T3 and RA check primers for the RA and T7 and LA check primer for the LA (Table 1). Design the PCR-Check-RA and PCR-Check-LA primers 100-200 bp towards the targeted region (Table 1). Positive colonies from PCR reactions using PCR-Check-RA with T3 or PCR-Check-LA with T7 will show a band that runs at 800-1,000 bp (Figure 3). NOTE: It is critical to test both arms since incorporation of one arm but not the other is sometimes observed.
- Grow PCR-verified colonies at 32 °C in 4 ml of LB+50 μg/ml ampicillin for overnight. Make a frozen stock of these cells for later use.
4. Replacing the Genomic Region with the Targeting Cassette
Day 4
- Amplify the RFP/KAN knockout cassette by PCR using pENTR-RFP/KAN (predigested with AscI and NheI to linearize) as a template and the 5'HA-RFP/Kan-F and 3'HA-RFP/Kan-R primers (Table 1). RFP/KAN cassette can be requested from reference 11.
- Gel purify the PCR product eluting in 20 μl of sterile water. The RFP-KAN cassette plus the homology arms should run around 3 kb.
- Inoculate 1 ml of the saturated SW102/P[acman]-KO culture (step 3.13) into 44 ml LB-Amp (50 μg/ml) and grow on 32 °C shaker to OD600 between 0.2-0.3. Include an additional identical sample for non-HS control. This sample will be treated just like the experimental one in every single aspect with the exception of the HS.
- After the SW102/P[acman]-KO culture reaches the desired density, follow steps from 3.5 to 3.10.
- Electroporate approximately 100 ng of targeting cassette into 90 μl of electrocompetent SW102/P[acman]-KO cells. Transfer cells into a 1 mm electroporation cuvette and electroporate at 1,800 V, 25 μF, 200 Ω. Add 300 μl of S.O.C. and allow cells to recover for 2 hr at 32 °C (shaking is not required). NOTE: It is highly recommended to use a fresh gel purified cassette.
- Plate cells in LB agar +Amp (50 μg/ml) +Kan (50 μg/ml) and incubate for 24-30 hr at 32 °C.
Day 5
- Grow 5 ml of culture from 5 different colonies at 32 °C O/N.
Day 6
- Perform a "dirty miniprep" and run half of the obtained DNA on a 1% agarose gel to look for the presence of a low-molecular weight plasmid. There should not be any plasmid that runs bellow 12 kb. (Figure 4)
- Make a 1:500 dilution of DNA from a positive clone and use 1 μl to electroporate into 50 μl of TransforMax EPI300. Important: Make sure to dilute the DNA to prevent electroporation of multiple plasmids into one cell. Plate cells in LB agar +Amp (50 μg/ml) +Kan (50 μg/ml) and incubate for 18-24 hr at 37 °C
Day 7
- Inoculate a single colony in 10 ml of LB + and grow O/N.
Day 8
- Seed 100 ml of LB media with saturated culture from step 4.10. Add appropriate antibiotics plus 100 μl of 1,000X copy control solution and incubate at 37 °C for 5-6 hr.
- Perform a maxiprep and verify insertion of the cassette in the correct site by sequencing.
- In addition to sequencing, perform a restriction enzyme digestion test with DNA from a clone that did not contain any low molecular weight plasmid and its parental DNA. The restriction enzymes selected will be different for each gene but the goal is to find a group of enzymes that allow to the investigator to characterize the recombineered vector. By way of example, the CG32095 targeting vector was serially digested with PacI, AscI, BamHI and AatII. The appearance of all the predicted bands and absence of any incorrect bands confirms that the targeting vector has been correctly recombineered (Figure 5).
5. Injecting Flies and Mobilizing Cassette In vivo
- Inject the cassette using ΦC31 integrase, into a pre-defined landing site of choice. Outside vendors, such as Rainbow Transgenics (http://www.rainbowgene.com), can be used for injection services. Important: The DNA must be freshly prepared before injection. Higher transformation efficiency is observed when the DNA is prepared on-site and injected the same day.
- To mobilize the cassette from the landing site, use Bloomington stock number 25680 or 25679. These stocks contain Flippase and I-SceI downstream of a heat shock promoter, on chromosome two and three, respectively. In addition, they contain a hs-hid transgene on the balancer chromosome and on the Y-chromosome. Collect males from the chosen stock and cross to virgin females carrying the KO cassette in the landing site. Set ~30 crosses and allow females to lay eggs for 2-3 days. NOTE: It is recommended to choose the stock that is on a different chromosome from the targeted locus.
- Flip parents into a new set of 30 vials, and heat shock larvae at 37 °C for 1 hr, twice a day, for three consecutive days. The heat shocks activate the production of the enzymes; flippase and I-SceI, and the cell death gene hid, killing off the males and flies carrying balancer chromosomes. Allow the parents in the newly flipped vials to lay eggs for 2-3 days, then repeat the heat shocks for those vials, after flipping the parents out into new vials again. Repeat this for several flips, until the parents do not lay eggs anymore.
- Collect virgin females from the F1 progeny of the heat-shocked flies, and cross to y-w-males. Aim for 150-200 crosses; three virgins and three males per cross.
- Screen the F2 progeny under a fluorescent scope that allows detection of RFP in the eye. Screen for RFP positive eyes that are mutant for the white and yellow genes (y-w-). This screening process positively selects for the presence of the RFP cassette and selects for the absence of the P[acman] vector (mini-white) and landing site, which is marked by the wild-type yellow gene. NOTE: It is less time-consuming to screen for RFP in the eye, than to first screen for yw flies. The mobilization events are very efficient during the heat shocks, resulting in many fewer flies containing RFP in the eye, as compared to y-w- flies.
- Set up single pair matings with each y-w-RFP+ fly, since each mobilization event may be different. Map the RFP to the correct chromosome. Correct chromosome targeting is typically observed in >95% of cases.
- Establish stocks and check for correct targeting events using standard techniques for Southern Blot Analysis. Design a 2 kb probe upstream (left arm) and downstream (right arm) of the gene being targeted, and 2 kb (or shorter if necessary) spanning the deleted ORF. The first two probes will yield a band in the homozygous and heterozygous knockouts, that is not present in the wildtype, while the ORF will not yield a band in the homozygous knockout. A quick PCR for the ORF is also possible, however this relies on the absence of a band.
Results
Amplification of the LA and RA homology arms should produce 500 bp products and the PCR-SOE reaction should yield a 1.0 kb product (Sections 2.1-2.4; Figure 2). The BP reaction performed in section 2.5 is typically very efficient and bacterial transformation of the product yields 5-100 colonies on average. Nearly all the colonies tested with PCR check show the expected PCR product.
During the first round of recombineering (Section 3) expect to get 20-40 colonies after transformation of the digested P[acman]-KO-1.0 containing the LA and RA arms into SW102 cells. 40-60% of these clones will carry the desired recombination product. The non-heat shock control should contain very few if any colonies as compared to the heat-shocked cells. The appearance of the same number of colonies on both sample and control plates usually indicates the presence of uncut plasmid or some other contaminant. In this case, the experiment should be discarded and repeated, this time digesting the P[acman]-KO-1.0 carrying the LA and RA arms to completion following the instruction described in step 3.2. An example of a PCR check for retrieval of genomic region of interest into P[acman]-KO 1.0 in which ≈60 colonies were obtained is shown in Figure 3. Here 60% of the colonies tested carried the desired recombination product.Care must be taken that the observed PCR products run at the predicted size, since aberrant PCR bands usually indicate incorrect recombineering events.
Occasionally no colonies will grow after the first round of recombineering. Using cells with sub-optimal efficiency represents the most common cause for a failed recombineering reaction. Keeping the cells cold at all times during their preparation and gentle handling during the washes (NEVER vortex) is critical for obtaining high quality competent cells. Try to always use freshly digested/purified P[acman]-KO-1.0 DNA to obtain the highest efficiency.
Sometimes the first round of the recombineering yields colonies but those colonies do not pass the PCR check assay. This usually indicates incorrect recombineering products but such a result may also indicate potential problems with one of the primer sets. To validate the PCR-Check-LA primer, set up a PCR reaction in combination with the attB1-I-SceI-LA-F. To verify PCR-check-RA set up a PCR reaction with the attB2-I-SceI-RA-R. In both cases use the original BAC as a template. These PCR reactions should yield products of the expected size, which will vary depending on the sequence of the check primers. Positive results from this control experiment will rule out the possibility that an inability to detect positive clones is due to primer inefficiency.
If you have tried the first round of recombineering several times following the above protocol without any success and have performed all the suggested controls, we recommend choosing a new pair of 500 bp homology arms. For reasons that are not clear at the present time, some genomic regions are highly resistant to DNA recombination. Simply designing new homology arms and moving them towards or away from the gene of interest sometimes solves these problems.
The second round of recombineering (section 4) is typically much more efficient. Normally 30-50 colonies are observed after transformation, with 90-95% of these containing the desired construct. The most common problem at this step is obtaining false-positive colonies. Figure 4 shows examples of true and false positive clones after second round of recombineering (section 4).
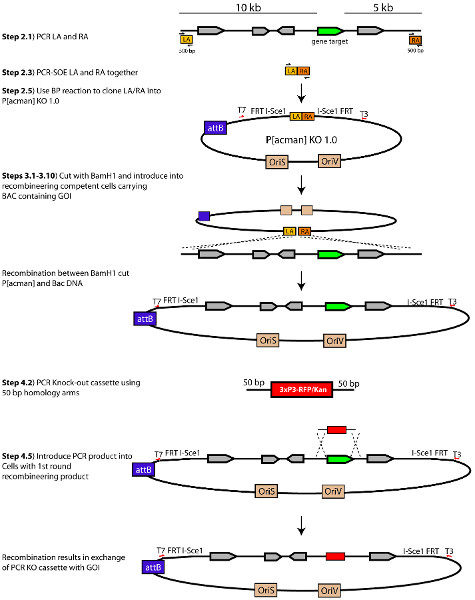
Figure 1. Schematic of the process to generate a P[acman]-KO 1.0 targeting vector. Modified from Chan, et al., (2012). Click here to view larger figure.

Figure 2. Schematic of splicing by overlapping PCR (PCR SOE) performed on step 2.3. Click here to view larger figure.
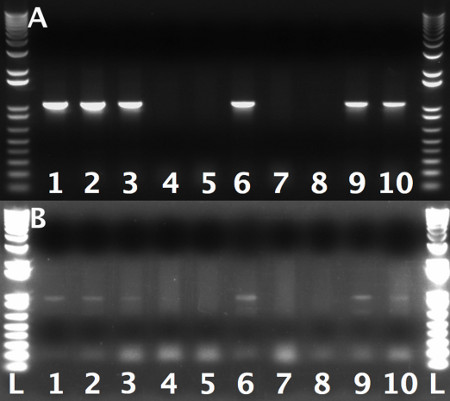
Figure 3. PCR-check gel showing efficiency of first recombineering event. Each lane represents a single colony. A: Colonies tested for left arm integration using T7 and LA-check primers. B: The same colonies as A, tested for right arm integration using T3 and RA-check primers.1 kb Plus DNA Ladder was used as a molecular weight marker. NOTE: Due to poor priming of T3 primer relative to T7, the bands of the right arm check were significantly darker. This should be taken into account when viewing the gel.

Figure 4. DNA agarose gel showing high molecular weight plasmids (P[acman]) and low molecular weight plasmid (undigested template) isolated from potential positive clones after second round of recombineering. DNA was isolated from 4 potential positive clones and ran on a 1% agarose gel stained with ethidium bromide. Clones 1,3 and 4 are true positive clones. Clone 2 is a false positive one as noted by the all the forms of a plasmid that runs bellow 12 kb (the largest size band in DNA ladder lane). 1 kb Plus DNA Ladder was used as a molecular weight marker.

Figure 5. Test for desired recombination event by restriction enzyme digest. In the example above is shown a computer-predicted banding pattern of the P[acman]-KO CG32095 before and after the second round of recombineering. Four different enzymes were used: PacI, AscI, AatII and BamHI. Correctly recombined product will have the same bands as its parental P[acman]-KO CG32095 except for the intended change (arrowheads). NEBcutter V2.0 was used to predict the band pattern.
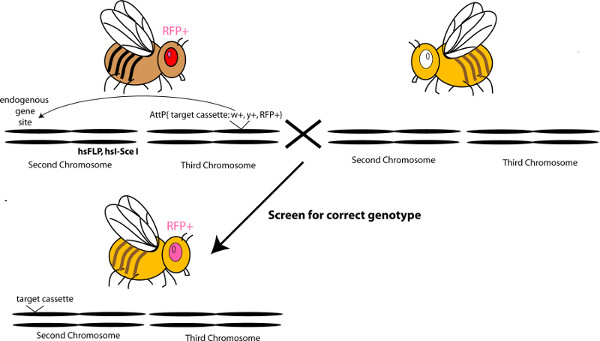
Figure 6. Schematic describing the mobilization of a targeting cassette in vivo. Click here to view larger figure.

Table 1. Primers. Click here to view larger table.
Discussion
The power of genetic model organisms in biomedical research is largely based on the tools available for genetic manipulation. The small models C. elegans and Drosophila in particular allow for inexpensive and fast molecular genetic analyses of complete pathways and gene families implicated in multicellular development or function. Recent years have seen significant advances in tool development for manipulating genes in Drosophila 14,15. For example, recombineering, which is widely used in mouse genetics to manipulate Bac DNA constructs, was recently adapted for Drosophila, through the use of the P[acman] vector and ΦC31-mediated transgenesis 6. Different selection cassettes allow for the insertion or deletion of specific sequences anywhere within a construct using recombination competent bacteria 4,13. We have modified the original P[acman] vector so that it can be used for constructing in vivo homologous recombination vectors that target endogenous loci in Drosophila. This new vector enables one to generate targeting cassettes with larger homology arms than those that can be easily made using cut and paste cloning methods. Furthermore, a Gateway-cloning cassette has also been incorporated into this vector, allowing one to rapidly and efficiently introduce new genomic DNA into the system.
Although most of the methodology described here is straightforward, there are several steps we have found critical for the success of the technique. Due to the size of the manipulated DNA (>15 kb), the first round of recombineering reaction (section 3) poses a significant technical hurdle. Hence, this round of recombineering requires a very efficient recombination protocol. Seemingly minor protocol adjustments, like growing the cells to lower density, washing the cells three times instead of two and with 10% glycerol instead of dH2O 13, have greatly improved the transformation efficiency of our recombineering competent cells. In addition, taking care to make sure the LA/RA containing P[acman] is cut to completion with BamHI (Step 3.1) eliminates many false positives.
Undesirable recombination events represent another fairly common problem during recombineering. For example, we have observed cases of duplications and translocations within various constructs. These events can lead to multiple problems during Drosophila transformation and in vivo gene targeting. PCR verification and sequencing analysis cannot detect all of these events. Hence, a restriction enzyme analysis must be performed on the final cloned product before injection of DNA into embryos. This final check has proven critical and has allowed us to avoid injecting constructs that contain undesired aberrations.
Maxi-prepping the targeting vector just before injection and never freezing the DNA greatly improves the transformation efficiency of these large constructs into Drosophila. In short, small details make a big difference in the success of these techniques. The protocols outlined and demonstrated here represent insights from a number of different sources and our own experience.
While we hope the protocol presented here helps others to adopt recombineering methods in their own laboratories, we believe further improvements and refinements to the methodology are still possible. Occasionally, specific genomic fragments are difficult to manipulate using recombineering for reasons that are not entirely obvious. Furthermore, despite our best efforts to eliminate unwanted background, we still find that certain pieces of genomic DNA tend to produce incorrectly recombineered end-products. A deeper understanding of the recombineering process may yield more optimal protocols in the future and open discussion of recombineering failures and success stories will foster the successful use of these powerful techniques by the broader research community.
Disclosures
The authors do not have any competing interests in regards to the techniques outlined here.
Acknowledgements
We would like to thank Hugo Bellen and the Bloomington Stock Center for reagents. We further thank Koen Venken, Hugo Bellen and all members of the Buszczak and Hiesinger labs for helpful discussions. This work was supported by grants from the National Institute of Health to ACR (T32GM083831), PRH (RO1EY018884) and to MB (RO1GM086647), a grant by the Cancer Prevention Research Institute of Texas to MB and PRH (RP100516), and the Welch Foundation (I-1657) to PRH. MB is an E.E. and Greer Garson Fogelson Scholar in Biomedical Research and PRH is a Eugene McDermott Scholar in Biomedical Research at UT Southwestern Medical Center.
Materials
| Name | Company | Catalog Number | Comments |
| Name of the reagent | Company | Catalogue number | Comments (optional) |
| SW102 Recombination competent bacteria | NCI-Frederick | Recombination Bacteria (SW102, SW105 and SW106) | http://ncifrederick.cancer.gov/research/brb/logon.aspx |
| TransforMax EPI300 electrocopmpetent E. coli | Epicentre | EC300110 | Includes CopyControl induction solution |
| PfuUltra II Fusion HS DNA Polymerase | Aligent Technology Inc. | 600670 | |
| BamHI-HF | New England Biolabs | R3136S | |
| Zymoclean Gel DNA Recovery Kit | Zymo Research | D4001 | |
| Use Gateway BP ClonaseII Enzyme kit | Invitrogen | 11789-020 | |
| P[acman]KO1.010 | Buszczak and Hiesinger Labs | Upon request | |
| pENTR RFP-Kan11 | Buszczak and Hiesinger Labs | Upon request | |
| Flystocks | Bloomington stock center | Stock numbers: 25680, 25679 | y1 w*/Dp(2;Y)G, P{hs-hid}Y; P{70FLP}11 P{70I-SceI}2B snaSco/CyO, P{hs-hid}4 y1 w*/Dp(2;Y)G, P{hs-hid}Y; P{70FLP}23 P{70I-SceI}4A/TM3, P{hs-hid}14, Sb1 |
| Electroporation machine | Biorad GenePulser Xcell with PC module | 165-2662 | |
| Cuvettes | Fisher Brand | #FB101 |
References
- Rong, Y. S., Golic, K. G. A targeted gene knockout in Drosophila. Genetics. 157, 1307-1312 (2001).
- Rong, Y. S., Golic, K. G. Gene targeting by homologous recombination in Drosophila. Science. 288, 2013-2018 (2000).
- Gong, W. J., Golic, K. G. Ends-out, or replacement, gene targeting in Drosophila. Proc. Natl. Acad. Sci. U.S.A. 100, 2556-2561 (1073).
- Warming, S., Costantino, N., Court, D. L., Jenkins, N. A., Copeland, N. G. Simple and highly efficient BAC recombineering using galK selection. Nucleic Acids Res. 33, e36 (2005).
- Copeland, N. G., Jenkins, N. A., Court, D. L. Recombineering: a powerful new tool for mouse functional genomics. Nat. Rev. Genet. 2, 769-779 (2001).
- Venken, K. J., He, Y., Hoskins, R. A., Bellen, H. J. P[acman]: a BAC transgenic platform for targeted insertion of large DNA fragments in D. melanogaster. Science. 314, 1747-1751 (2006).
- Venken, K. J., et al. Versatile P[acman] BAC libraries for transgenesis studies in Drosophila melanogaster. Nat. Methods. 6, 431-434 (2009).
- Groth, A. C., Fish, M., Nusse, R., Calos, M. P. Construction of transgenic Drosophila by using the site-specific integrase from phage phiC31. Genetics. 166, 1775-1782 (2004).
- Bischof, J., Maeda, R. K., Hediger, M., Karch, F., Basler, K. An optimized transgenesis system for Drosophila using germ-line-specific phiC31 integrases. Proceedings of the National Academy of Sciences of the United States of America. 104, 3312-3317 (2007).
- Chan, C. C., et al. Systematic discovery of Rab GTPases with synaptic functions in Drosophila. Current Biology: CB. 21, 1704-1715 (2011).
- Chan, C. C., Scoggin, S., Hiesinger, P. R., Buszczak, M. Combining recombineering and ends-out homologous recombination to systematically characterize Drosophila gene families: Rab GTPases as a case study. Commun. Integr. Biol. 5, 179-183 (2012).
- Wu, N., Matand, K., Kebede, B., Acquaah, G., Williams, S. Enhancing DNA electrotransformation efficiency in Escherichia coli DH10B electrocompetent cells. Electronic Journal of Biotechnology. 13, (2010).
- Wang, S., Zhao, Y., Leiby, M., Zhu, J. A new positive/negative selection scheme for precise BAC recombineering. Mol. Biotechnol. 42, 110-116 (2009).
- Venken, K. J., Bellen, H. J. Transgenesis upgrades for Drosophila melanogaster. Development. 134, 3571-3584 (2007).
- Dietzl, G., et al. A genome-wide transgenic RNAi library for conditional gene inactivation in Drosophila. Nature. 448, 151-156 (2007).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved