Method Article
Advanced Glycation End-Products Sensitize Human Sensory-Like Neuron Cells to Capsaicin-Induced Calcium Influx
* These authors contributed equally
In This Article
Summary
Increased collagen-derived advanced glycation end products (AGEs) are consistently linked to painful diseases. Here, we investigated whether glycation sensitizes sensory neurons to capsaicin excitation.
Abstract
Increased collagen-derived advanced glycation end products (AGEs) are consistently linked to painful diseases, including osteoarthritis, diabetic neuropathy, and neurodegenerative disorders. Human sensory-like neurons differentiated from the SH-SY5Y cell line gain pro-nociceptive functions when exposed to AGEs by releasing substance P and upregulating the transient receptor potential vanilloid 1 (TRPV1) expression. Here, we investigated whether this receptor was functionally active and whether the glycation process sensitizes sensory neurons to capsaicin excitation. Sensory-like neuron cells were obtained from the differentiation of SH-SY5Y cells with all-trans-retinoic acid and brain-derived neurotrophic factor. Incubation with glycated collagen extracellular matrix (ECM-GC) simulated a pro-nociceptive stimulus. Control cells were incubated with a non-glycated extracellular collagen matrix (ECM-NC). Fluo-8 Calcium Flux Assay Kit was used to assess calcium influx, which was stimulated by capsaicin. The results show that glycation increases calcium influx compared with cells treated with normal collagen, suggesting that sensory-like neurons express functional TRPV1 channels and that glycation increases capsaicin excitation. These data indicate AGEs hypersensitive sensory-like neuron cells, triggering pro-nociceptive signaling. Together, our results suggest that we established a functional model responsive to capsaicin that can be useful for screening candidates for managing painful conditions.
Introduction
Glycation is a non-enzymatic, irreversible, and spontaneous process in which proteins, such as collagen, bind to reducing sugar molecules, resulting in advanced glycation end products (AGEs). AGEs may activate cellular membrane receptors, triggering intracellular pathways activation, such as extracellular signal-regulated protein kinase (ERK) 1/2, p38 mitogen-activated protein kinase (MAPK), and c-jun n-terminal kinases (JNKs), rho-GTPases, phosphoinositol-3-kinase (PI3K), Janus kinase/signal transducer and activator of transcription (JAK/STAT), and protein kinase C (PKC), increasing proinflammatory molecules release and oxidative stress1. Glycated collagen also impairs the structure and properties of the extracellular matrix, and increased collagen-derived AGEs are consistently linked to painful diseases, including osteoarthritis, diabetic neuropathy, and neurodegenerative disorders2,3.
Our group previously demonstrated that the SH-SY5Y cell line can be differentiated into sensory-like neuron cells since these cells express channels involved in nociception, such as sodium channels (Nav 1.7, Nav 1.8, and Nav 1.9) and transient receptor potential vanilloid type 1 (TRPV1), markers typically found in peripheral sensory neurons4. TRPV1 is a nonselective cation channel permeable to calcium ions and sensitive to capsaicin stimulus. Importantly, when the sensory-like neuron cells are exposed to glycated collagen matrix (ECM-GC), they gain pro-nociceptive functions by increasing c-Fos expression, a transcription factor involved in neuronal activation, and substance P release, a neuropeptide widely involved in neuroinflammation and pain. These cells respond to analgesics, such as morphine, the prototype opiate, decreasing ECM-GC-induced substance P release. Together, these data indicate that this model is responsive to a pro and anti-nociceptive molecule4,5.
Monitoring intracellular Ca2+ concentration changes is essential for studying numerous cellular processes. In neurons, it can be a useful tool to predict neuronal damage and neuroprotective properties of drugs. Capsaicin, the pungent active ingredient of hot chili peppers, is the most studied agonist of the TRPV1 receptor5 and a valuable tool for studying the pain mechanisms and screening potential new analgesics. Previous studies demonstrated that primary sensory neurons from the dorsal root ganglia of rodents incubated with high glucose exhibit a significant increase in capsaicin-induced calcium influx6. However, whether the TRPV1 channel was functionally active in our cell model and whether the glycated collagen sensitizes sensory-like neuron cells to capsaicin excitation, which may activate nociceptive signaling pathways, remain unknown. Therefore, we aimed to develop a cost-effective protocol utilizing simple tools for real-time calcium monitoring in sensory-like cells while ensuring reliable analysis. Here, we provide a comprehensive protocol for helping researchers go through the steps to differentiate SH-SY5Y cells in sensory-like neuron cells and how to sensitize them to pro-nociceptive stimuli. This method can contribute to the discovery of new analgesic or neuroprotective compounds.
Protocol
1. SH-SY5Y culture and differentiation into sensory-like neuron cells
NOTE 1: All steps present in this section need to be done under a laminar flow hood, and all solutions and supplies need to be sterile.
- First, prepare a culture flask (25 cm2) by adding 5 mL of culture medium.
NOTE: For thawing, expansion, and maintenance of this cell type, use a mixture of culture medium: Dulbecco Modified Eagle's and Ham's Medium F12 (DMEM/F12) supplemented with 10% heat-inactivated fetal bovine serum and 1% penicillin-streptomycin. - Then, remove the cryotube containing the SH-SY5Y cells from the liquid nitrogen and keep it on ice. Thaw the cells in a water bath at 37 °C for 2 min.
- Add 1 mL of complete culture medium to the cryovial, transfer the cells to a 15 mL tube, add 3 mL of complete culture medium (DMEM/F12 supplemented with 10% heat-inactivated fetal bovine serum), and mix by repeatedly drawing it up and dispensing it back into the tube using a pipette.
- Pipette the cells into the 15 mL tube and centrifuge at 290 g. Discard the supernatant to remove dimethyl sulfoxide (DMSO), a cryoprotective agent used to prevent ice crystal formation during the freezing cells.
- Add 1 mL of culture medium to the cell pellet, mix them well using a pipette, and place them in a 25 cm2 culture flask to expand the cells. Place the cells in an incubator in a humidified atmosphere of 5% CO2 at 37 °C.
NOTE: Cells can be used when they reach approximately 80% confluence. For neuronal differentiation, it is recommended to use SH-SY5Y cells up to the cell passage twenty. - Prepare the extracellular collagen matrix (100 µg/mL) by diluting rat tail type I collagen in sterile PBS, and pour 1 mL of this solution into a Petri dish (35/10 mm) for a coating.
- Incubate this in an incubator in a humidified atmosphere of 5% CO2 for approximately 1 h.
- After this period, wash the plate twice with sterile PBS, then count cells using the Neubauer chamber.
- To count cells, first wash the 25 cm2 culture flask containing the cell with 5 mL of sterile PBS to remove all the culture medium. Then remove the sterile PBS, add 3 mL of 0.05% trypsin to the cells, and place them in an incubator at 37 °C and 5% CO2 for approximately 3-5 min to complete cell detachment.
- After that, remove the cells from the incubator, add 6 mL of culture medium to the culture flask, and transfer this solution to a 15 mL tube. Centrifuge at 290 g for 5 min.
- Remove the supernatant and resuspend the cells well in 1 mL of complete culture medium.
- Finally, count the cells using a Neubauer counting chamber. Mix 20 µL of cells and 20 µL of trypan blue, and add 10 µL of this mixture to a Neubauer chamber. Count the live cells that do not stain in blue.
- Plate 5 × 104 cells/mL cells in a Petri dish (35/10 mm) on the same culture medium described before. Use the total volume of 1 mL per plate.
- Then, wait for 24 h to start the neuronal differentiation protocol. For that, remove the culture medium and replace it with 1 mL of DMEM/F12 supplemented with 2% heat-inactivated fetal bovine serum, 1% penicillin-streptomycin, and 10 µM all-trans retinoic acid (RA 10 µM).
- Wait for 48 h and replace the culture medium every day for 3 days. Place the cells in an incubator in a humidified atmosphere of 5% CO2 at 37 °C.
- On the fifth day, remove the medium from the cells and add 1 mL of serum-free culture medium supplemented with human brain-derived neurotrophic factor (BDNF 50 ng/mL) in each plate.
NOTE: BDNF from different companies (see table of material) was tested. They all worked in the same way. - On the seventh day of differentiation, wash the cells with sterile PBS once and use sensory-like neuron cells for experiments. If it is impossible to use these cells immediately for experiments, add a serum-free culture medium to keep them alive until the use.
NOTE: At the end of neuronal differentiation, it is necessary to wash cells with sterile PBS to remove the remaining BDNF, avoiding cell toxicity.
2. Glycated collagen and glycation process
NOTE: All steps in this section must be done under a laminar flow hood, and all solutions and supplies must be sterile.
- Prepare the collagen glycation by incubating collagen type I from rat tail fibrillar state (3.89 mg/ mL) with 200 mM D-ribose, 160 mM D-glucose, and 200 mM D-threose at 4 °C for 7 days.
NOTE: To prepare glycated collagen, weigh the sugars based on their molecular weight and mix them with collagen type I from rat tail fibrillar state (3.89 mg/mL). - Incubate sensory-like neuron cell culture for 24 h with glycated collagen extracellular matrix (ECM-GC, 100 µg/mL) or normal collagen extracellular matrix (ECM-NC, 100 µg/mL) prepared in DMEM/F12 culture medium without serum and antibiotic supplementation.
3. Calcium influx assay
- For calcium influx, use the calcium flux assay kit and prepare the solutions according to the manufacturer's instructions, as indicated below.
- Initially, prepare 1 mL of Fluo-8 dye-loading by adding 2 µL of Fluo-8 stock solution in 998 µL of 1x assay buffer (a mix of HBSS buffer and 10x Pluronic F127 Plus.
NOTE: This working solution is stable for at least 2 h at room temperature (RT). - Later, incubate 250 µL of this solution in each plated dish of sensory-like neuron cells previously differentiated and treated with ECM-NC or ECM-GC for 30 min in a humidified atmosphere of 5% CO2 at 37 °C. Then, remove the dish from the incubator and keep it at RT (in the dark) for 30 min.
NOTE: This solution becomes cytotoxic if the 2 h incubation is extrapolated. Prepare the capsaicin solution during the incubation period.
4. Capsaicin induction
NOTE: Capsaicin, a TRPV1 agonist, was used to induce calcium influx in the cells.
- Weigh and dilute capsaicin in 1% ethanol and ultrapure water. Then, prepare a solution at a concentration of 2 µM, which was also diluted in ultrapure water.
NOTE: Capsaicin solution must be made on the day of use. Dilute capsaicin first in ethanol and then in water; otherwise, it will precipitate. - Add 250 µL of 2 µM capsaicin solution to each 35/10 mm Petri dish so that the final concentration is 1 µM.
NOTE: The stock solution concentration of capsaicin was 2 µM to ensure better dispersion and avoid isolated fluorescence peaks.
5. Calcium influx imaging and confocal microscopy analysis
NOTE: Imaging was performed in a confocal microscope equipped with a 20x/0.75NA objective and a 488 nm excitation laser (0.5% intensity). Emission was detected at 520 nm. Cells were scanned in xy axes (512 x 512 pixels) over time (t) at a speed of 600 Hz with an acquisition interval of 433 ms and a total acquisition time of 5 min. Imaging was performed at 37 °C to maintain the physiological condition of the cells using the microscopy software.
- Prepare a syringe with 250 µL of 2 µM capsaicin solution (the final concentration in the plate will be 1 µM) and connect it to a scalp vein set - Butterfly type 23 GA sterile. Secure the syringe using a lab stand and clamp.
- Put the Petri dish on the microscope stage and, with extreme caution, place the scalp needle in the Petri dish, avoiding contact with the bottom, the tip of the needle should not be submerged in the liquid.
NOTE: An adhesive tape can be used to fix the scalp position. - Find a field with an adequate number of cells (more than 20) and adjust the focus using bright field light.
- Start Live mode with a 488 nm laser and adjust focus and illumination parameters.
- Start the acquisition recording, and after the baseline period (t = 0 to t = 60 seconds), apply 250 µL of 2 µM capsaicin and gently push the syringe plunger to stimulate calcium influx into cells.
NOTE: The final recording acquisition time was approximately 300 s per plate.
6. Post-processing/data analysis
- Perform the cell analysis using the microscopy software in the Quantification mode. First, analyze fluorescence spike cells before and after the capsaicin stimulus.
- Create a region of interest (ROI) in each responsive cell, i.e., cells that display a fluorescence spike after capsaicin. Make sure to draw ROI in the whole cell area, as demonstrated above (Figure 1).
- Export the data in a comma-separated values (CSV) file for subsequent analysis.
- Alternatively, perform the analysis using the freely available software FIJI7 (https://fiji.sc).
- To open the image files, use the Bio-Formats plugin by navigating to File > Import > Bio-Formats and selecting the file.
- Next, use the Magnifying Glass tool to zoom in and the Freehand Selection tool to draw a Region of Interest (ROI) around the responsive cells.
- After drawing each ROI, press the T key to add it to the ROI Manager. Repeat this process for all cells of interest (Figure 2).
- Once all ROIs are added, go to the ROI Manager window, select all ROIs, and then navigate to Analyze > Set Measurements in the main menu.
- Then, return to the ROI Manager and select More > Multi Measure to measure the intensity across all frames. The results will appear in a new window; export as a CSV file by going to File > Save As.
NOTE: The CSV file may be analyzed in Excel, Google Sheets, and GraphPad software. Use the equation ΔF/F0 = (F(t) - F0)/F0 to convert fluorescence intensity values obtained with the Fluo-8 Calcium Flux Assay Kit into a relative measure of calcium influx change. Here, F0 represents the baseline fluorescence, calculated as the average intensity from images captured from t = 0 s to t = 60 s before stimulation with capsaicin. F(t) is the value of the maximum fluorescence intensity observed after the stimulus, which allows for assessing the peak calcium influx. This approach highlights the increase in fluorescence relative to the baseline, where a rise in ΔF/F0 indicates an elevated intracellular calcium level.
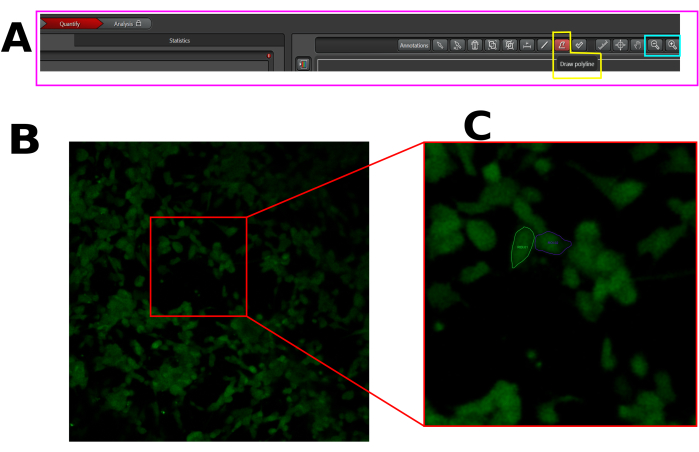
Figure 1: Example of ROI in the selected cells for calcium influx analysis in LAS X software. (A) LAS X interface in quantify mode. The pink rectangle shows the quantification tab. The yellow rectangle shows the draw polyline tool, and the cyan rectangle shows the zoom-out and zoom-in tools. (B) Field of view (FOV) captured. (C) Zoom in on FOV to facilitate the drawing of ROI in the entire cell. Please click here to view a larger version of this figure.
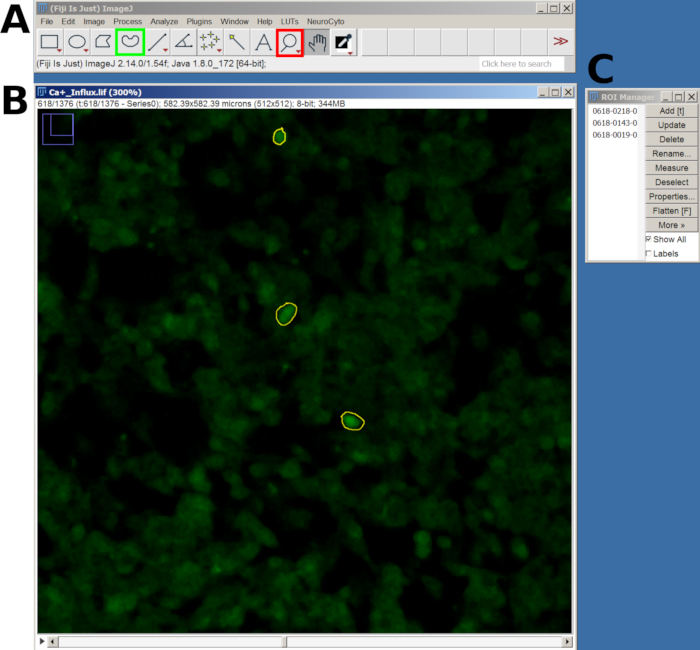
Figure 2: Example of ROI in the selected cells for calcium influx analysis in FIJI software. (A) FIJI interface showing the main menu and tool. The red square shows the magnifying glass tool, and the green square shows the Freehand selection. (B) Field of view (FOV) captured with zoom-in and a drawn ROI. (C) The ROI manager window. Please click here to view a larger version of this figure.
7. Troubleshooting
- If the image is out of focus, try the following:
- Check the adhesion of the tape; if it has loosened, this may cause the needle to shift and affect the focus.
- Reattach the needle with additional tape for increased stability.
- Replacement of standard needles. Use pre-bent dental needle tips to improve alignment on the plate.
- If the cells are exhibiting a saturated signal, try the following steps:
- Decrease laser intensity and gain settings.
- Verify reagent incubation time. If it exceeds the manufacturer's recommendation, it may lead to signal saturation, complicating calcium influx detection.
- Follow these additional suggestions for trouble shooting:
- Regular Calibration: Ensure the imaging equipment is regularly calibrated to maintain focus and signal detection quality.
- Document changes: Keep a log of any adjustments made to the protocol for future reference and to facilitate troubleshooting.
- Reactive adjustments: If issues persist, consider testing with different reagent concentrations or alternative methods of signal detection.
Results
SH-SY5Y cells differentiation into sensory-like neuron
High-content screening images demonstrate that the protocol of neuronal differentiation changes SH-SY5Y cell morphology. The sensory-like neuron cells (differentiated cells) display a rounded cell body that projects an extensive network of neurofilaments. They form branches of more elongated neurite projections connecting surrounding neurons, which is consistent with mature neuron features (Figure 3)4. Importantly, these cells display increased levels of TRPV1 when compared to undifferentiated SH-SY5Y cells (Figure 4).
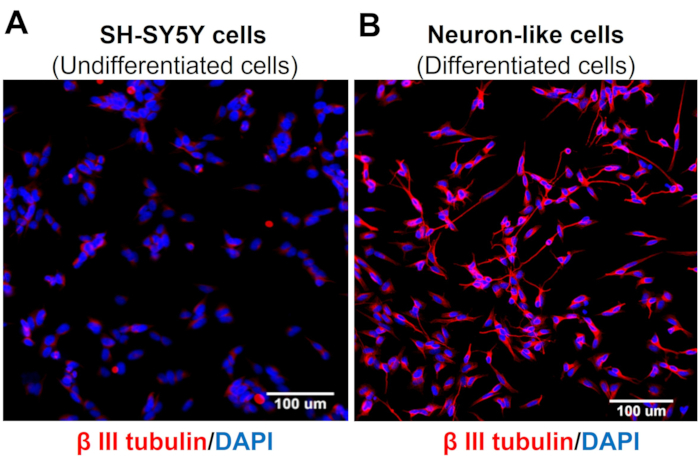
Figure 3: β-III tubulin expression in undifferentiated and differentiated cells. High Content Screening (Molecular Devices, San Jose, CA, EUA) immunofluorescence images of specific neuronal markers (β-III tubulin) in (A) undifferentiated cells (SH-SY5Y cells) and (B) differentiated cells (sensory neuron-like cells). Representative images of five areas. Magnification: 20x. Scale bar: 100 µm. Please click here to view a larger version of this figure.
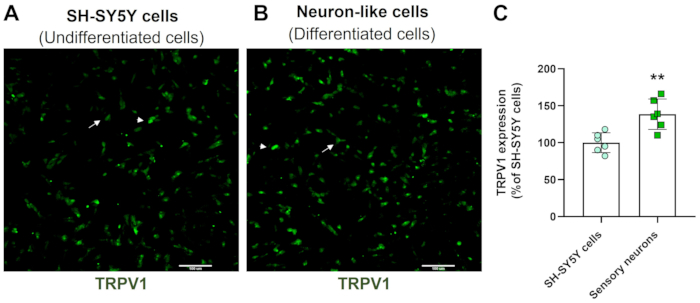
Figure 4: TRPV1 expression in undifferentiated and differentiated cells. HCS immunofluorescence images of TRPV1 expression in (A) undifferentiated cells (SH-SY5Y cells) and (B) differentiated cells (sensory neuron-like cells). Representative images of four areas. The arrow labels low-expression TRPV1 cells, and the arrowhead labels high-expression TRPV1 cells. Magnification: 10x. Scale bar: 100 µm. (C) Quantification of TRPV1 expression in SH-SY5Y cells and sensory neurons. Unpaired Student's t-test, **p<0.005 compared to EMC-NC, n= 6. Please click here to view a larger version of this figure.
Collagen glycation interacting with sensory-like cells mimics a painful microenvironment4. Here, we showed that glycated collagen enhances capsaicin-induced calcium influx when compared to cells treated with normal collagen (Figure 5). These results suggest that AGEs are sufficient to increase capsaicin-evoked currents.
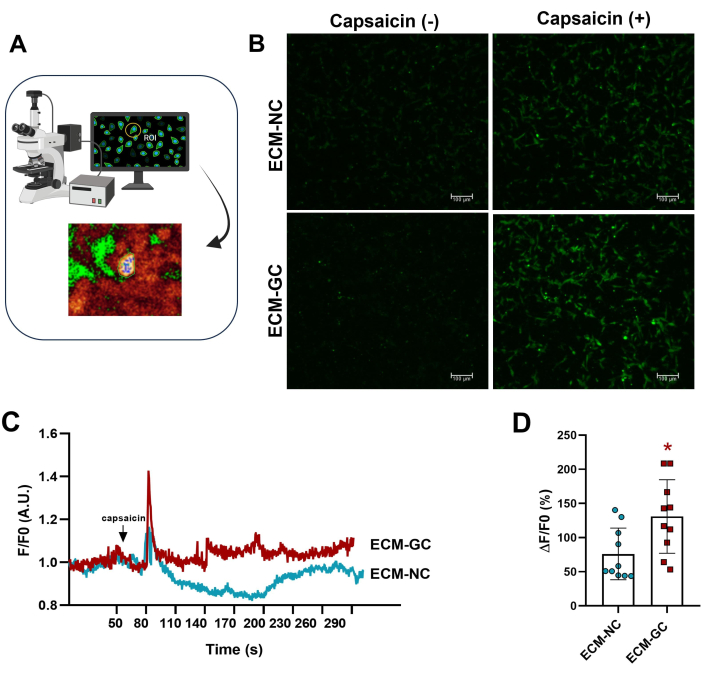
Figure 5: Intracellular calcium measurements. (A) Identifying fluorescence region of interest (ROI). (B-D) Calcium influx is indicated by increased fluorescence before and after capsaicin application (1 µM, final concentration). The arrow indicates capsaicin application. Increases in intracellular calcium were determined as ΔF/F0 (F0 is basal fluorescence). Paired Student's t-test (Wilcoxon matched-pairs), *p < 0.05 compared to ECM-NC, n= 10 cells. Please click here to view a larger version of this figure.
Discussion
Nociceptors are specialized subsets of sensory neurons that mediate pain. These cells express voltage-gated and ligand ion channels, such as TRPV1, whose activation leads to calcium influx and the release of neuropeptides and neurotransmitters that regulate nociceptive transmission. Here, we describe a protocol for differentiating SH-SY5Y into sensory-like neuron cells to evaluate capsaicin-induced calcium influx8,9. Importantly, we showed that mimicking a pro-nociceptive environment with glycated collagen, there is an up-regulation of capsaicin-induced calcium influx, confirming that TRPV1 channels are functional, and AGEs may sensitize these channels by increasing their responsiveness to a noxious stimulus.
Evaluating calcium influx in sensory neurons has been a valuable tool for screening analgesic candidates10. Progress has been made in the design of molecular calcium indicators, with the development of stable calcium dyes like Fluo-8. Fluo-8 (no washing method) has advantages over other fluorescent dyes, including increased brightness, working at room temperature, and showing higher sensitivity to lower calcium concentrations. In this protocol, capsaicin was applied through a scalp vein set. Previously, we used a regular pipette, but it often displaced the cell plate and interrupted the image acquisition. Another possibility would be using a perfusion pump to deliver capsaicin slowly. Interestingly, the approach in this protocol utilizes readily available and cost-effective components that can be implemented in any laboratory, providing a significant advantage over traditional perfusion chambers for monitoring calcium influx. Moreover, the apparatus described here is universally compatible with most fluorescence microscopes commonly used in research settings, eliminating the need for specialized equipment or modifications. This adaptability facilitates seamless integration across diverse imaging configurations, enhancing the accessibility of advanced calcium imaging techniques to a broader range of laboratories, especially in laboratories with limited budgets. However, different from a benchtop multi-mode microplate reader of automated fluorescence measurements, one limitation of the method described here is that it requires high-resolution imaging equipment and manual pipetting, which is more time-consuming. However, confocal microscopy holds the advantage of detecting lower fluorescence emission.
Methods for studying sensory neurons mostly use primary cell culture or pluripotent stem (iPS) cells. Primary human sensory neurons are only rarely accessible and generating iPS is labor-intensive and requires expensive reagents and expert technicians. Therefore, despite the limitation involved in differentiating SH-SY5Y into neuronal cells, which do not completely retain the physiological characteristics of an intact human neuron, they are morphologically similar to primary neurons9,10, and maintain important features, such as expression of sodium channels, substance P release, and responsiveness to capsaicin and morphine4,11,12,13.
Pain is a complex condition, and finding drugs that impair cultured neuron excitation may not be sufficient to block pain sensation. However, the previous data showing that morphine decreases glycated collagen-induced substance P release suggests that our model is useful for finding new analgesic molecules. Moreover, here we demonstrate that glycated collagen is sufficient to hypersensitize neurons to capsaicin-induced calcium influx, indicating that calcium imaging in the presence of AGEs may be useful in searching for new therapeutic compounds for painful inflammatory and degenerative diseases. Of note, we used 1 µM capsaicin to induce Ca2+ influx, a standard concentration for Ca2+ imaging in neurons8,14,15. However, a dose-response curve should be conducted in future studies to optimize the experimental conditions.
The method described here for evaluating calcium influx may have broader applications beyond neuron-like cells. It is also suitable for primary sensory cell cultures and can be adapted for other cell types, such as cardiomyocytes and macrophages. Furthermore, given that glycation collagen occurs with aging and in pathological conditions such as diabetic complications, this approach may serve as a valuable tool for investigating kidney disease and ocular disorders.
A primary modification in our protocol is the replacement of the standard needle with pre-bent dental needle tips. These curved needles allow for better adjustment on the plate, minimizing handling, reducing the amount of adhesive tape needed, and lowering the risk of the tip touching the bottom of the plate.
Some problems that may occur during the procedure and their solutions are detailed in section 7 of the protocol. If the image is out of focus after adjusting the needle on the plate, check if the adhesive tapes have loosened, causing the needle to shift the plate. It is recommended that the needle be reattached with additional tape for better stability. If cells exhibit saturated signals, one should try to decrease the laser intensity and gain parameters. Verify the reagent incubation time. Our experience indicates that if the incubation time exceeds the manufacturer's recommendation, the signal becomes excessively intense, which may interfere with calcium influx detection. For unexpected fluorescence peaks and cell detachment after liquid injection, the liquid must be injected slowly to prevent sudden fluorescence spikes and avoid disrupting cell adhesion. Rapid injections can generate turbulence, leading to transient signal artifacts and detachment of cells from the well bottom.
Disclosures
MCB, AMCT, and VOZ own a patent on the process of identifying molecular entities involved in osteoarthritis pain (BR102018008561-1).
Acknowledgements
This work was supported by Fundação Amparo à Pesquisa do Estado de São Paulo FAPESP Grant number 2015/50040-4 and 2020/13139-0, São Paulo Research Foundation and GlaxoSmithKline, FAPESP 2022/08417-7 and 2024/04023-0.
Materials
| Name | Company | Catalog Number | Comments |
| All-trans retinoic acid | Tocris | 695 | |
| BDNF | Tocris | TOCR-2837 | |
| BDNF | Sigma-Aldrich | B3795 | |
| Butterfly type 23GA sterile | Beckton Dickinson Asepto | 38833814 | Scalp vein set |
| Capsaicin | Sigma-Aldrich | M2028 | |
| D-glucose | Sigma-Aldrich | G5767 | |
| DMEM/F12 | Gibco | 12500062 | Basal medium |
| D-ribose | Sigma-Aldrich | R7500 | |
| D-threose | Sigma-Aldrich | T7392 | |
| Fluo-8 Calcium Flux Assay Kit | Abcam | ab112129 | No wash |
| Heat-inactivated fetal bovine serum | Gibco | A5670801 | |
| High Content Screening | Molecular Devices | ||
| LASX software | Leica Microsystems | Microscopy software | |
| Leica TCS SP8 | Leica Microsystems | Leica TCS SP8 | Confocal microscope |
| Penicillin-streptomycin | Gibco | 15140130 | |
| Petri dish (35/10 mm) | Greiner bio-one | 627965 | |
| Rat tail type I collagen | Corning | 354236 | |
| SH-SY5Y | Merck | 94030304-1VL | Neuroblastoma cell line |
References
- Bierhaus, A. et al. Understanding RAGE, the receptor for advanced glycation end products. J Mol Med. 83 (11), 876-886 (2005).
- Hudson, B. I. et al. Blockade of receptor for advanced glycation endproducts: A new target for therapeutic intervention in diabetic complications and inflammatory disorders. Arch Biochem Biophys. 419 (1), 80-88 (2003).
- Dandia, H., Makkad, K., Tayalia, P. Glycated collagen - a 3D matrix system to study pathological cell behavior. Biomater Sci. 7 (8), 3480-3488 (2019).
- Bufalo, M. C. et al. Human sensory neuron-like cells and glycated collagen matrix as a model for the screening of analgesic compounds. Cells. 11 (2), 247 (2022).
- Frias, B., Merighi, A. Capsaicin, nociception and pain. Molecules. 21 (6), 797 (2016).
- Lam, D. et al. RAGE-dependent potentiation of TRPV1 currents in sensory neurons exposed to high glucose. PLoS One. 13 (2), e0193312 (2018).
- Schindelin, J. et al. Fiji: an open-source platform for biological-image analysis. Nat Methods. 9 (7), 676-682 (2012).
- Li, F. et al. TRPV1 activity and substance P release are required for corneal cold nociception. Nat Commun. 10 (1), 5678 (2019).
- Mickle, A. D., Shepherd, A. J., Mohapatra, D. P. Sensory TRP channels:the key transducers of nociception and pain. Prog Mol Biol Transl Sci. 131, 73-118 (2015).
- Iseppon, F., Linley, J. E., Wood, J. N. Calcium imaging for analgesic drug discovery. Neurobiol Pain. 11, 100083 (2022).
- Vetter, I. et al. Characterisation of Nav types endogenously expressed in human SH-SY5Y neuroblastoma cells. Biochem Pharmacol. 83 (11), 1562-1571 (2012).
- Dekker, L. V. et al. Analysis of human Nav1.8 expressed in SH-SY5Y neuroblastoma cells. Eur J Pharmacol. 528 (1-3), 52-58 (2005).
- Lam, P. M. W., Hainsworth, A. H., Smith, G. D., Owen, D. E., Davies, J., Lambert, D. G. Activation of recombinant human TRPV1 receptors expressed in SH-SY5Y human neuroblastoma cells increases [Ca2+]i initiates neurotransmitter release and promotes delayed cell death. J Neurochem. 102 (3), 801-811 (2007).
- Koplas, P. A., Rosenberg, R. L., Oxford, G. S. The role of calcium in the desensitization of capsaicin responses in rat dorsal root ganglion neurons. J Neurosci. 17 (10), 3525-3537 (1997).
- Tominaga, M. et al. The cloned capsaicin receptor integrates multiple pain-producing stimuli. Neuron. 21 (3), 531-543 (1998).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionExplore More Articles
This article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved