Imaging Biological Samples with Optical and Confocal Microscopy
Przegląd
Source: Peiman Shahbeigi-Roodposhti and Sina Shahbazmohamadi, Biomedical Engineering Department, University of Connecticut, Storrs, Connecticut
Optical microscopes have been around for centuries, and while they reached their theoretical limitation of resolution decades ago, new equipment and techniques, such as confocal and digital image processing, have created new niches within the field of optical imaging. The best optical microscopes will typically have a resolution down to 200 nm in ideal conditions. However, optical microscopes are limited by the diffraction of waves, a function of the wavelength, which is around 500 nm for visible light. While the resolution of optical microscopes does not reach that of electron microscopes, they are the most valuable tools in the imaging of biological macrostructures and are a staple in any biological lab.
In conventional light microscopes, the signal produced from the imaged object is from the full thickness of the specimen, which does not allow most of it to be in focus to the observer. This causes the image to have "out of focus blur". The confocal microscope, on the other hand, illuminates the sample through a pin-hole, and is therefore able to filter out the out-of-focus light from above and below the point of focus in the object.
This demonstration provides an introduction to image acquisition using optical and confocal microscopy methods. Here, a sectioned piece of mouse brain will be studied. Image acquisition and analysis, including the tools to generate topographical maps and composite images, will be covered. The advantages and disadvantages of different imaging methods as they relate to resolution, depth of focus and sample type will also be discussed. The purpose of this demonstration is to provide more information on optical and confocal microscopes to determine if these microscopy modules are the best fit for a type of biological sample.
Zasady
Optical microscopes function using at least two elements of magnification. The primary lens, called the objective, determines the total magnification, and the secondary lens, called the eyepiece, focuses the virtual image for viewing. The total magnification is determined by multiplying the magnifications of the two lenses. The focusing of light through these sources, coupled with the focusing of light from the lamp onto the sample, give a specified plane of focus where the magnification and the lamp light all meet at the same point, which gives the best resolution in the image. The figure below demonstrates how the specimen's focal plane is created through the different lenses. Objects outside of the focal plane will have beams of light interfering from other parts of the sample due to the larger area of illumination. This causes blur in the image. Therefore, to focus on different z-positions of a sample with widely varying heights, the z-direction slices must be moved into the focal plane.

Figure 1. Optical microscopy lenses and focal planes.
Digital microscopes work on the same principle as optical micropscopes, except that it doesn't rely on an eyepiece. It's an optical microscope equipped with a digital camera. The digital camera acts as a detector, and images are displayed on a computer monitor. These microscopes are ideal for the analysis and documentation of samples during research and development (R&D), manufacturing and inspection, quality control and assurance (QC/QA), as well as failure analysis (FA). They usually offer software that allow users to analyze the sample image. Figure 2 shows a typical digital microscope set-up.

Figure 2. Main components of digital microscope.
The main components of the system are:
- Optical engine: Contains the image acquisition sensor and lenses for zooming in on the image.
- Objective: Acquires and focuses the light from the sample. Three different objectives are available for various image acquisition tasks.
- Scanning stage: Location where the sample is to be placed.
- Microscope stand: Provides the support for the optical engine and the scanning stage. Also controls the communication between the attached components and the computer.
- Computer: Supports user software and allows images to be viewed on monitor.
- Controller: Controls the microscope and workflow using multi-touch gestures and touch-sensitive, context-specific icons. The control knobs control the zoom, focus, and microscope image position.
A confocal microscope, or confocal laser scanning microscopy (CLSM), is a microscope with increased optical resolution and contrast. Confocal means "having the same focus". The object and its image are "confocal."
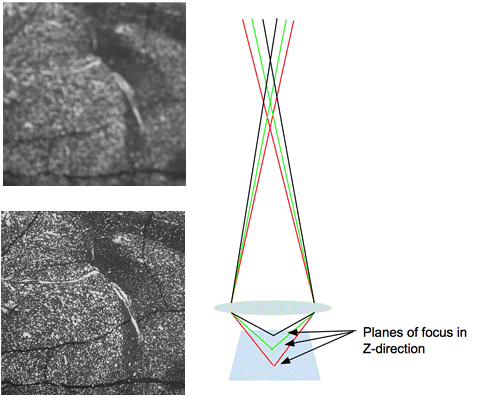
Figure 3. Blur and its effect on an image. The left image shows an out of focus image with blurred edges. The right image demonstrates the path of light through the lens when imaging a sample that is in focus.
As opposed to general light microscopes that illuminate and image the entire sample in view, confocal microscopes utilizes a pinhole between the sample stage and detector so that only a smaller beam of light is focused at one narrow depth level at a time. Thus, the only visible area of the sample is the in-focus point. The confocal microscope then raster scans the surface of the sample with this much more focused beam of light (or a laser). The data is then assembled into one 2D image that has better resolution than classical optical microscopy. Also, because the light is focused on a very narrow range of heights, the user can put different planes into focus as the Z-direction is moved. Through image processing techniques and automation software confocal microscopes aid in the 3D reconstruction of multi-plane focused composite images.
Confocal microscopes have the ability through image processing to give Z-direction data about a sample that previously wasn't available in optical microcopy. For example, in the demonstration described below, the user can define the upper and lower ranges of focus for a sample, and then not only develop a heat map showing measurements of the z-direction but also create a composite image that shows all parts of the image in focus. These features are especially helpful when obtaining 3-D data about a sample.

Figure 4. Main components of a confocal microscope.
The main components of the confocal microscope include:
- Scan head with fine Z-drive and 4-megapixel camera, stand with coarse Z-drive
- Objectives: 2.5x/ 5x/ 10x/ 20x/ 50x/ 100x
- Stages: Scanning stage and fixed stage
- Computer System: PC system imaging software
- Controller: x, y, z motion
Procedura
1. Confocal Imaging
- Load the sample onto the stage. Center it beneath the lens. It should not exceed the weight limitation of the stage, which in this case is 5 kg. The sample should not be more than 100 mm thick.
- Open the imaging software and select "Create Job."
- Under the Topographies Column, choose the assistant button.
- Create an overview image at the lowest magnification, 2.5X. Before switching the magnifications, ensure that the sample is in focus by changing the Z position until you see a clear image. This can be done by pushing down or pulling up on the 3D microscope manipulator. Finer Z motion is attained by engaging the button on the side, which causes a blue light around the edges.
- Slowly increase the lens magnification, continuously playing with the light intensity and the focus until you are at the desired magnification. If desired, choose a different area of interest by moving the stage in the x and y directions by using the manipulator.
- Once an overview image is taken at the low magnification, hit the next button to proceed to the Reference Point step. If desired, specify a certain reference point for the sake of measurement (i.e., the corner of the sample), though for this purpose the default reference point is fine.
- Hit the next arrow to proceed to the next part of the wizard.
- Change the objective as desired to see a resolution appropriate for your sample. In this case, the 50X objective lens is used to visualize the cells in the sample. The 50X is the lens that is closest to the sample, so gradually move up to the 50X objective ensuring that there is still room to decrease the working distance after the 20X lens.
- On the "Measuring Range Definition" page, move the Z position slightly (clicking the side button on the manipulator for fine adjustments) so that only the very top of the sample is in focus and click "Set Last". Then move the stage in the Z direction (downwards) until only the very bottom of the sample is in focus and press "Set First". Make sure that the number of calculated slices does not exceed 1000 or the program will fail.
- Make sure that the intensity of light does not oversaturate (cause red pixels) in the image at any of the levels and then hit done. This will take the tomography image and open the tomography software.
- In the tomography software, open tabs such as the Studies tab to view the data in 3D and take measurements in 2D or 3D space.
2. Digital Optical Microscope Imaging
- Load the sample onto the stage. Center the sample beneath the lens. The sample weight should not exceed the weight limitation of the stage, which in this case is 4 kg. The sample should be no higher than 12 cm.
- Open the imaging software.
- Select a Job from the list of templates provided. It is also possible to work outside of a job by pressing Free Examination, which allows you to study a sample outside of a job.
- Acquire an overview image that shows the entire stage. This will serve as a map later on to display the part of the sample that is being viewed. While acquiring the image, use the controller to change the focus and the image position.
- Place a coordinate system. The default coordinate system is from the back-left corner of the stage and is fine for this application. If the sample is crooked, you can adjust the coordinates here.
- Name the sample and the job, this adds it to the list of jobs so that other users can return to it.
- Select the camera button under Acquire. Take an initial image, and then press the live button as you navigate the sample.
- Move the focus downwards until the sample is clearly in focus. It may be necessary to also adjust the lighting under the Lighting and Aperture" tab.
- Optimize the image with the tools under the Image Optimization panel. You can play with different parameters under the Image Enhancements tab, such as the tilt of the lens, the lighting levels on the sample, and brightness and contrast, until the image has the desired crispness.
- Perform measurements by tapping the Pencil tool on the software. From there, you have access to several measurement tools, including distances, angles, and area. Use the distance and area tools to measure the size of the sample.
- Go to the Results Workflow tab and check the workflow layout to configure the layout of the report
- Tap the save button to save your job so that others can use the same workflow.
Wyniki
The following images give an overview of results that can be obtained of a mouse brain using a confocal microscope. They show how varying levels of information can be obtained and how a topographical map of the results reveals the height of the sample.
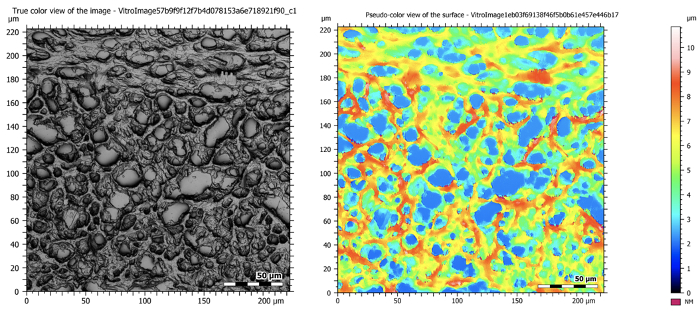
Figure 5: Confocal images at 50X magnification showing a sectioned mouse brain. The image on the left is a composite image that takes all of the in-focus planes during tomography and creates one high resolution and deeply focused image. The image on the right shows the topographical map of the sample.

Figure 6: As a better representative example of the 3D applications of the confocal microscope, a hole in plastic was imaged and analyzed. The original topographical map is on the left and the 3D reconstruction is on the right.

Figure 7: Shows the extent of the ConfoMap software analysis for observing a profile from a 3D reconstruction. The amplitude parameters, roughness profile, curve characterization are shown.
The following images give an overview of results that can be obtained from using a digital optical microscope on the same mouse brain slice. The digital microscope gives a larger field of view but lower resolution images from the confocal microscope, which is ideal for looking at larger components or biological structures. The software has useful analysis tools for measuring the sample.

Figure 8: Overview image showing whole organ slice.

Figure 9: Zoomed in image of a sectioned mouse brain. Here is a 300 micron field of view obtained with mixed coaxial and ring lighting as well as electronic image stabilization.

Figure 10: Demonstration the measurement capabilities of the digital optical microscope. The diameter of the sample is measured on the left, and a user-defined outline that is used to calculate the internal area of the sectioned mouse brain is shown on the right. These tools are useful when analyzing biological samples, which may not have edges that are the same as pre-defined shapes.
Wniosek i Podsumowanie
In this demonstration, the depth of focus, field of view and maximum resolution and magnification of optical and confocal microscopes were optimized to view biological samples. This demonstration was designed to help the participant decide which microscopy module is the best for a certain application. Both modes of microscopy have advantages in analyzing biological samples for their ease of preparation and high resolution composite images.
The applications for optical and confocal microscopy are far-reaching. Because of the limited sample preparation and the ability to integrate planes of motion and use above-sample light techniques, these tools are able to obtain information from most data sets. Microscopy has been a very popular option when imaging live cells, such as those treated with fluorescence, but the applications can range from imaging surfaces of biomedical devices to detecting defects and roughness before implantating them in the body. Confocal and optical microscopy are the current standard for imaging biological samples.
Finally, confocal microscopy offers improved imaging with fluorescence techniques. Fluorophores in a sample have a limited lifetime and can photo-bleach when exposed to high amounts of light. In traditional light microscopy, the entire sample is illuminated during imaging, which results in rapid photo-bleaching. However, since only a tiny portion of the sample is illuminated at one time with confocal microscopy, the lifetime of the fluorophore is longer and there are fewer challenges associated with photo-bleaching.
Tagi
Przejdź do...
Filmy z tej kolekcji:

Now Playing
Imaging Biological Samples with Optical and Confocal Microscopy
Biomedical Engineering
35.7K Wyświetleń

SEM Imaging of Biological Samples
Biomedical Engineering
23.5K Wyświetleń

Biodistribution of Nano-drug Carriers: Applications of SEM
Biomedical Engineering
9.3K Wyświetleń

High-frequency Ultrasound Imaging of the Abdominal Aorta
Biomedical Engineering
14.4K Wyświetleń

Quantitative Strain Mapping of an Abdominal Aortic Aneurysm
Biomedical Engineering
4.6K Wyświetleń

Photoacoustic Tomography to Image Blood and Lipids in the Infrarenal Aorta
Biomedical Engineering
5.7K Wyświetleń

Cardiac Magnetic Resonance Imaging
Biomedical Engineering
14.7K Wyświetleń

Computational Fluid Dynamics Simulations of Blood Flow in a Cerebral Aneurysm
Biomedical Engineering
11.7K Wyświetleń

Near-infrared Fluorescence Imaging of Abdominal Aortic Aneurysms
Biomedical Engineering
8.2K Wyświetleń

Noninvasive Blood Pressure Measurement Techniques
Biomedical Engineering
11.9K Wyświetleń

Acquisition and Analysis of an ECG (electrocardiography) Signal
Biomedical Engineering
104.8K Wyświetleń

Tensile Strength of Resorbable Biomaterials
Biomedical Engineering
7.5K Wyświetleń

Micro-CT Imaging of a Mouse Spinal Cord
Biomedical Engineering
8.0K Wyświetleń

Visualization of Knee Joint Degeneration after Non-invasive ACL Injury in Rats
Biomedical Engineering
8.2K Wyświetleń

Combined SPECT and CT Imaging to Visualize Cardiac Functionality
Biomedical Engineering
11.0K Wyświetleń
Copyright © 2025 MyJoVE Corporation. Wszelkie prawa zastrzeżone