Cardiac Magnetic Resonance Imaging
Przegląd
Source: Frederick W. Damen and Craig J. Goergen, Weldon School of Biomedical Engineering, Purdue University, West Lafayette, Indiana
In this video, high field, small-bore magnetic resonance imaging (MRI) with physiological monitoring is demonstrated to acquire gated cine loops of the murine cardiovascular system. This procedure provides a basis for assessing left-ventricular function, visualizing vascular networks, and quantifying motion of organs due to respiration. Comparable small animal cardiovascular imaging modalities include high-frequency ultrasound and micro-computed tomography (CT); however, each modality is associated with trade-offs that should be considered. While ultrasound does provide high spatial and temporal resolution, imaging artifacts are common. For example, dense tissue (i.e., the sternum and ribs) can limit imaging penetration depth, and hyperechoic signal at the interface between gas and liquid (i.e., pleura surrounding the lungs) can blur contrast in nearby tissue. Micro-CT in contrast does not suffer from as many in-plane artifacts, but does have lower temporal resolution and limited soft-tissue contrast. Furthermore, micro-CT uses X-ray radiation and often requires the use of contrast agents to visualize vasculature, both of which are known to cause side effects at high doses including radiation damage and renal injury. Cardiovascular MRI provides a nice compromise between these techniques by negating the need for ionizing radiation and providing the user with the ability to image without contrast agents (although contrast agents are often used for MRI).
This data was acquired with a triggering Fast Low Angle SHot (FLASH) MRI sequence that was gated off of the R-peaks in the cardiac cycle and expiratory plateaus in respiration. These physiological events were monitored through subcutaneous electrodes and a pressure-sensitive pillow that was secured against the abdomen. To ensure the mouse was properly warmed, a rectal temperature probe was inserted and used to control the output of a MRI-safe heating fan. Once the animal was inserted into the bore of the MRI scanner and navigation sequences were run to confirm positioning, the gated FLASH imaging planes were prescribed and data acquired. Overall, high field MRI is a powerful research tool that can provide soft tissue contrast for the study of small animal disease models.
Zasady
Magnetic resonance imaging is a technique that exploits the paramagnetic properties of tissue to visualize soft tissue contrast. The bore of an MRI machine is conventionally wrapped using a solenoid coil that provides a constant homogeneous magnetic field (B0) when an electric current is applied. In the presented high field murine imaging, a 7 Tesla (T) magnetic field strength, which is approximately 140,000 times that of the Earth's magnetic field and more than double the common clinical 3T and 1.5T scanner field strengths, is used. This homogeneous magnetic field causes the hydrogen protons inherent to almost all living tissues to align their axes of rotation. These spins can then be "tipped" using radio-frequency (RF) waves to a certain angle relative to the axis of rotation (i.e., the flip angle). As the protons then attempt to relax back to their original orientation, the component of their spin perpendicular to their main axis induces a detectable electric signal.
Furthermore, magnetic gradients can be applied that perturb the main magnetic field and allow for spatially-isolated RF excitations to localize the received signal. Specific to the methods described here, the FLASH sequence uses repetitive low flip angle excitations to induce a steady-state pattern in the proton motion. This paradigm allows tissues that are inherently dynamic, such as in the cardiovascular system, to be imaged rapidly and achieve relatively stable snapshots within the cardiac cycle. Through triggering the FLASH sequence with physiologic signals, images of cardiovascular system can be acquired that highlight both cardiac, vascular, and respiratory motion.
Procedura
1. Animal Preparation
- Identify the mouse to be imaged in its cage and transfer it to the anesthesia induction chamber.
- Anesthetize the mouse using isoflurane and confirm knockdown using a toe-pinch technique. Take the paw between your thumb and index finger and pinch firmly to check for a response. If the animal withdraws their foot, you should either wait or redose with anesthesia according to the approved protocol.
- Check that all staff entering the imaging facility are MR safe. This includes removing any magnetic clothing/accessories, confirming no magnetic implants or pacemakers, and removing metal containing piercings.
- Open isoflurane flow to the nosecone in the MRI room. This allows the longer tubing to be primed with anesthetic before mouse transfer to ensure the animal does not wake up.
- Close isoflurane flow to the anesthesia induction chamber and transfer the mouse to the imaging stage. Place the mouse on the stage such that the approximate location of the heart is aligned with the center of the magnet.
- Secure the nose cone and reconfirm knockdown using the toe-pinch technique.
- Insert the three electrocardiogram leads subcutaneously with one lead to the left and right of the heart and one at the base of the left hind limb.
- Insert the rectal temperature probe using a sterile probe sheath and lubricant.
- Place the pillow respiration sensor on the epigastric region of the abdomen and secure it in place using a cardboard plate. The cardboard will allow ensure a pressure-sensitive signal.
- Confirm all physiological signals are being acquired through the monitoring software outside the scanner room. If a heart rate, respiratory rate, or temperature outside of the normal range is detected, pause imaging and assess whether the animal is appropriately anesthetized. If the animal is found to be in distress, anesthesia administration should cease and the animal should be returned to the cage to recover.
- Set-up the heating module and fan and begin warming airflow to the animal. Secure all the air tubing in place so that the warm air blows toward the mouse starting just past the tip of the tail.
- Place the gradient coil over the animal and make sure all cables/tubing are secured.
2. Cardiac Magnetic Resonance Imaging - This section can be adapted for other applications.
- Tune and match the gradient coil outside the bore of the magnet to ensure the maximal signal is being detected from the subject.
- Slowly insert the imaging stage into the bore of the magnet such that the animal is positioned directly in the center of the bore. This includes making sure the gradient coil has equal spacing along all radial directions. This is the position where the main magnetic field will be most homogeneous.
- Run a localizer/navigator scan to confirm the location of the mouse within the scanner. Some segment of the heart should be visualized within all three planes (i.e., axial, sagittal, and coronal). If this is not the case, repeat the process of repositioning the mouse and running localizer/navigation scans until the desired position is achieved.
- Set-up the parameters for the FLASH sequence. For example: TR/TE = 8.0/2.0 ms, FA = 20°, FOV = 35 x 35 mm, matrix-size = 192 x 192, and NEX = 6. Then, select the external triggering to be "on".
- On the monitoring software, configure the external triggers such that the MRI sequences are started upon identifying the R-peaks in the cardiac cycles and while respiration is stable during the expiratory phase. As those two conditions are met, the sequence can be serially run and data will be acquired.
- Prescribe and run an initial FLASH sequence slice in the coronal view such that the slice plane follows the axis from the apex of the heart through the aortic valve. This initial cine loop will provide a two-chamber view of the heart.
- Referencing the results from the two-chamber view, prescribe and run a new FLASH sequence along the apex-aortic valve axis to visualize a four-chamber view.
- Finally, prescribe a short-axis slice that is perpendicular to the apex-aortic valve axis approximately halfway through the heart. The papillary muscles should be distinctly visible within the cine loop output at this location.
- Additional slices can be acquired parallel to this one to build time-synced volumes of the heart. These volumes are created by concatenating adjacent cine loops in post-processing.
- Once imaging is completed, transfer acquired data to an appropriate location for analysis and remove the animal from the scanner.
Wyniki
Figure 1 shows a cine loop of a short-axis view of the left ventricle, which is directly perpendicular to the base-apex axis of the heart and at a position that includes the papillary muscles.

Figure 1: Bright blood cine imaging of a mouse heart with 14 short-axis view snapshots across the cardiac cycle, including end diastole (t = 8) and peak systole (t = 13). The regions of dropout signal within the lumen of the left ventricle indicates fast blood moving, which was originally out of plane and not tagged by the RF wave excitation.
The second representative image shows a 4-chamber view of the heart with bright-blood inflow through the mitral and tricuspid valves, which then flows out through the aortic and pulmonary valves, respectively.
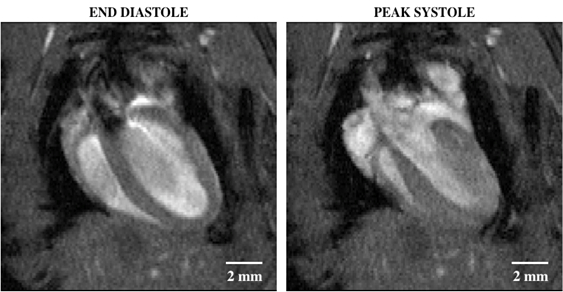
Figure 2: Bright blood cine imaging of a mouse heart with a four-chamber view showing end diastole (left) and peak systole (right). The regions of dropout signal within the lumen of the left ventricle indicates fast blood moving, which was originally out of plane and not tagged by the RF wave excitation.
Finally, a third representative result is a maximum intensity projection (MIP) that shows how the multiple slices can be spatially combined to visualize the cardiovascular system of the whole mouse body.
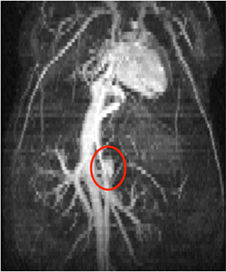
Figure 3: Maximum intensity projection of a three-dimensional stack of time-synced two-dimensional bright blood images, showing the thoracic and abdominal regions of a mouse. The heart, inferior vena cava, and small abdominal aortic aneurysm (red circle) can be seen from this view.
Wniosek i Podsumowanie
Here, cardiac MRI is used in conjunction with cardiac- and respiration-gating to acquire cine loop data of the murine heart. While the heart was the focus of demonstration, additional regions of the cardiovascular system can be imaged following the same methodology. Even though MRI does not suffer from the same artifacts commonly seen with other imaging modalities, there is a noticeable trade-off with spatial resolution achieved per acquisition duration. This trade-off is of concern when the mouse cannot withstand longer durations of anesthesia, such as in severe disease models. Still, MRI has the advantage of visualizing the underlying tissue without the risk of ionizing radiation damage inherent to micro-CT. Using MRI techniques, an in vivo assessment of the cardiovascular can be performed, laying the foundation for longitudinal studies of both disease progression and associated therapy response in small animal models.
As an extension of the described technique, this technology can be used to compare the kinematics of healthy versus diseased hearts. Murine models of cardiac dysfunction can be far more controlled than those found in the clinic, allowing researchers to identify specific factors contributing to heart disease as well as study the remodeling process after mechanical injury. Furthermore, a comparable research endeavor can be performed with a vascular focus such as that with abdominal aortic aneurysm (AAA) formation. Given that blood gives a high intensity signal under the described methods, the contrast can be exploited to assess the expansion of a AAA and measure changes to the vessel's biomechanical properties. Finally, studies looking at vascularization of the brain can be conducted to compare angiogenic responses to traumatic brain injury or stroke. Ideally, as with most pre-clinical imaging, techniques such as high field cardiovascular MRI can further our understanding of human disease processes as well as spark innovation towards the next generation of diagnostic technology.
Tagi
Przejdź do...
Filmy z tej kolekcji:

Now Playing
Cardiac Magnetic Resonance Imaging
Biomedical Engineering
15.0K Wyświetleń

Imaging Biological Samples with Optical and Confocal Microscopy
Biomedical Engineering
36.3K Wyświetleń

SEM Imaging of Biological Samples
Biomedical Engineering
24.1K Wyświetleń

Biodistribution of Nano-drug Carriers: Applications of SEM
Biomedical Engineering
9.6K Wyświetleń

High-frequency Ultrasound Imaging of the Abdominal Aorta
Biomedical Engineering
14.8K Wyświetleń

Quantitative Strain Mapping of an Abdominal Aortic Aneurysm
Biomedical Engineering
4.6K Wyświetleń

Photoacoustic Tomography to Image Blood and Lipids in the Infrarenal Aorta
Biomedical Engineering
5.9K Wyświetleń

Computational Fluid Dynamics Simulations of Blood Flow in a Cerebral Aneurysm
Biomedical Engineering
12.0K Wyświetleń

Near-infrared Fluorescence Imaging of Abdominal Aortic Aneurysms
Biomedical Engineering
8.4K Wyświetleń

Noninvasive Blood Pressure Measurement Techniques
Biomedical Engineering
12.1K Wyświetleń

Acquisition and Analysis of an ECG (electrocardiography) Signal
Biomedical Engineering
107.0K Wyświetleń

Tensile Strength of Resorbable Biomaterials
Biomedical Engineering
7.7K Wyświetleń

Micro-CT Imaging of a Mouse Spinal Cord
Biomedical Engineering
8.3K Wyświetleń

Visualization of Knee Joint Degeneration after Non-invasive ACL Injury in Rats
Biomedical Engineering
8.3K Wyświetleń

Combined SPECT and CT Imaging to Visualize Cardiac Functionality
Biomedical Engineering
11.2K Wyświetleń
Copyright © 2025 MyJoVE Corporation. Wszelkie prawa zastrzeżone