Near-infrared Fluorescence Imaging of Abdominal Aortic Aneurysms
Przegląd
Source: Arvin H. Soepriatna1, Kelsey A. Bullens2, and Craig J. Goergen1
1 Weldon School of Biomedical Engineering, Purdue University, West Lafayette, Indiana
2 Department of Biochemistry, Purdue University, West Lafayette, Indiana
Near-infrared fluorescence (NIRF) imaging is an exciting optical technique that utilizes fluorescent probes to visualize complex biomolecular assemblies in tissues. NIRF imaging has many advantages over conventional imaging methods for noninvasive imaging of diseases. Unlike single photon emission computed tomography (SPECT) and positron emission tomography (PET), NIRF imaging is rapid, high-throughput, and does not involve ionizing radiation. Furthermore, recent developments in engineering target-specific and activatable fluorescent probes provide NIRF with high specificity and sensitivity, making it an attractive modality in studying cancer and cardiovascular disease. The presented procedure is designed to demonstrate the principles behind NIRF imaging and how to conduct in vivo and ex vivo experiments in small animals to study a variety of diseases. The specific example shown here employs an activatable fluorescent probe for matrix metalloproteinase-2 (MMP2) to study its uptake in two different rodent models of abdominal aortic aneurysms (AAAs).
Zasady
As the name suggests, NIRF imaging utilizes light within the first near-infrared window, ranging from 650 nm to 900 nm, to deliver photons into tissue. The energy, E, of a photon is characterized by Equation 1, where h is the Planck's constant, c is the speed of light in a vacuum, and λ is the wavelength of light.
 =
=  (Equation 1)
(Equation 1)
Target-specific fluorescent molecules called fluorophores are typically introduced into the animal through genetic engineering or via tail vein injection prior to imaging. These fluorophores absorb photon energy, which raises the energy of the molecules from the ground state, S0, to the unstable, excited state S1'. Due to the instability of the S1' state, the molecules relax to the lowest vibrational energy level within that state and release energy in the form of heat. The fluorophores, now in the relaxed, excited state S1, then return to the ground state S0, emitting light at a specific wavelength. The emitted light, which has a longer wavelength due to the dissipation of energy in the form of heat, is then captured and recorded using a fluorescence imaging system. The fundamental shift between the absorption and emission spectra is called the Stokes shift and is important as it makes it possible to differentiate between the excitation and emission light.
Procedura
The following procedure provides detailed steps needed to collect in vivo and ex vivo NIRF images from small animals:
1. Experimental Setup
- Connect a fiber optic light source to the fluorescence imaging system using a fiber optic light guide.
- Select the appropriate excitation filter for the experiment. The excitation filter determines the wavelength of light to be delivered to the sample and should be chosen to match the excitation spectrum of the fluorophore introduced into the sample.
- Select the appropriate emission filter. The emission filter blocks undesired spectral components, which may be attributed to autofluorescence, and should be chosen to match the emission spectrum of the fluorophore.
2. Sample Preparation
- In Vivo
- Anesthetize the animal in an induction chamber using isoflurane at a concentration of 3-4% on the flowmeter dial.
- Transfer the animal to a nose cone that is fixed on the imaging stage, and maintain isoflurane at a concentration of 1-2%. A heat source is not necessary because the animals are typically only imaged for a short period of time (> 5 min), and their body temperature does not substantially decrease.
- Secure the animal's paws to minimize motion artifacts. Remove hair from the region of interest by applying a depilatory cream.
- Apply depilatory cream to the smallest area necessary. After 30 s, wipe it off with a gauze pad. Wipe the area a second time with an ethyl alcohol moistened gauze pad to completely remove the depilatory cream.
- Apply ophthalmic ointment to the eyes to prevent drying of the corneas.
- Inject the activatable fluorescent molecular probe into the animal. For this specific application, MMP2 activatable probes were injected intravenously into the tail vein. At this point, the mouse can be imaged. Proceed to the "Image Acquistion" of this protocol to continue. Monitor the animal for regular breathing throughout the brief procedure.
- Ex Vivo
- Following injection of the fluorescent probe, euthanize the animal in a humane way according to the 2013 AVMA Guidelines for the euthaniasia of animals. Carbon dioxide (CO2) overdose is a standard practice for euthanizing small animals.
- Surgically extract the tissue or organ of interest and carefully remove excess adipose tissue with forceps.
- Rinse the tissue in phosphate buffered saline to remove residual blood and place the sample directly on the imaging stage.
3. Image Acquisition
- Open the molecular imaging software and turn on both the fiber optic light source and the fluorescence imaging system.
- Open the acquisition window and specify the type of exposure appropriate for the study. Available exposures include: Standard Exposure to capture a single image, Time Lapse Exposure to capture a series of images over a fixed time interval, and Progressive Exposure to capture a continuous sequence of exposures at different exposure times.
- Select UV-Transillumination as the illumination source.
- Using the previewed image as a reference, adjust the focus of the lens, the field of view, and the f-stop/aperture in the capture system chamber to optimize the sampled image quality. Adjust the exposure time and position of the sample.
- Close the preview window and ensure that all parameters on the acquisition window match the camera and filter settings.
- Click 'Expose' to acquire and save the image.
- Standard molecular imaging software typically provides a variety of analytical, measurement, and image correction tools to quantify fluorescence signals for image analysis.
- At the end of the imaging session, remove the sample/animal, turn-off the system, and clean the imaging stage to minimize damage to the system.
Wyniki
Representative in vivo and ex vivo NIRF images taken from rodents with abdominal aortic aneurysms (AAAs) are shown in Figures 1-2. An activatable fluorescent probe was injected systemically via the tail vein to visualize matrix metalloproteinase-2 (MMP2) activity. MMP2 is an elastolytic enzyme involved in the degradation of the extracellular matrix that plays a major role in the initiation and progression of AAA. All images were acquired using a 625 nm excitation filter, a 700 nm emission filter, and 60 seconds exposure time.
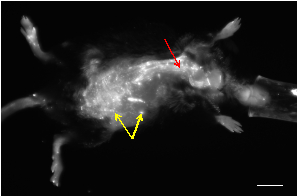
Figure 1: A representative in vivo NIRF image of an apolipoprotein E-deficient mouse that developed an AAA following infusion of angiotensin-II. Most of the small spots showing high signal are from skin autofluorescence (yellow arrows). The vasculature can be visualized as tubular structures with high fluorescence signals (red arrow). Scalebar: 1 cm.
Figure 2 shows an increase in MMP2 activity in the aneurysmal region of the abdominal aorta, as seen by the observed increase in signal intensity relative to healthy regions of the abdominal aorta. This result is consistent with results in the literature that show elevated MMP2 levels within AAAs. Excess fluorescent probes were filtered and accumulated in the kidneys, leading to bright fluorescent signals.

Figure 2: NIRF images of AAAs from two different animal models: (A) a suprarenal AAA in angiotensin II-infused apolipoprotein-E deficient mouse and (B) an infrarenal AAA in rat infused with porcine pancreatic elastase. Yellow arrows point to the AAAs. Scalebars: 3 mm.
Wniosek i Podsumowanie
NIRF imaging relies on fluorescent probes to quantify and visualize biomolecular assemblies in tissues. Absorbed photon energy from near-infrared light excites fluorescent molecules to a higher energy state, and the emitted light with a longer characteristic wavelength is captured by a fluorescence imaging system. Here, the application of NIRF imaging to study MMP2 activity in abdominal aortic aneurysms was demonstrated in vivo and ex vivo. Unlike SPECT or PET, which are considered to be the gold standards in studying metabolic processes in the body noninvasively, NIRF imaging is a rapid and high-throughput imaging technique that does not involve ionizing radiation. One of the limitations of this modality is its relatively small penetration depth. Although this limitation makes clinical imaging of deep tissues challenging, NIRF imaging plays an important role studying tumors and cardiovascular diseases in small animals.
Given the appropriate fluorescent probe, many molecular structures can be visualized using the presented NIRF imaging procedures to study both disease initiation and progression in small animal models. Specific ex vivo and in vivo applications include 1) evaluation of MMP activity in rodent vasculature, 2) early tumor detection in different types of cancers, and 3) assessment of nanoparticle pharmacokinetics and biodistribution for therapeutic applications. In addition to increased MMP2 activity within AAAs, other MMP fluorescent probes have been utilized to study atherosclerosis progression and to characterize cardiac extracellular matrix composition following a myocardial infarction. Furthermore, the fluorophore indocyanine green has been used to study tissue perfusion in murine models of hindlimb ischemia. To elaborate more on the application of NIRF imaging on early cancer detection, tumor-targeting NIRF dyes can be used to assess tumor margins and assist in resection procedures. The integration of near-infrared fluorophores into nanoparticles developed for drug delivery allows scientists to develop more effective nanoparticle-based therapeutics for a variety of diseases. Lastly, the ability to spatially localize the fluorescent signal in whole animals or intact tissue is a clear advantage over other conventional enzymatic assays (gel zymography) and protein analysis (western blot) that requires animals to be sacrificed and tissues to be homogenized.
Tagi
Przejdź do...
Filmy z tej kolekcji:

Now Playing
Near-infrared Fluorescence Imaging of Abdominal Aortic Aneurysms
Biomedical Engineering
8.4K Wyświetleń

Imaging Biological Samples with Optical and Confocal Microscopy
Biomedical Engineering
36.3K Wyświetleń

SEM Imaging of Biological Samples
Biomedical Engineering
24.1K Wyświetleń

Biodistribution of Nano-drug Carriers: Applications of SEM
Biomedical Engineering
9.6K Wyświetleń

High-frequency Ultrasound Imaging of the Abdominal Aorta
Biomedical Engineering
14.8K Wyświetleń

Quantitative Strain Mapping of an Abdominal Aortic Aneurysm
Biomedical Engineering
4.6K Wyświetleń

Photoacoustic Tomography to Image Blood and Lipids in the Infrarenal Aorta
Biomedical Engineering
5.9K Wyświetleń

Cardiac Magnetic Resonance Imaging
Biomedical Engineering
15.0K Wyświetleń

Computational Fluid Dynamics Simulations of Blood Flow in a Cerebral Aneurysm
Biomedical Engineering
11.9K Wyświetleń

Noninvasive Blood Pressure Measurement Techniques
Biomedical Engineering
12.1K Wyświetleń

Acquisition and Analysis of an ECG (electrocardiography) Signal
Biomedical Engineering
107.0K Wyświetleń

Tensile Strength of Resorbable Biomaterials
Biomedical Engineering
7.7K Wyświetleń

Micro-CT Imaging of a Mouse Spinal Cord
Biomedical Engineering
8.3K Wyświetleń

Visualization of Knee Joint Degeneration after Non-invasive ACL Injury in Rats
Biomedical Engineering
8.3K Wyświetleń

Combined SPECT and CT Imaging to Visualize Cardiac Functionality
Biomedical Engineering
11.2K Wyświetleń
Copyright © 2025 MyJoVE Corporation. Wszelkie prawa zastrzeżone