Method Article
Genome Editing in the Yellow Fever Mosquito Aedes aegypti using CRISPR-Cas9
W tym Artykule
Podsumowanie
Here, we describe a detailed protocol for genome editing through embryonic microinjection in the mosquito A. aegypti using the CRISPR-Cas9 technology.
Streszczenie
The emergence of the clustered, regularly interspersed, short palindromic repeats (CRISPR)-Cas9 technology has revolutionized the genetic engineering field and opened the doors for precise genome editing in multiple species, including non-model organisms. In the mosquito Aedes aegypti, loss-of-function mutations and DNA insertions have been accomplished with this technology. Here, we describe a detailed protocol for genome editing through embryonic microinjection in the mosquito A. aegypti using the CRISPR-Cas9 technology, focusing on both the generation of gene knockout and knockin lines. In this protocol, quartz needles are filled with a mixture of guide RNA, recombinant Cas9, and a plasmid containing a DNA cassette encoding a gene for a fluorescent marker, if gene knockin is desired. Embryos at the preblastoderm stage are lined up onto a strip of double-sided sticky tape placed onto a coverslip, which is subsequently mounted onto a glass slide. With the help of a microinjector, the needles are inserted gently into the posterior end of the embryos and a small volume of the CRISPR mixture is dispensed. When the embryos are hatched, the larvae are checked under the fluorescent scope, and the pupae are sex-sorted and separated in different cages. Once the adults emerge, these are reciprocally crossed with wild-type individuals, blood-fed, and placed for egg laying. Once these eggs are hatched, the fluorescent larvae collected represent individuals with stable insertion of the DNA cassette into their genome. These larvae are then grown to the adult stage, outcrossed to wild-type individuals, and then further assessed through molecular techniques to confirm that the exact sequence of the DNA cassette is present at the desired site of the mosquito genome. Homozygous lines can also be obtained by following the provided pipeline of crossing schema and molecular screening of the mutations.
Wprowadzenie
Precise genome editing has become arguably easier, but possible, with the establishment of the CRISPR-Cas technologies of molecular scissors1. These technologies take advantage of a mechanism that the prokaryotic immune system uses to fight against phage infections2. Amongst these systems, clustered, regularly interspersed, short palindromic repeats (CRISPR) along with the Cas9 nuclease usually rely upon 20 base pair RNAs, the guide RNAs (gRNAs), with sequences homologous to the targeted DNA, which are followed by an NGG protospacer adjacent motif (PAM) sequence3. The gRNAs loaded onto the Cas9 guide these nucleases precisely to specific target sites in the genome, triggering double-strand DNA breaks3.
DNA double-strand breaks induce the repair mechanisms to patch the double-helix4. Whereas any DNA repair is expected to be precise, there exist less accurate DNA repair mechanisms that can leave behind sequence scars and, in turn, loss-of-function mutations4. Among the error-prone DNA repair mechanisms, the Non-Homologous End-Joining (NHEJ) can cause frame-shift mutations, including small deletions, insertions, and nucleotide changes (SNPs), that can result in loss-of-function mutation. The Homology Directed Repair (HDR) mechanism, on the other hand, relies upon the homologous chromosome as a template to copy the exact sequence of the undamaged allele and make a perfect repair of the targeted DNA sequence4.
Based on this knowledge, the CRISPR-Cas9 technology has been developed to precisely edit genomes, arguably at any sequence containing a PAM site3. In mosquitoes, the CRISPR-Cas9 technology has been used to knock out a variety of genes, through embryonic microinjection of a mixture of Cas9 and gRNAs, taking advantage of the NHEJ repair mechanism5,6. Similar germline mutagenesis is obtained with the injection of gRNA + Cas9 mix into the hemolymph of adult female mosquitoes7. This technology was coined ReMOT control and relies upon a modified version of a Cas9 tagged with a peptide that is taken up by the ovaries through endocytosis during the process of egg development (vitellogenesis)7. Knocking in specific gene cassettes into a genome is only possible through embryonic microinjection of a mixture of gRNA and Cas9 (or a plasmid expressing those molecules) along with a plasmid encoding a desired DNA cassette8. Taking advantage of the HDR mechanism, the plasmid containing the DNA cassette of interest flanked by homologous sequences (500-1,000bp)9,10 upstream and downstream of the target site is used as a template to rewrite the double-strand break, copying also the DNA cassette into the target sequence9.
The CRISPR-Cas9 technology has been used to knock out multiple genes primarily involved with the sensory systems in the mosquito Aedes aegypti11, but also genes associated with male fertility and female viability (PgSIT) for population control12. Knocking out target genes has also been accomplished by knocking in genes encoding fluorescent markers into the coding sequences of specific genes13,14. This strategy has the advantage of not only inducing frame-shift mutations but also allowing the use of fluorescent light to sort the individuals of the new knockout line13,14. The A. aegypti genome has also been edited with sequences of binary expression systems, such as the Q-system (QF-QUAS)11. Knocking in the gene encoding the QF transactivator downstream to a promoter of a specific gene assures defined spatiotemporal expression of the transactivator15,16. Once a QF-expressing mosquito line is crossed to another mosquito line containing the binding sites (QUAS) for QF, the latter binds to it and triggers the expression of genes downstream to the QUAS sequence15,16. This system, overall, allows tissue- and time-specific expression of such effector genes, which can be fluorescent markers used for cell localization or detection of neuronal activity, and even Cas9 nucleases for disrupting genes in specific tissues (i.e., somatic knockout)11.
Given all the information available for A. aegypti genetic transformation, we provide herein a detailed protocol with step-by-step directions to performing genome editing with the CRISPR-Cas9 system through embryonic microinjection. Strategies for generating both knockout, through frame-shift mutations and deletions mediated by NHEJ, and knockin lines, by HDR-mediated gene cassette insertions, are discussed.
Protokół
Details related to the equipment and reagents used in this protocol are listed in the Table of Materials. All animals were handled following the Guide for the Care and Use of Laboratory Animals, as recommended by the National Institutes of Health. The procedures were approved by the UCSD Institutional Animal Care and Use Committee (IACUC, Animal Use Protocol #S17187) and UCSD Biological Use Authorization (BUA #R2401).
1. gRNAs and donor plasmid design
- For making knockout mutants, design two gRNAs spaced by ~20-100 bp (Figure 1A).
- Design 20 bp gRNAs for CRISPR-Cas9, excluding the PAM sequence (NGG) by using an online tool (Figure 1A), such as CHOPCHOP ( https://chopchop.cbu.uib.no/)17or Benchling (benchling.com) or CRISPOR (http://crispor.gi.ucsc.edu/)18. Select the most specific and off-target-free gRNAs for experiments, as suggested by the online tool.
- Ensure an efficient restriction enzyme cutting site is included within the sequence between the gRNA cut sites (Figure 1A). Include a restriction enzyme site between gRNA cut sites to allow for quick visual confirmation of successful edits.
NOTE: When the deletion occurs, the restriction site is removed, so the enzyme will not cut the sequence, producing a single, uncut band on a gel to confirm the deletion.
- For HDR-mediated gene cassette insertions, design multiple gRNAs using the tools mentioned for making knockout mutants and select the most effective one after later evaluation.
- Design donor plasmids for Homology-Directed Repair (HDR) containing target site homology arms, the cargo DNA sequence, a fluorescent marker, the origin of replication, and an antibiotic resistance gene (Figure 1B).
- Choose the homology arms from the upstream and downstream regions of the target site, each spanning 500-1,000 bp10 (Figure 1B).
- Select the cargo sequence, which may include a fluorescent marker, a gene of interest, or a regulatory element (e.g., QF2).
- Select a fluorescent marker. Common fluorescent markers used for A. aegypti are included in a DNA cassette containing a promoter, a fluorescent marker-encoding gene, and a 3' UTR sequence. See the discussion for further details.
2. Injection mix preparation
- Dilute the Cas9 protein with the Cas9 dilution buffer to 1 µg/µL.
NOTE: Do not thaw and freeze Cas9 more than 2x. It is advisable to make aliquots of the Cas9 protein. - Purchase the gRNAs or produce them in-house.
NOTE: For in-house production, use standard RNA decontamination practices and RNAse-free materials.- Design 4-6 gRNAs and choose the best two gRNAs for injection (see in vitro cleavage assay below).
- Design a forward primer that includes the T7 promoter sequence upstream of the gRNA sequence. Use the universal gRNA reverse primer for the non-template PCR reactions, as described below. The overlapping sequence base pairs to the corresponding sequence in the universal reverse primer (bold and underlined), creating a template for the DNA polymerase to amplify their sequences.
NOTE: For these PCR reactions, the forward primer should contain the T7 promoter (bold), followed by two guanines (important for transcription initiation via T7 RNA polymerase), the 20-nucleotide sequence of the gRNA (N20; without the PAM sequence), and the primer overlapping sequence (underlined).
Primer forward: 5'- GAAATTAATACGACTCACTATAGGN20 GTTTTAGAGCTAGAAATAGC- 3'
Primer reverse: 5''-AAAAGCACCGACTCGGTGCCACTTTTT CAAGTTGATAACGGACTAGC
CTTATT TTAACTT GCTATTTCTAGCTCTAAAAC - 3' - Synthesize the DNA template by non-template PCR. Set up multiple reactions (each containing 12.5 µL of the 2x master mix, 1.25 µL of the 10 µM solution of forward primer, 1.25 µL of the 10 µM solution of reverse primer, and 10 µL of ultrapure water) so that enough PCR product (300 ng) is available for the in vitro transcription reaction. Use the following PCR conditions: initial denaturation for 30 s at 98 °C; 35 cycles of amplification for 10 s at 98 °C, 10 s at 62 °C, and 10 s at 72 °C; final extension at 72 °C for 2 min; and storage at 4 °C.
- Confirm amplification of a single DNA fragment (122 base pairs) on an agarose gel (2%).
- Clean up the PCR template using a PCR Purification kit, following the manufacturer's recommendations.
- Perform an in vitro transcription reaction with a T7 Transcription Kit. Mix 2 µL of 10x reaction buffer, 2 µL each of nucleotides (ATP, CTP, UTP, and GTP), 2 µL of T7 RNA polymerase, 3 µL of the template DNA (100 ng/µL), and 5 µL of ultrapure water. Incubate the mixture at 37 °C for at least 2 h (not more than 16 h) to overnight (12 h).
NOTE: In this reaction, the T7 RNA polymerase binds to the T7 promoter included in the primer forward sequence, which leads to the transcription of the gRNA. - Treat the transcription reaction with DNase by adding 1 µL of DNase (mix well) and incubating at 37 °C for 15 min.
- Purify the synthesized sgRNAs with a transcription cleanup kit by following the manufacturer's recommendations.
- Perform an in vitro cleavage assay to assess the cutting efficiency of the selected gRNAs (Figure 1C).
- Design primers to amplify a DNA fragment (500-1,000 bp) flanking the gRNA cutting site.
- Set up the PCR reactions as follows: 12.5 µL of the 2x master mix, 1.25 µL of the 10 µM solution of forward primer, 1.25 µL of the 10 µM solution of reverse primer, 9 µL of ultrapure water, and 1 µL of 5 ng/µL template DNA.
- Use the following PCR conditions: conditions: initial denaturation for 30 s at 98 °C; 35 cycles of amplification for 10 s at 98 °C, 15-30 s at X °C (ideal annealing temperature for primers), and 35 s at 72 °C; final extension at 72 °C for 2 min; and final long-term storage at 4 °C.
- Refer to the manufacturer's guidelines for the best amplification temperatures and time span. Pool at least five reactions or scale up the volumes of the reagents so that enough PCR product (1.5-2 µg) is obtained after PCR cleanup (single band) or agarose gel PCR band purification.
- Set up Cas9 cleavage reactions with recombinant Cas9 by incubating the following mixture at 37 °C for 1 h: 1 µL of 10x Cas9 reaction buffer, 0.35 µL of recombinant Cas9 (1 µg/µL), 1 µL of gRNA (100 ng/µL), 6.65 µL of ultrapure water, and 1 µL of 300 ng/µL of template DNA.
- Set up a negative control reaction without any gRNA.
- Check for cleavage efficiency by running reactions on an agarose gel (1.5-2.0%; Figure 1C).
3. Assembling the donor plasmid
- PCR-amplify the homology arms from the genomic DNA of A. aegypti.
- For primer design, refer to the cloning kit manufacturer's guidelines. Use an online tool (e.g., Benchling), which provides support for plasmid design and assembly using different cloning strategies (e.g., Gibson, Golden Gate, and restriction enzyme-based cloning).
- Amplify the cargo sequence from A. aegypti genomic DNA or order a commercially synthesized gene fragment.
- Amplify fluorescent markers from plasmids in-house.
- Ligate DNA fragments from steps 3.1.1 to 3.1.3 into the backbone of an existing plasmid by Gibson assembly. Incubate a mixture of 10 µL of 2x master mix, X µL of all DNA fragments, 10-X µL of ultrapure water at 50 °C for 1 h.
NOTE: Refer to the manufacturer's guidelines for calculating the ideal ratio of inserts to plasmid backbone.- Calculate the concentrations of each cloning fragment in pico mols: pmols = (weight in ng) × 1,000/(base pairs × 650 daltons).
- Use 50-100 ng of plasmid backbone and 2-3-fold molar excess of each insert.
- If assembling 2-3 fragments, add 0.02-0.5 pmols of each fragment into the Gibson reaction. If assembling 4-6 fragments, add 0.2-1.0 pmols of each one.
- Use 3-5 µL of the Gibson assembly reaction to transform E. coli competent cells JM109.
NOTE: JM109 was chosen for Gibson assembly due to its recA- genotype, which reduces undesirable recombination events and prevents nuclease carryover during cell harvest, ensuring the integrity of the assembled DNA fragments
For plasmids larger than 10 kb, we recommend transformation of DH5α competent cells using the extended protocol, following the manufacturer's recommendations. - Expand the transformed bacteria and purify the plasmid using a miniprep kit.
- For confirmation of correct plasmid assembly, run a diagnostic restriction enzyme digest with the purified plasmid DNA and visualize by agarose gel electrophoresis (Figure 1D,E).
NOTE: We suggest the use of an online tool for the selection of a couple of restriction enzymes that can cut the plasmid on a single site. Software like Benchling has a built-in tool that performs virtual digestion of plasmid sequences with the selected restriction enzymes, displaying the expected DNA band pattern on a virtual electrophoresis gel (Figure 1D).- Carry out the plasmid restriction enzyme digestion. Incubate a mixture of 1 µL of 10x restriction digest buffer, 0.5 µL of restriction enzyme 1, 0.5 µL of restriction enzyme 2, 1 µL of plasmid DNA (300 ng/mL), and 7 µL of ultrapure water at 37 °C for 2 h.
- Run the restriction digest reactions on an agarose gel (1.5%).
NOTE: The DNA band pattern (Figure 1E) should resemble the virtual digest band pattern (Figure 1D). Plasmids can also be sent for whole plasmid sequencing if service is available.
- Culture the plasmid clone into 150 mL of LB media.
- Carry out plasmid maxiprep, following the manufacturer's guidelines.
- Suspend the plasmid in ultrapure water.
4. Mixing the injection construct
NOTE: The suggested final concentration ranges for each construct are provided in Table 1. Start with a ratio of 2:1:2 (ng Cas9: ng sgRNA: ng donor plasmid) and adjust as necessary to optimize HDR efficiency. Monitoring the outcomes will help identify the most effective combination.
- For making knockout mutants,
- Dilute the aliquot of Cas9 protein to the desired concentration using the Cas9 dilution buffer and dilute the aliquot of gRNA with ultrapure water.
- Premix the Cas9 protein with each gRNA to form a Ribonucleoprotein (RNP) complex. Combine the premixed solutions.
- For HDR-mediated gene cassette insertions,
- Dilute and mix Cas9 protein and gRNAs as suggested for making knockout mutants.
- Dilute the donor plasmid with ultrapure water.
- Combine all the constructs.
5. Pulling and loading microinjection needles
- Use quartz filament to pull the injection needles (Figure 2). Make sure the filament is 10 cm long, with an outer diameter of 1 mm and an inner diameter of 0.7 mm.
- Pull the needles using a laser micropipette puller with one of the following two programs:
Program 1: Heat 805, Filament 4, Velocity 50, Delay 145, Pull 145
Program 2: Heat 650, Filament 4, Velocity 40, Delay 150, Pull 156
NOTE: Program 1 results in a thinner but longer needle tip, making it suitable for injections with lower concentration constructs, where a finer tip is beneficial for precision. Program 2 yields a shorter but thicker needle tip, ideal for higher concentration injections, as the construct is thicker and requires a sturdier needle.
6. Embryo harvesting
- Prepare an electric or manual aspirator to collect mosquitoes and use plastic vials for collection and embryo harvesting.
- Moisten white circle filter papers and place them on the inside wall or on top of moist cotton in the collector.
- Place 5-10 female mosquitoes that were blood-fed 5-10 days ago into the collector. Place the collector in the dark for 45 min.
- Take out the filter papers for embryo harvesting.
7. Embryo line up
- Use a wildtype strain (Liverpool) of A. aegypti for embryo injection.
- Select preblastoderm stage embryos, particularly those that are light gray in color, from the harvesting paper (Figure 3).
- Transfer a few embryos with a wetted brush to the double-sided sticky tape placed on top of a coverslip. Align the embryos in parallel while their surrounds are still wet ensuring they are side by side, with all posterior ends facing the front.
- Once positioned correctly, allow the environment to dry slightly to secure the embryos in place.
- Add Halocarbon oil 700 onto the embryos during alignment to prevent desiccation.
NOTE: Ensure the embryos are not surrounded by water when adding the oil, as this would cause the embryos to float in the oil.
8. Embryo microinjection
NOTE: Injection is conducted at room temperature or at 18 °C. The 18°C temperature is recommended for practical reasons, as it delays embryo development.
- Set up a microinjector with the following parameters: compensation pressure (Pc) 300 hPa, injection pressure (Pi) 500 hPa. Adjust these conditions whenever necessary.
- Load 3 µL of the mixed construct into a needle using a microloader.
- Place the cover glass with aligned embryos on top of a microscope slide (Figure 3). Position the cover glass and slide under the microscope for injection. Make sure the posterior end of the embryo is positioned toward the needle.
- Place one needle into a needle holder together with a micromanipulator and position the needle at a 10° angle towards the posterior end of the aligned embryos, keeping it stationary (Figure 4A). Gently open the needle under a microscope and lightly touch it with the edge of a coverslip.
NOTE: Another option is to create a small opening in the needle by tapping it with an embryo. However, a slightly wider needle opening is recommended, as the Cas9 protein's stickiness can clog the needle. - Move the slide glass towards the needle for injection (Figure 5).
9. Injected embryo aftercare
- Use lint-free, disposable wipes to remove the oil surrounding the embryos.
- Add deionized water to rinse the embryos, and move the embryos onto a wet filter paper. Place the wet filter paper on a wet tissue inside Karat 9 oz cups (Figure 4B and Figure 6A,B).
- Place wet cotton at the bottom of the cup to maintain moisture (Figure 6B).
- Preserve the injected embryos on wet filter paper.
10. Embryo hatching and G0 larva screening
- At least 4 days after injection, transfer the filter paper with embryos to approximately 3 L of deionized water in Sterilite 6-quart pans for hatching.
NOTE: Eggs typically hatch more efficiently within 2 weeks after injection. Ensure the eggs remain moist during this period. The filter paper should not be too wet, as excessive moisture may lead to early hatching of the embryos. While longer preservation may improve hatching rates, do not exceed a month for optimal results. Using deoxygenated water may also help with hatching. - Once the G0 larvae hatch, add fish food mixed with water to the pan as food.
- Screen the G0 larvae for the fluorescent marker at the 3rd to 4th instar larval stage (Figure 7).
- Maintain the larvae separately based on their fluorescent status, with fluorescent-positive and fluorescent-negative larvae kept in separate pans.
11. Sex sorting pupae and outcrossing to wild type
- Separate the injected mosquitoes by sex when they pupate, using size and sex-specific structures at the genital lobe for identification:
- Males: Smaller size, a more prominent and pointed genital lobe, and broader paddles (structures at the tail end of the pupae; Figure 8A1,A2).
- Females: Larger size, a smaller and less pronounced genital lobe, and narrower paddles (Figure 8B1,B2).
- Pool fluorescent-positive or fluorescent-negative mosquitoes of each sex.
- Outcross the pooled mosquitoes with mosquitoes of the opposite sex from the Liverpool strain. Use a 3:1 to 5:1 ratio of wild type to G0 mosquito.
- Cross the mosquitoes for 4 days.
- Provide the females with a blood meal after crossing.
12. G1 screening
- Three days after blood feeding, provide egg cups by placing a paper towel inside the wall of Karat 9 oz cups and adding around 3 oz of deionized water.
- After 3-4 days, harvest and hatch the G1 embryos. Screen the G1 larvae for the fluorescent marker at the 3rd to 4th instar stages (Figure 7).
- Collect the fluorescent larvae, suggesting an HDR insertion. When the fluorescent G1 individuals reach the pupal stage, separate them by sex and place each sex in separate cages.
- Outcross the fluorescent G1 mosquitoes to individuals of the opposite sex of the Liverpool strain.
13. G1 insertion site confirming
- After providing the blood meal, set up G1 females for single female egg laying by placing a small piece of paper towel in individual Drosophila vials and adding 3 mL of deionized water.
- For knockout mutants:
- After egg laying, water evaporates, and the paper towel dries out, hatch the eggs from females that clearly exhibit knockout mutations and obtain the G2 generation.
- Collect the body of the G1 mothers have successfully hatched G2 offspring and extract DNA.
- Use the DNA as a template for PCR to amplify the sequence covering the target site. Make sure the primers amplify a DNA fragment of ~200 bp. Set up the reactions (each containing 12.5 µL of the 2x master mix, 1.25 µL of the 10 µM solution of forward primer, 1.25 µL of the 10 µM solution of reverse primer, 9 µL of ultrapure water, and 1 µL of template DNA [5 ng/µL]). Use the following PCR conditions: initial denaturation for 30 s at 98 °C; 35 cycles of amplification for 10 s at 98 °C, 15-30 s at X °C (primers' ideal annealing temperature), and 10 s at 72 °C; final extension at 72 °C for 2 min; and final long-term storage at 4 °C.
- Purify PCR fragments and perform restriction enzyme digestion of the PCR fragments by incubating a mixture of 1 µL of 10x restriction digest buffer, 0.5 µL of restriction enzyme 1, 0.5 µL of restriction enzyme 2, 1 µL of PCR fragment (200 ng/mL), and 7 µL of ultrapure water at 37 °C for 30 min.
NOTE: Certain restriction enzymes can be used in the PCR mix without prior purification. - Visualize the PCR fragment digestion by gel electrophoresis to confirm whether cleavage has occurred.
- Sequence the undigested PCR fragment to identify the knockout mutations.
- For HDR-mediated insertions, after egg laying:
- Collect the body of the G1 mothers and extract DNA.
- Use DNA as a template for PCR to amplify the sequence covering the target site.
- Perform sequencing of the amplified DNA fragment to confirm the integrity of the inserted DNA cassette.
- Hatch the egg and obtain the G2 generation.
14. Expanding the new CRISPR lines
- For knockout mutant lines:
- Set up G2 females for egg-laying, with 20 females per G1. Each G1 represents one line.
- After egg laying, confirm knockout mutations in the same manner as for G1.
- Proceed with five G1 lines that exhibit clearly identified mutations in both G1 and G2. Hatch the eggs collected from G2 of each line, giving rise to the G3 generation.
- From the five outcrossed lines, select two lines with clearly identified mutations and high fitness or the desired phenotype.
- Outcross G3 females from each selected line with males from the Liverpool strain.
- Set up 50 single females for egg-laying from each line. Confirm knockout mutations as done previously for G1.
NOTE: Increase the number of females in the setup to maintain diversity. - Repeat the procedures for two additional generations with the two lines (Figure 4J).
- For new HDR-mediated insertions lines:
- Verify the presence of fluorescent individuals in the G2 generation.
- Outcross G2 mosquitoes, from each G1 individual, with the Liverpool strain. Each G1 individual represents one line.
- Proceed with three more generations of outcrossing. Continuously check the fluorescent marker and observe the fitness19 of each line (Figure 4x).
- Select one line with correct insertions and high fitness and the desired phenotype.
15. Make homozygous lines
- For knockout mutant lines:
- Generation G6:
- Hatch the eggs produced by G5 and obtain the G6 generation.
- Intercross G6 adults within their respective lines (Figure 4K and Figure 9A).
- Set up 50 single G6 females for egg-laying per line, and hatch the eggs as previously described (Figure 9B).
- Collect the G6 females that have successfully hatched G7 offspring, extract DNA, and perform PCR and restriction enzyme digestion as done for G1 (Figure 9C).
NOTE: A simple and inexpensive squishing buffer (SB) can be used at this step for DNA extraction.- Using a handheld grinder, macerate a single mosquito in 100 µL of 1x SB (10 mM Tris-HCl pH 8, 1 mM EDTA, and 25 mM NaCl). Add Proteinase K (200 µg/mL) and incubate at 37 °C for 30 min, followed by 95 °C for 2 min. Spin down at 10,000 × g for 5 min and collect the supernatant20.
- Confirm mutation by visualizing the results on a gel.
- Discard the eggs produced by females without mutations (Figure 9D).
- Maintain larvae from females with confirmed mutations to establish several G7 lineages, and discard broods without mutations (Figure 9D).
- Generation G7:
- Intercross females and males within each G7 lineage (Figure 9E).
- Set up 10 single G7 females for egg-laying per line, and hatch the eggs as previously described (Figure 9F).
- Collect the G7 females that have successfully hatched G7 offspring, extract DNA, and perform PCR and restriction enzyme digestion (Figure 9G).
- Detect knockout mutations on both alleles or a single allele by visualizing the results on a gel.
- Maintain larvae from G7 females confirmed to have knockouts in both alleles, producing the G8 generation (Figure 9H). Discard broods without mutations (Figure 9H).
NOTE: This step ensures that the females are homozygous. In G8, testing the homozygosity of the progeny confirms whether the fathers are also homozygous."
- Generation G8:
- Cross G8 adults per each G7 female lineage (Figure 9I).
- Randomly pick five G8 males, extract DNA, and perform PCR and restriction enzyme digestion (Figure 9J).
- Provide a blood meal and hatch the eggs from G8 females that were crossed with G8 males confirmed to have knockouts in both alleles.
- Continue with the G7 lineages where all detected males have knockouts in both alleles, indicating these lineages are homozygous.
- Generation G6:
- For HDR-mediated insertions lines:
- Hatch the eggs produced by G5 and obtain the G6 generation. Screen larvae at the G6 stage for fluorescent markers.
- Discard larvae without fluorescent markers (Figure 4xi).
- Intercross mosquitoes that exhibit fluorescent markers.
- Continue this procedure for each subsequent generation until no non-fluorescent larva is observed. At this point, the mosquito line will be homozygous.
Wyniki
Design and validation of gRNA-mediated gene targeting for HDR homology recombination
To ensure the desired gene is accurately targeted, we recommend selecting a couple of gRNAs and positioning the 5' and 3' homology arms close to the cutting site for HDR-mediated homologous recombination (Figure 1A). For example, we designed two gRNAs to target both sides of the start codon of the gene of interest and used the QF2-Hsp70-OpIE2-ECFP-SV40 cassette as a marker, inserting it into the targeted gene via 1 kb homology arms through the HDR mechanism (Figure 1B). The double-strand breaks lead to a deletion of a short DNA fragment between the cutting sites of the gRNAs (Figure 1A).
The cleavage efficiency of gRNAs and their ability to cut the target gene was tested by in-vitro Cas9 cleavage assay. The PCR fragments from the target gene were cleaved into two fragments by Cas9, loaded with the gRNAs (Figure 1C). Additionally, restriction enzymes can be used to verify the integrity of the donor plasmid before moving to the downstream steps of the protocol. Plasmid digestion results should display one or two bands on the gel, with the presence of a small band indicating successful digestion into a short fragment (Figure 1D,E).
Preparation of microinjection needles
Before starting the injection process, it is important to prepare fresh microinjection needles. The needles should have a protuberant shape with a slight curve. About 2/3 of the needle should be narrow at the top, while the remaining 1/3 wider. The front end of the needle tip should not exceed 0.5 cm in length from the narrow to the wide part (Figure 2A). After using the edge of the coverslip to open the needle, a bevel-tipped needle is preferable for microinjection, as it is easier to use after breaking the needle by gently touching the surface of the black mosquito embryo (Figure 2B). On the other hand, a blunt-tipped needle can make it difficult to pierce light gray embryos during microinjection (Figure 2C). The needles should neither be too soft nor too hard to penetrate the mosquito embryos effectively to avoid breaking the needle.
Injection of embryos
To ensure successful injection, begin by harvesting light gray mosquito embryos and aligning them so that the posterior pole faces the same direction (Figure 3A). This directional alignment helps inject the plasmid close to the germline cells (posterior pole), which is crucial for producing mutants that can be inherited by the next generation. Select fresh, uniform, light gray embryos from females that were blood-fed 5-10 days prior (Figure 3A1). Avoid using embryos that are unevenly gray or brown (Figure 3A2), as these may not develop properly.
Align the mosquito embryos one by one on the double-sided tape to facilitate accurate and efficient injection. Apply halocarbon oil before the eggs desiccate and darken to a medium or dark gray color (Figure 3B).
When injecting, focus on the surface of the posterior pole of each embryo. The anterior side of the embryo (right side in Figure 5), on the other hand, bears a flat and hard surface (micropyle). Gently use a beveled needle containing the injection mix to pierce the embryo (Figure 5). The embryo's surface depresses and rebounds, indicating that the needle has penetrated the embryo. Upon successful injection, the embryo's color lightens within a few seconds (yellow curve in Figure 5, top-right).
After injection, carefully use the brush to pick up and transfer the embryos to another wet filter paper using a laboratory wipe to remove excess oil (Figure 6A). Keep the embryos on a wet paper towel for several days at rearing temperature (Figure 6B). Excess oil can reduce the hatching rate of mosquito embryos. Use cotton balls with ultra-pure water to maintain hydration. Embryos that remain gray or brown (yellow arrow) after 2 h post injection are unlikely to hatch (Figure 6C).
Screening and outcrossing
Once the embryos have matured, add ultrapure water to the egg cup and place it in a 27 °C incubator for hatching. It typically takes 5-7 days for the larvae to develop to the 3rd instar stage. Initially, we prescreened the G0 larvae that exhibited fluorescence, though it was weaker or partial compared to other mutant lines. This ensured that the marker was expressed correctly and that the plasmids were successfully injected into the embryos.
These larvae were then crossed with wild-type (WT) mosquitoes. During subsequent screenings, we selected and retained the G1 larvae that displayed stronger and complete fluorescence expression for rearing, expanding the line stock, and conducting further experiments. The G1 fluorescent larvae can be identified based upon the pattern of fluorescence protein expression provided by the combination of reporter promoter and fluorescent protein: promoter Hr5Ie1-eGFP (Figure 7A) drives expression of green fluorescent protein throughout the entire body; Hr5Ie1-DsRed (Figure 7B) leads to expression of red fluorescence in the body; OpIE2-CFP (Figure 7C) induces expression of cyan (blue) fluorescence protein in only specific part of the body. When the 3xP3-TdTomato combination is used, red fluorescent protein is specifically expressed in the eyes (Figure 7D). This specific line of transgenic mosquito also contains the OpIE2 promoter driving the expression of CFP in the entire body (Figure 7D).
Special process of homozygous establishment for knockout mutants
To simplify line maintenance and ensure that all mosquitoes are mutants, we implemented a pipeline to generate non-fluorescent homozygous mutant mosquitoes. As described in protocol step 15, after outcrossing with WT mosquitoes, we placed the G6 mutant mosquitoes in the same cage for intercrossing (Figure 9A). Following intercrossing, females were collected into individual vials for egg-laying (Figure 9B). This step allowed for the separation of females with different genotypes.
After collecting eggs to obtain G7, genomic DNA was extracted from G6 females, followed by PCR and gel electrophoresis of PCR fragments, treated or untreated with a restriction enzyme (Figure 9C). Lines showing digested PCR fragments only, indicating no mutations, were discarded. While those lines showing double-PCR bands, indicating the presence of mutations and, in turn heterozygous females, were kept and hatched (Figure 9D). The G7 mosquitoes were then allowed to mature, and all adults were placed in the same cage for another round of intercrossing to increase the likelihood of establishing a homozygous line (Figure 9E). Blood-fed G7 females were again collected individually for G8 egg collection, representing three potential genotypes (Figure 9F). Genomic DNA was extracted from G7 females, and PCR followed by gel electrophoresis was performed to determine which were homozygous (Figure 9G). Eggs from non-mutant or heterozygous females were discarded, and only those with homozygous results were kept (Figure 9H). G8 eggs from step H were hatched, and the resulting adults were intercrossed in a separate cage (Figure 9I). From each cage, 10 adult males were randomly selected for gel electrophoresis and sequencing (Figure 9J). This step should yield homozygous individuals, which were then maintained as the stock (Figure 9K).
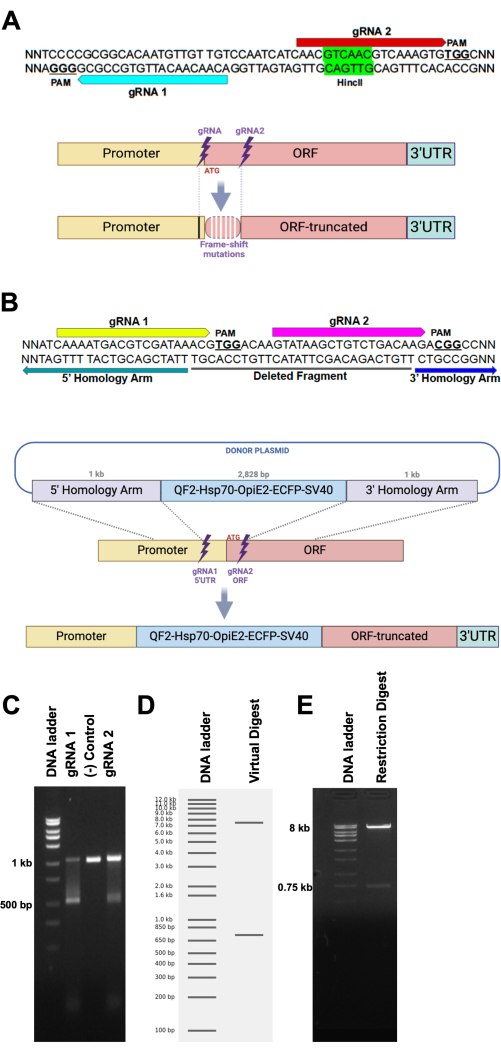
Figure 1: Schematic of gRNA and HDR donor plasmid design. (A, top) Hypothetical DNA sequence target sequence for two gRNAs (blue and red arrows and their associated PAM sites along with a restriction enzyme target site (HincII). (A, bottom) Schematic representation of a target site subjected to NHEJ, leading to the emergence of frame-shift mutations (white vertical bars) due to a small sequence deletion or insertion. Mutations can extend beyond the cleavage site of the gRNAs (black vertical bar). (B, top) Hypothetical DNA sequence depicting the target sites for gRNA 1 (yellow arrow) and gRNA 2 (magenta arrow), followed by PAM sites (NGG, bold and underlined). The potentially deleted DNA sequence between both gRNAs is underlined by a black bar (deleted fragment). Green and blue arrows highlight the most 3' sequence of the 5' homology arm and most 5' sequence of the 3' homology arm, respectively. (B, bottom) Schematic representation of an HDR recombination of a DNA cassette from a plasmid to a homologous site in the genome. (C) In-vitro cleavage assay. Incubation of a 1 kb DNA fragment is digested by Cas9 loaded with either gRNA 1 (left lane) or gRNA 2 (right lane), resulting in a fragment of 500 bp. The center lane shows the negative control reaction, which was devoid of any gRNA. (D) Virtual digest of a plasmid (8.75 kb) with two single-cut restriction enzymes, resulting in virtually two bands (8 kb and 750 bp) on a hypothetical gel. (E) An agarose gel loaded with the restriction digest reaction of a plasmid, depicting the expected results provided by the virtual digest. Please click here to view a larger version of this figure.
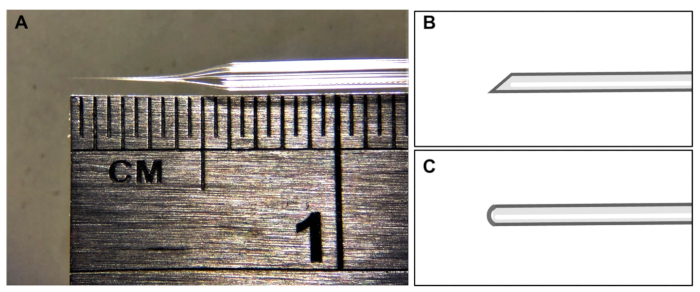
Figure 2: Microinjection needle shape. (A) Representative image of a needle tip. (B) Ideal shape of needle tip for microinjection of mosquito eggs. (C) Needle tip shape that should be avoided. Please click here to view a larger version of this figure.

Figure 3: Harvesting embryos. (A) Collecting light gray and (A1) homogeneous but not (A2) uneven embryos for injections; Scale bar=500 µm. (B) Image for the aligned embryos; Scale bar=500 µm. Please click here to view a larger version of this figure.
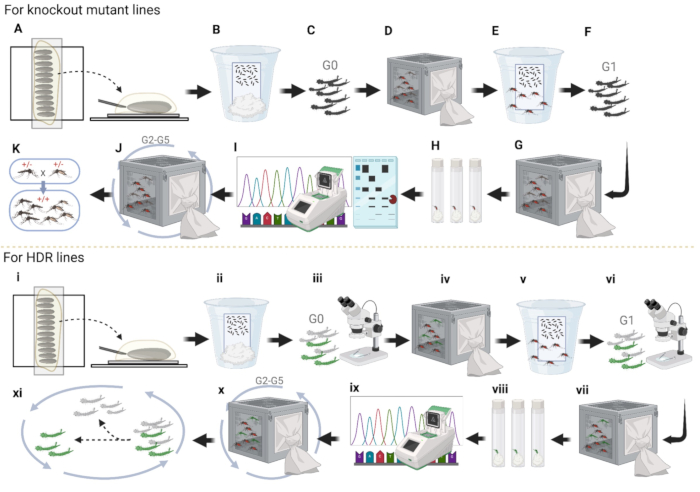
Figure 4: Pipeline of mosquito embryo microinjection. (A-K) Knockout Line injections. (A) Align mosquito embryos and inject the plasmid into the posterior end. (B) Injected embryo storage in the cup with wet paper; (C) G0 larvae hatching and separating to both sexes. (D) Both sexes of adults crossing to WT in separate cages; (E) collecting females' eggs in the egg cup. (F) G1 larvae hatching and separating to both sexes. (G) Both sexes of adults crossing to WT in separate cages. (H) Collect G1 female eggs individually in plastic vials and hatch the eggs. (I) PCR, run the gel with enzyme-treated fragments, and sequencing for G1 single females that have successfully hatched eggs. (J) Outcross G2 with WT for three more generations and sequence the mosquitoes in the same manner as done for G1. (K) Establish homozygous by intercrossing. (i-xi) HDR line injections. (i) Alignment of embryos. (ii) Injected embryo storage in the cup with wet paper. (iii) Prescreen of G0 larvae for fluorescence. (iv) Grow fluorescent larvae into adults and cross with WT in a cage. (v) Collection of G1 eggs in the egg cup. (vi) Screening and selection of fluorescent G1 larvae. (vii) G1 fluorescent adults crossed with WT. (viii) Blood-fed fluorescent G1 adult females laying eggs individually in plastic vials. (ix) Collection and sequencing of fluorescent G1 adults individually. (x) Outcross with wild type for three more additional generations and (xi) dispose of larvae without fluorescence to establish homozygotes. Please click here to view a larger version of this figure.

Figure 5: Injecting embryos. The moment of plasmid injection into mosquito embryos. Inset: injection mix visualized as a cloudy liquid inside the embryo (yellow dotted line); Scale bar=200 µm. Please click here to view a larger version of this figure.
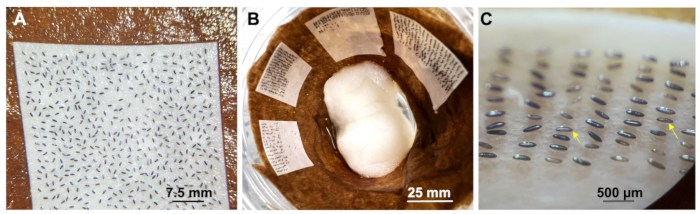
Figure 6: Embryo collection. (A) Harvesting embryos after injection and transferring them onto a wet filter paper. Scale bar=7.5 mm. (B) Maintaining injected embryos in a cup. Scale bar=25 mm. (C) Dark embryos after injection; Scale bar=500 µm. Please click here to view a larger version of this figure.
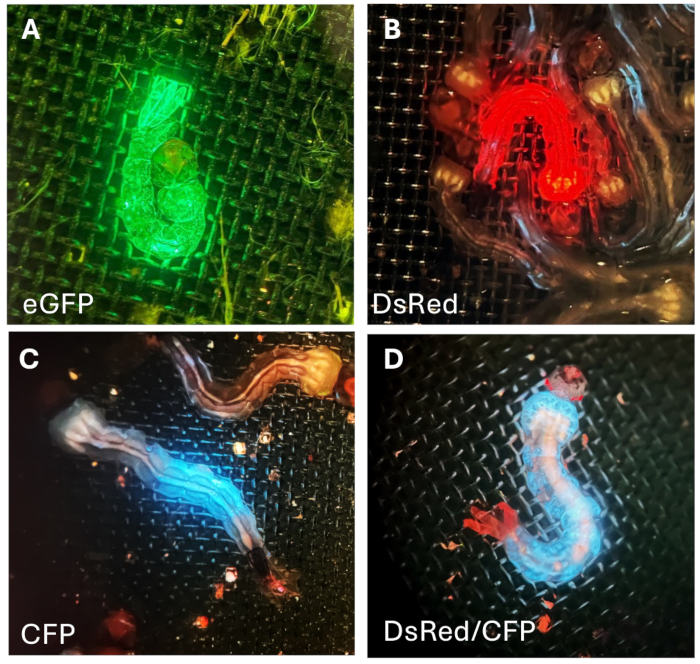
Figure 7: Fluorescence in G1 larvae. (A) Green, (B) red, and (C) bluefluorescent proteins on the body of mosquito larva. (D) Larva exhibiting eye specific-red fluorescence and body-specific blue fluorescence. Please click here to view a larger version of this figure.
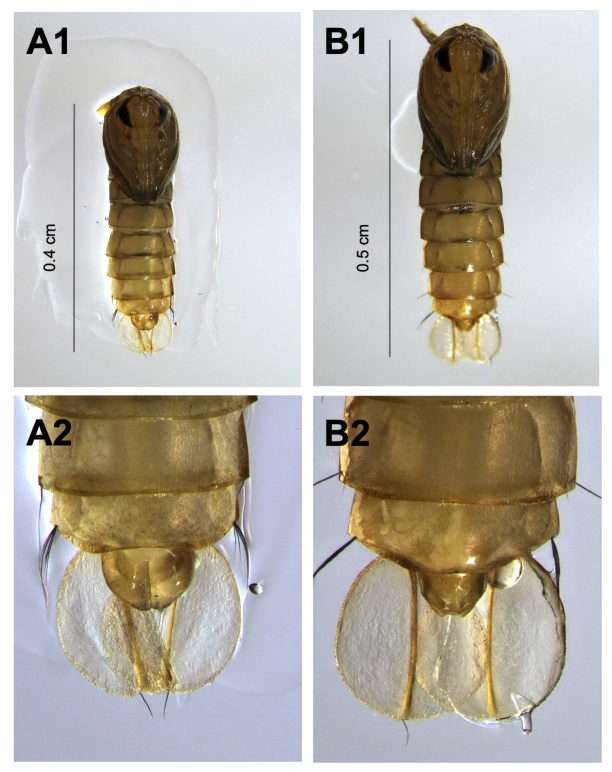
Figure 8: Sexual dimorphism in pupal morphology. (A1,A2) Male pupae are characterized by a smaller body size, a prominent and pointed genital lobe, and broader paddles at the tail end. (B1,B2) Female pupae display a larger body size, a smaller, less pronounced genital lobe, and narrower tail-end paddles. Please click here to view a larger version of this figure.
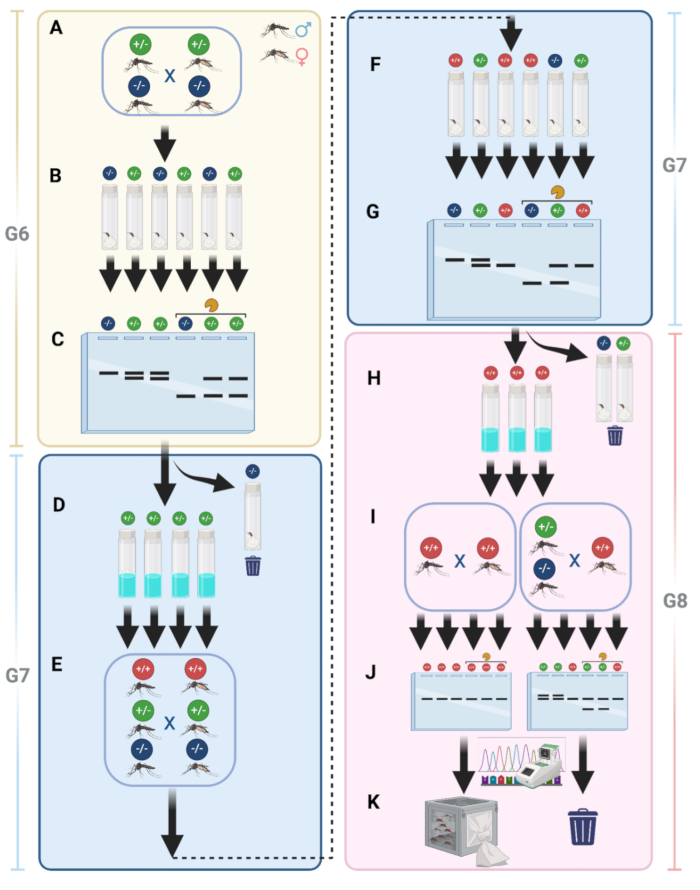
Figure 9: Details of homozygous establishment for knockout mutant lines. (A) Mutant mosquitoes are intercrossed in the same cage after outcrossing with WT. (B) After intercrossing, individual females are collected in vials for egg-laying. (C) Genomic DNA is extracted from G6 females, followed by PCR and gel electrophoresis with enzyme-treated and untreated fragments. (D) G7 eggs with positive PCR results (heterozygous) are hatched. (E) G7 adults are intercrossed again to increase the chances of obtaining homozygous individuals. (F) Blood-fed G7 females are individually collected for G8 egg collection, representing three genotypes. (G) Genomic DNA extraction, PCR, and gel electrophoresis are performed on G7 females to identify homozygous individuals. (H) Eggs from non-mutant or heterozygous females are discarded; homozygous eggs are kept. (I) G8 eggs are hatched and intercrossed in a separate cage. (J) Ten adult males are randomly selected from each cage for gel electrophoresis and sequencing. (K) Homozygous mosquitoes are identified and maintained as stock. Please click here to view a larger version of this figure.
| Injection construct | Final concentration |
| Cas9 protein | 100-300 ng/µL |
| gRNA | 50-100 ng/µL |
| donor plasmid | 100-500 ng/µL |
Table 1: The suggested final concentration ranges for each injection construct.
Dyskusje
CRISPR-Cas technology has changed the landscape of genome editing by promoting target-specific changes in chromosomes1. Even though transposable elements were essential for the generation of the first transgenic mosquitoes, their insertion sites are somewhat random, and the expression of the cargo construct (promoter + gene) may not correspond to the expression profile of the actual gene due to a genome positional effect (i.e., insertion site), which usually leads to ectopic expression21. Predating the emergence of the CRISPR-Cas9 molecular scissors, technologies that promote DNA double-strand breaks at specific target sites were developed and relied upon the DNA-binding properties of specific protein domains tagged to the FokI restriction enzyme, such as TALEN22 and Zinc Finger23. Although the latter technology was successfully utilized for mosquito genetic engineering24,25, the high cost of engineering such domains and the potential for off-target cleavage led to the discontinuation of such technologies. Since then, CRISPR-Cas9 technology has become the molecular scissors of choice11.
Genetic engineering of mosquitoes has been, for the most part, carried out by injection of the transformation mix into embryos26,27. Crafting mosquito embryonic microinjection requires focus, patience, and attention to detail. During the 2 h after egg laying, these embryos should be lined up, injected, and washed as carefully as possible so as not to disrupt their development. Focus is an essential skill to reduce the possibility of eggs being damaged or embryos getting desiccated during lineup. In the face of unexpected incidents like needle clogging, tip breakoff, and injection mix leakage, which happen quite often when Cas9 protein is in the mix, patience is another essential skill to manage these situations. Pocking embryos when the needle tip is too large or too much injection mix is leaking can only damage the embryo. The best solution is to replace the needle and carry on with the injections. No less important is the attention to detail. Younger female mosquitoes (6-10 days old) lay more eggs than older ones, and that is helpful in order to harvest healthy embryos. Choose deionized or ultrapure water and brown (non-bleached) paper towels to store the embryos after microinjection, as any contact with contaminated water or bleached surfaces can increase embryo mortality significantly. Light gray (<1 h old) embryos are softer, which facilitates the injection procedure, increasing the chances to get the mutant line. In addition, injected embryos should rest in humidified filter paper for at least 5 days before being hatched to allow full recovery and healing.
The quality and purity of the injection mix is fundamental for embryo survival. Neither buffers, salts nor alcohols from plasmid extraction kits or gRNA synthesis kits should be carried over to the injection mixes. Even though costly, the option of ordering the gRNAs may increase the likelihood of proper RNA folding. We also recommend injecting recombinant Cas9 protein over a plasmid encoding Cas9, as we believe the recombinant version is already loaded and ready to perform double-strand breaks as compared to a plasmid source that needs to the transcribed and translated and then loaded with gRNAs to start acting upon the DNA.
Although we described the protocol that works best in our hands as far as the use of CRISPR-Cas9 technology for genome editing in the mosquito A. aegypti, alternative reagents have been described in the literature that can also be used as replacement if needed. For instance, we suspend all reagents and dilute injection mixes with ultrapure water. Alternatively, injection mixes can be diluted in an injection buffer made of 5 mM KCl and 0.1 M NaPO4 (pH = 6.8)26,28. Besides using halocarbon oil 700 to protect embryos from desiccation after lining them up, halocarbon oil 2729 as well as a mixture of 27 and 70030 provide similar protection. The 10x concentrated version of the injection buffer described above has alternatively been used to prevent embryo desiccation26. As mentioned above, plasmids encoding Cas9 are commercially available (addgene) and have been used for CRISPR-Cas9-mediated genome editing in mosquitoes31.
Amongst the strategies to obtain a mosquito transgenic line, NHEJ-mediated knockout and HDR-mediated knockin approaches are available. Whereas the success rate of the former is much higher than the latter32,33, the crossing scheme and the screening process is much more time-consuming to obtain a knockout than a knockin line, as described in Figure 4. As an alternative approach to obtain a knockout line without going through complicated crossing schemes, HDR-mediated knockin of a gene cassette encoding a fluorescent marker offers the possibility of disrupting the coding sequence of a gene and selecting the transgenic line of mosquito using the fluorescent marker14,34.
Although this protocol focuses only on the basic steps for genetic transformation of mosquitoes, designing donor plasmids for HDR is an essential step that needs consideration. It is important to use promoters such as 3xP3, OpIE2, Hr5-IE1, and Pub6,35,36,37,38. 3xP3 is a synthetic promoter commonly used to drive expression in the nervous system and eyes of insects. OpIE2 and Hr5-IE1 are promoters derived from baculovirus that are used to drive strong expression in insect cells. Pub promoter drives expression throughout the body. U6 promoter is used for the expression of gRNAs. Fluorescent markers such as dsRed (and TdTomato), eGFP, CFP, and YFP are also used for donor plasmids for HDR. These are fluorescent proteins that emit red, green, cyan, and yellow fluorescence, respectively. For 3' UTRs, p1039 and SV4040 are often used to enhance mRNA stability and translation efficiency in transgenes.
As a summary of the microinjection procedure, for the knockout mutant lines, first, align mosquito embryos on double-sided tape side by side for injection (Figure 4A). Second, remove most of the halocarbon oil to facilitate the survival rate of embryos and transfer the embryos from the double-sided tape to a clean wet filter paper. Keep them in a cup with ultrapure water to maintain high moisture for a few days, waiting for the embryos to fully develop (Figure 4B). Third, hatch the injected embryos, separate the pupae into both sexes, and put them into different cages (Figure 4C). Fourth, place all fluorescent males into one cage to cross with 5- to 7 days old WT females, and vice versa (Figure 4D). Fifth, collect G1 eggs from the crossed WT females to expand the mosquito number at the beginning (Figure 4E). Sixth, hatching G1 eggs and separating pupae into both sexes (Figure 4F). Seventh, G1 adults cross with WT again (Figure 4G). Eighth, collect G1 adult females into vials for individual G2 egg collection and maintain ultra-pure water at the bottom of the vials using cotton balls or a paper towel for a few days (Figure 4H). Ninth, collect G1 females after they laid eggs and extract the genomic DNA from G1 females for PCR and sequencing (Figure 4I). Tenth, hatching correct G2 eggs for line-keeping and outcrosses with WT for three more generations to remove most unlinked background mutations (Figure 4J). Eleventh, establishing a homozygous mosquito line by using PCR, restriction enzyme assay, gel electrophoresis, and sequencing to screen for mutations (Figure 4K).
For the establishment of HDR lines, align mosquito embryos on double-sided tape side by side for embryo microinjection (Figure 4i). Second, after injections, remove most of the halocarbon oil and store the embryos in a cup with ultra-pure water to maintain moisture for a few days and wait for the embryos to mature (Figure 4ii). Third, hatch injected embryos and screen G0 larvae to sort those with or without fluorescence (Figure 4iii); larvae with fluorescence means that the plasmid was injected into mosquito embryos successfully. Fourth, allow the fluorescent larvae to mature into adults and place all fluorescent males into one cage to cross with WT females, and vice versa, to expand mosquito number (Figure 4iv). Fifth, collect eggs from the crossed WT females (Figure 4v). Sixth, hatch, screen, and sort G1 fluorescence larvae and dispose of the larvae without fluorescence (Figure 4vi). Seventh, cross G1 fluorescence adults with WT (Figure 4vii). Eighth, collect G2 eggs in plastic vials individually to separate the different insertion sites of the plasmid in mosquitoes (Figure 4viii). Ninth, hatch eggs, check mosquito fitness, and carry out sequencing (Figure 4ix). Tenth, select two lines with stronger phenotype, and outcross with WT for three more generations (Figure 4x). Eleventh, selecting larvae with fluorescence to intercross for two more generations to establish a homozygous line for further experiments (Figure 4xi).
Here we provided the most comprehensive protocol for A. aegypti genome editing with CRISPR-Cas9 that we have used to generate multiple transgenic lines. We suggest this protocol be used as a starting point for other investigators, who should make changes based on the experience obtained with their microinjection trials. Different reagents and conditions should always be tested. The success rate of gene editing in mosquitoes is yet to be fully optimized and very few laboratories have been able to make transgenic mosquitoes via CRISPR-Cas9.
Ujawnienia
O.S.A. is a founder of Agragene, Inc. and Synvect, Inc. with an equity interest. The terms of this arrangement have been reviewed and approved by the University of California, San Diego in accordance with its conflict-of-interest policies. The remaining authors declare no competing interests.
Podziękowania
The authors thank Judy Ishikawa and Ava Stevenson for helping with mosquito husbandry. This work was supported by funding from NIH awards (R01AI151004, RO1AI148300, RO1AI175152) awarded to O.S.A. and K22AI166268 to N.H.R. Figures were created using BioRender.
Materiały
| Name | Company | Catalog Number | Comments |
| 10x Cas9 reaction buffer | PNA Bio | CB01 | |
| Benchling software | Benchling | N/A | www.benchling.com |
| Cas9 dilution buffer | PNA Bio | CB03 | |
| Cas9 protein | PNA Bio | CP01-50 | |
| DH5α E. coli Competent Cells | New England Biolabs | C2987 | |
| Double-sided sticky tape | Scotch Permanent | 3136 | |
| Drosophila vials | Genesee Scientific | 32-109 | |
| Filter papers | GE Healthcare Life Science | 1450-042 | |
| Fish food | Tetra | B00025Z6YI | goldfish flakes |
| Flugs | Genesee Scientific | AS273 | |
| Fluorescent microscope | Leica Microsystems | M165 FC | |
| Gene fragment | Integrated DNA Technologies | N/A | |
| gRNA | Synthego | N/A | |
| Halocarbon oil 700 | Sigma-Aldrich | H8898 | |
| Injection microscope | Leica Microsystems | DM2000 | |
| JM109 E. coli Competent Cells | Zymo Research | T3005 | |
| Microinjector | Eppendorf | FemtoJet 4x | |
| Microloader Tips for Filling Femtotips | Eppendorf | E5242956003 | |
| Micromanipulator | Eppendorf | TransferMan 4r | |
| Micropipette Pullers | Sutter Instrument | P-2000 | |
| Microscope Cover Glass | Fisherbrand | 12-542-B | |
| Microscope slide | Eisco | 12-550-A3 | |
| Mouse blood (live mice used for feeding) | University of California | IACUC, Animal Use Protocol #S17187 | Used for mosquito blood feeding; details comply with animal ethics protocols |
| NEB Q5 High-Fidelity DNA polymerase | New England Biolabs | M0491S | |
| PCR Purification Kit | Qiagen | 28004 | |
| Plasmid Miniprep Kit | Zymo Research | D4036 | |
| Quartz filament | Sutter Instruments | QF100-70-10 | |
| Transcription Clean-Up Kit | Fisher Scientific | AM1908 | |
| Ultra-pure water | Life Technologies | 10977-023 |
Odniesienia
- Anzalone, A. V., Koblan, L. W., Liu, D. R. Genome editing with CRISPR-Cas nucleases, base editors, transposases and prime editors. Nat Biotechnol. 38 (7), 824-844 (2020).
- Garneau, J. E., et al. The CRISPR/Cas bacterial immune system cleaves bacteriophage and plasmid DNA. Nature. 468 (7320), 67-71 (2010).
- Jinek, M., et al. A programmable dual-RNA-guided DNA endonuclease in adaptive bacterial immunity. Science. 337 (6096), 816-821 (2012).
- Shen, H., Li, Z. DNA double-strand break repairs and their application in plant DNA integration. Genes (Basel). 13 (2), 322 (2022).
- Vinauger, C., et al. Modulation of host learning in Aedes aegypti mosquitoes. Curr Biol. 28 (3), 333-344.e8 (2018).
- Li, M., Bui, M., Yang, T., Bowman, C. S., White, B. J., Akbari, O. S. Germline Cas9 expression yields highly efficient genome engineering in a major worldwide disease vector. Proc Natl Acad Sci U S A. 114 (49), E10540-E10549 (2017).
- Chaverra-Rodriguez, D., et al. Targeted delivery of CRISPR-Cas9 ribonucleoprotein into arthropod ovaries for heritable germline gene editing. Nat Commun. 9 (1), 3008 (2018).
- Rouyar, A., et al. Transgenic line for characterizing GABA-receptor expression to study the neural basis of olfaction in the yellow-fever mosquito. Front Physiol. 15, 1381164 (2024).
- Ang, J. X. D., et al. Considerations for homology-based DNA repair in mosquitoes: Impact of sequence heterology and donor template source. PLoS Genet. 18 (2), e1010060 (2022).
- Zhang, J. -. P., et al. Efficient precise knockin with a double cut HDR donor after CRISPR/Cas9-mediated double-stranded DNA cleavage. Genome Biol. 18 (1), 35 (2017).
- Coutinho-Abreu, I. V., Akbari, O. S. Technological advances in mosquito olfaction neurogenetics. Trends Genet. 39 (2), 154-166 (2023).
- Li, M., et al. Targeting sex determination to suppress mosquito populations. eLife. 12, RP90199 (2024).
- Zhan, Y., Alonso San Alberto, D., Rusch, C., Riffell, J. A., Montell, C. Elimination of vision-guided target attraction in Aedes aegypti using CRISPR. Current Biol. 31 (18), 4180-4187.e6 (2021).
- Greppi, C., et al. Mosquito heat seeking is driven by an ancestral cooling receptor. Science. 367 (6478), 681-684 (2020).
- Potter, C. J., Tasic, B., Russler, E. V., Liang, L., Luo, L. The Q system: a repressible binary system for transgene expression, lineage tracing, and mosaic analysis. Cell. 141 (3), 536-548 (2010).
- Riabinina, O., et al. Improved and expanded Q-system reagents for genetic manipulations. Nat Methods. 12 (3), 219-222 (2015).
- Labun, K., et al. CHOPCHOP v3: expanding the CRISPR web toolbox beyond genome editing. Nucleic Acids Res. 47 (W1), W171-W174 (2019).
- Concordet, J. -. P., Haeussler, M. CRISPOR: intuitive guide selection for CRISPR/Cas9 genome editing experiments and screens. Nucleic Acids Res. 46 (W1), W242-W245 (2018).
- Williams, A. E., et al. Quantifying fitness costs in transgenic Aedes aegypti mosquitoes. J Vis Exp. , e65136 (2023).
- Bassett, A. R., Tibbit, C., Ponting, C. P., Liu, J. -. L. Highly efficient targeted mutagenesis of Drosophila with the CRISPR/Cas9 system. Cell Rep. 4 (1), 220-228 (2013).
- Coutinho-Abreu, I. V., Zhu, K. Y., Ramalho-Ortigao, M. Transgenesis and paratransgenesis to control insect-borne diseases: current status and future challenges. Parasitol Int. 59 (1), 1-8 (2010).
- Christian, M., et al. Targeting DNA double-strand breaks with TAL effector nucleases. Genetics. 186 (2), 757-761 (2010).
- Kim, Y. G., Cha, J., Chandrasegaran, S. Hybrid restriction enzymes: zinc finger fusions to Fok I cleavage domain. Proc Natl Acad Sci U S A. 93 (3), 1156-1160 (1996).
- DeGennaro, M., et al. orco mutant mosquitoes lose strong preference for humans and are not repelled by volatile DEET. Nature. 498 (7455), 487-491 (2013).
- McMeniman, C. J., Corfas, R. A., Matthews, B. J., Ritchie, S. A., Vosshall, L. B. Multimodal integration of carbon dioxide and other sensory cues drives mosquito attraction to humans. Cell. 156 (5), 1060-1071 (2014).
- Lobo, N. F., Clayton, J. R., Fraser, M. J., Kafatos, F. C., Collins, F. H. High efficiency germ-line transformation of mosquitoes. Nat Protoc. 1 (3), 1312-1317 (2006).
- Kistler, K. E., Vosshall, L. B., Matthews, B. J. Genome engineering with CRISPR-Cas9 in the mosquito Aedes aegypti. Cell Rep. 11 (1), 51-60 (2015).
- Handler, A. M., Harrell, R. A. 2nd Transformation of the Caribbean fruit fly, Anastrephasuspensa, with a piggyBac vector marked with polyubiquitin-regulated GFP. Insect Biochem Mol Biol. 31 (2), 199-205 (2001).
- Harrell, R. A. . 2nd Mosquito embryo microinjection under halocarbon oil or in aqueous solution. 2024 (7), (2024).
- Sun, R., Raban, R., Akbari, O. S. Generating mutant strains with transgenic Cas9. Cold Spring Harb Protoc. 2023 (9), 671-678 (2023).
- Giraldo, D., et al. An expanded neurogenetic toolkit to decode olfaction in the African malaria mosquito Anopheles gambiae. Cell Rep Methods. 4 (2), 100714 (2024).
- Liu, G., Lin, Q., Jin, S., Gao, C. The CRISPR-Cas toolbox and gene editing technologies. Mol Cell. 82 (2), 333-347 (2022).
- Chu, V. T., et al. Increasing the efficiency of homology-directed repair for CRISPR-Cas9-induced precise gene editing in mammalian cells. Nat Biotechnol. 33 (5), 543-548 (2015).
- Laursen, W. J., et al. Humidity sensors that alert mosquitoes to nearby hosts and egg-laying sites. Neuron. 111 (6), 874-887.e8 (2023).
- Weng, S. -. C., Antoshechkin, I., Marois, E., Akbari, O. S. Efficient sex separation by exploiting differential alternative splicing of a dominant marker in Aedes aegypti. PLoS Genet. 19 (11), e1011065 (2023).
- Li, M., et al. Development of a confinable gene drive system in the human disease vector. eLife. 9, e51701 (2020).
- Dalla Benetta, E., et al. Engineered Antiviral Sensor Targets Infected Mosquitoes. The CRISPR journal. 6 (6), 543-556 (2023).
- Li, H. -. H., et al. C-Type lectins link immunological and reproductive processes in Aedes aegypti. iScience. 23 (9), 101486 (2020).
- van Oers, M. M., Vlak, J. M., Voorma, H. O., Thomas, A. A. M. Role of the 3' untranslated region of baculovirus p10 mRNA in high-level expression of foreign genes. J Gen Virol. 80 (Pt 8), 2253-2262 (1999).
- Salem, T. Z., et al. The influence of SV40 polyA on gene expression of baculovirus expression vector systems. PloS One. 10 (12), e0145019 (2015).
Przedruki i uprawnienia
Zapytaj o uprawnienia na użycie tekstu lub obrazów z tego artykułu JoVE
Zapytaj o uprawnieniaThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. Wszelkie prawa zastrzeżone