Method Article
A Swimming-Induced Zebrafish Exercise Apparatus for Versatile Training Approaches
W tym Artykule
Podsumowanie
The exercise apparatus designed for less fortunate fish facilitates the implementation of diverse exercise protocols with varying intensities by manipulating water flow speed, achievable through rheotaxis.
Streszczenie
To comprehensively investigate the effects of exercise on health and disease, animal models play a pivotal role. Zebrafish, a widely utilized vertebrate model organism, offers a unique platform for such studies. This study introduced the development of a cost-effective apparatus tailored for zebrafish exercise studies utilizing readily available materials. The device is founded on the principles of a swim tunnel and encompasses a network of pipes and valves linked to a submersible pump. Water flow is meticulously monitored by a sensor and regulated via valves. To assess the apparatus's effectiveness, two training protocols were implemented: moderate-intensity continuous training (MICT) and high-intensity interval training (HIIT). Fish were trained collectively, and their swimming performance was assessed through an endurance test. Both training protocols led to improvements in swimming performance following 30 days of training and induced alterations in the molecular response to exercise compared to a sedentary control group. Notably, HIIT demonstrated superior efficiency over MICT. The zebrafish training system proved to be a valuable tool for investigations in exercise physiology and further advances the utility of the zebrafish model in this field.
Wprowadzenie
Physical exercise encompasses any bodily movement performed by skeletal muscles that result in increased energy expenditure, with exercise being a structured and repetitive subset of physical activities1. Exercise, a multifactorial and cost-effective activity involving the entire body, yields numerous health benefits, such as preventing metabolic syndrome and sarcopenia2. Consequently, the field of exercise physiology holds significant interest as it seeks to elucidate how the body adapts to the acute stress of exercise, the chronic stress of physical training, and the overall impact of exercise on health1.
Conducting exercise physiology studies in humans can be both expensive and time-consuming due to challenges in experimental design and participant monitoring3. Therefore, the use of animal models in laboratory settings has been highly recommended due to their genetic and physiological uniformity. Moreover, under controlled laboratory conditions, animals typically have sedentary lifestyles and regulated food intake4. Among animal models, rodents have been the most widely employed in research involving physical exercise1. However, zebrafish (Danio rerio; Hamilton, 1822) is a complementary model to murine and other species for exercise studies5,6,7,8.
In zebrafish research, physical exercise can be conducted using commercially available or custom-built swim tunnels. Among the commercially available options, the Blazka-type tunnel, developed by the Loligo System, is the most frequently utilized7,9,10. This system induces forced swimming through a propeller coupled to an electric motor, generating a continuous water flow within the tunnel. This swimming capability is rooted in the principle of rheotaxis, an innate behavior in fish that drives them to swim against water currents and maintain their position11. Rheotaxis enables the measurement of critical swimming speed (Ucrit), representing the maximum velocity a fish can sustain for a specific duration. However, it is worth noting that this equipment, while valuable for assessing swimming behavior and oxygen consumption, comes with a significant cost12.
Researchers have developed alternative apparatus for exercising zebrafish, often based on the Blazka-type mechanism10,13,14 or simpler mechanisms8,15,16. Nonetheless, these methods may be constrained by the technical demands of the protocol, including extended durations, substantial equipment expenses, and limitations in throughput and precision. Consequently, the primary objective of the study was to design an affordable and user-friendly zebrafish exercise system using readily available materials, providing a new alternative apparatus for physical exercise in fish. A secondary goal was to implement both aerobic and anaerobic exercise regimes in zebrafish, further advancing the utilization of the zebrafish model as an intervention strategy in exercise research.
Protokół
The procedures received prior approval from the Animal Use Ethics Committee of the Federal University of São Paulo (CEUA/UNIFESP no. 9206260521). Only adult female wild-type Danio rerio, aged 6 months and weighing 2.5-3 g, were employed in this study. The equipment and reagents needed for the study are listed in the Table of Materials.
1. Custom-made Zebrafish exercise apparatus
NOTE: The exercise apparatus was custom-built. For details, see Figure 1, Supplementary Table 1, Supplementary File 1, and Supplementary File 2.
- Place a submersible pump (N) inside a water tank (O) (≥30 L). Ensure that the water meets the following conditions: pH of 7.2 ± 0.5 and 400 ± 50 µS, 28 ± 1 °C.
- Flowing Supplementary Table 1 and Figure 1, connect Pipe (I) to point Tee Pipe (B), and attach a small pipe (G) to the side of B. From G, establish connections to Globe Valve (F), then to another G, and in sequence to Pipe Elbow (A) and I, thereby completing the segment responsible for regulating water pressure within the system. This regulation is achieved through a return flow to the water tank (O).
- In the alternate section of B, link it to a Pipe (J), followed by connections to A and G. Utilize Socket Pipe Fitting (D) to connect the Gate Water Valve (E) to G.
- Integrate a fish entry port into the system by connecting B to G at one end and attaching another G at the opposite end. Subsequently, connect Socket Pipe Fitting (C) to this second G, establishing a sequence that connects to the Acrylic Pipe (K), which is crucial for visualizing swimming behavior.
- To link K to the Water Flow Sensor (M), utilize pipes C, G, and D. Proceed to connect M to G using D, and then integrate A, G, and H to facilitate the water's return to the reservoir.
NOTE: Insert a mosquito screen in the short pipe segment between the gate and globe valve (F2) to prevent fish from accessing other sections of the apparatus. The globe valve (F) serves a dual purpose. The first globe valve (F1) controls the water flow returning to the reservoir before entering the remainder of the apparatus, acting as a system pressure control valve. The globe valve (F2) is an entry and exit point for the zebrafish within the system.
- To link K to the Water Flow Sensor (M), utilize pipes C, G, and D. Proceed to connect M to G using D, and then integrate A, G, and H to facilitate the water's return to the reservoir.
- Attach a water flow sensor downstream of the acrylic pipe.
NOTE: The flow sensor should be connected to an LCD display and programmed using an Arduino (Figure 1). Details of the Arduino setup are provided in Supplementary File 2.
2. Apparatus operation
- To safely introduce the fish into the system, it is essential to interrupt the water flow. To achieve this, close the Gate Valve (E) while keeping the Globe Valve (F1) open. Subsequently, open the Globe Valve (F2), which serves as the entrance to the system, gently introduce the fish, and promptly close the F2 valve. Finally, open the E valve to fill the exercise area with water.
- Use the globe valve to control flow speed, diverting water back to the reservoir when needed.
- Utilize the gate valve (F2) for precise flow adjustment and to manage fish access.
- To remove the fish at the end of the test, close valve (E) upon observing exhaustion criteria. Then, open valve F and rotate it 180° relative to the axis of the acrylic tube; this will facilitate the drainage of water carrying the exhausted fish along with it.
- Perform the flow monitoring.
NOTE: It is necessary to monitor the water flow speed through a system that incorporates an Arduino Nano, a 16 x 2 LCD screen, a 10 kΩ, 0.25 W through-hole resistor, and a 10 kΩ potentiometer. The flow sensor continuously monitors water flow speed based on Hall Effect technology17. Each pulse of current corresponds to one revolution of the sensor flopper, resulting in a frequency (Hz) of 6.6 x Q (flow rate in L/min).- Connect the appropriate wires of the flow sensor to the 5 V, GND, and D2 pins of the Arduino Nano (Supplementary Table 1). Load the provided sketch (Supplemental File 1) into the Arduino using the Arduino IDE. Power the system through the Arduino USB port.
NOTE: The flow measurements are displayed on the 16 x 2 LCD screen. The calibration of the water flow sensor is depicted in Figure 2. The schematics of the Arduino connections to the LCD are illustrated in Figure 3.
- Connect the appropriate wires of the flow sensor to the 5 V, GND, and D2 pins of the Arduino Nano (Supplementary Table 1). Load the provided sketch (Supplemental File 1) into the Arduino using the Arduino IDE. Power the system through the Arduino USB port.
3. Endurance test
NOTE: This step outlines the procedure for the Endurance test to determine the maximum swimming speed (Umax) of zebrafish.
- First, allow the fish to adapt for 60 min per day to a low water flow speed (0.06 m/s) inside the swimming tunnel for two weeks.
NOTE: After a 24 h preconditioning period, individual zebrafish will undergo the sustained swimming performance test. The purpose of this test is to establish the Umax of each fish. - Place the zebrafish individually in the apparatus.
- Testing conditions: Position the fish against a water flow with an initial speed of 0.06 m/s for 10 min.
- Velocity Increments: Increase the water flow in discrete stages, with velocity increments of 0.02 m/s occurring every minute for 40-50 min.
- Umax determination: Recpod the maximum swimming speed (Umax) when the fish meet the exhaustion criteria.
NOTE: Exhaustion is defined when the first of the following situations is observed: (1) Inability to maintain its position against the water flow for more than three instances, or (2) Inability to sustain its position for longer than 5 s. - Close the valve (E) upon observing exhaustion criteria. Then, open valve F and rotate it 180° relative to the axis of the acrylic tube. This will facilitate the drainage of water, carrying the exhausted fish.
4. Exercise groups and procedure
NOTE: To establish distinct exercise protocols, it is essential to include a sedentary group exposed to identical experimental conditions to compare the effects of exercise protocols, albeit without undergoing high-intensity exercise. It is also essential to establish the Umax because the fractions of the Umax value are necessary to determine the intensity of exercise protocols.
- Sedentary (SED) group: Subject the fish to forced swimming against the water flow at 0.06 m/s for 60 min.
NOTE: The apparatus generates a continuous water flow, compelling the fish to swim against this current based on the principle of rheotaxis11. - Moderate-Intensity Continuous Training (MICT) group: Subject the fish to forced swimming against the water flow at 60% of Umax, as determined in the maximal capacity test, for 35 min.
NOTE: This protocol was adapted from Húngaro et al.18. During the first 10 min, the fish was acclimated to the same speed as the sedentary group (0.05 m/s). - High-Intensity Interval Training (HIIT) group: Subject the fish to forced swimming alternating the swimming speeds: 2 min at 90% of Umax followed by 2 min at 30% of Umax, repeated for 18 min (9 cycles). This protocol was adapted from Marcinko et al.19.
NOTE: During the first 10 min of the exercise period, it is necessary to acclimate the fish to the same speed as the sedentary group (0.06 m/s). - Implement all exercise protocols for 5 days a week over a four-week period.
NOTE: Fish should be housed in aquariums that provide suitable conditions, and they should only be introduced into the exercise apparatus during designated exercise periods. Fish should be provided with tropical fish-flaked food three times daily, and the water in maintenance aquariums should undergo a partial change every 2 days. - Repeat the endurance test at the end of each week, with latency and velocity data at the point of fatigue as indicators of physical conditioning parameters.
- To induce overtraining effects, increase the water flow velocity weekly based on the results of the endurance test conducted after each 4-day cycle of training. Training durations must be adjusted to account for the distance traveled (velocity × time), and these durations should remain consistent between the exercised groups.
- Adjust the swimming time in response to the increased water flow, thereby standardizing the training load across the exercised groups.
5. Body measurements
- Anesthetize the fish with 0.0075% tricaine (w/v) by immersion for conducting body measurements (weight and size)20.
- Photograph and weigh the fish to determine body dimensions using ImageJ software.
- Express the data in terms of Body Condition Indices (weight [g]/standard length [mm]2; BMI) and Body Condition Scoring (BCS)20.
- To eliminate size and weight variations caused by egg formation, subject the fish to standard breeding20, followed by measurements and weighing.
Wyniki
The exercise apparatus demonstrated remarkable efficiency in regulating flow velocity. To gradually enhance swimming speed, water flow was incrementally increased weekly for all groups, except for the SED group, which was maintained at a constant flow velocity of 0.06 m/s. Notably, the apparatus allowed for a remarkable level of precision, achieving flow velocity adjustments as fine as 0.001 m/s. However, the error rate was 30% at low velocities, such as 0.06 m/s. At high velocities, such as 0.3 m/s and 0.5 m/s, the error rate was 3%-4% (Figure 2). The maximum velocity reached during the training was 0.4 m/s in the SED, 0.44 m/s in the MICT, and 0.49 m/s in the HIIT groups in the last endurance.
The physical performance of zebrafish was evaluated weekly using Umax in the endurance test. The results revealed significant enhancements in physical performance for zebrafish subjected to MICT and HIIT compared to the SED group (Figure 4). Both MICT and HIIT were submitted to the same distance traveled during training; however, the HIIT group induced rapid improvements, with a significant increase in Umax observed after just two weeks (p = 0.0003). Over the training period, the HIIT group demonstrated a consistent 10% weekly improvement, resulting in an overall enhancement of approximately 30%. In contrast, MICT training led to more gradual gains, with a notable ~10% increase in Umax recorded only in the third week of training, followed by no further improvement in the subsequent week (p = 0.0024). These findings highlight the differential effects of HIIT and MICT training protocols on zebrafish physical performance.

Figure 1: Apparatus design. (A) Schematics of the swimming apparatus. The blue arrows indicate the direction of water flow. The relevant pipe lengths are depicted. The letters represent each apparatus component described in Supplementary Table 1. (B) Photograph of the apparatus. Please click here to view a larger version of this figure.
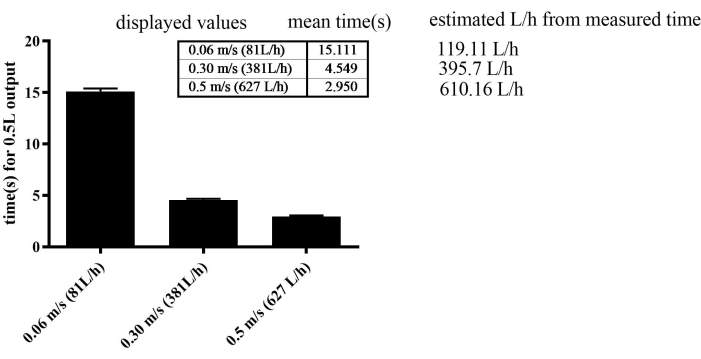
Figure 2: Calibration of the water flow sensor by measuring the time required for a 0.5 L flow. The SEM was 0.277 in the 0.06 m/s, 0.123 in the 0.3 m/s, and 0.109 in the 0.5 m/s groups. Please click here to view a larger version of this figure.
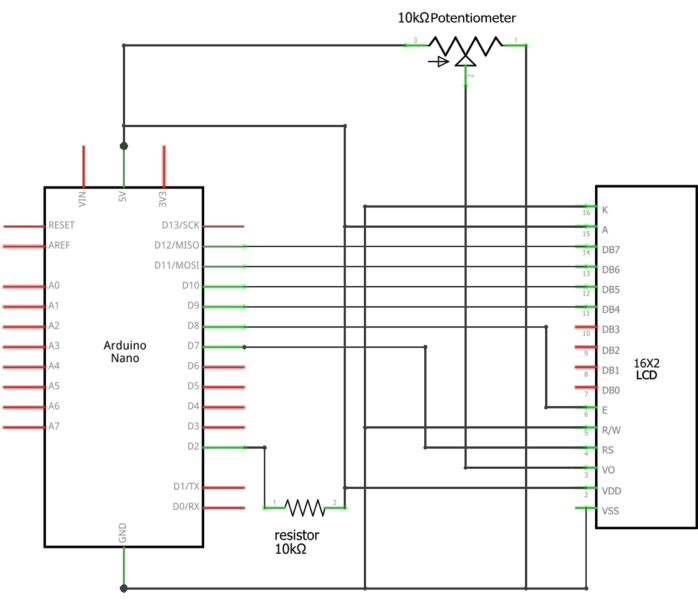
Figure 3: Schematics of the Arduino connections to the LCD. The appropriate wires of the sensor should be connected or soldered between the D2 pin of the Arduino and the 10 kΩ resistor (Signal wire), the Ground of the Arduino (black wire), and 5 V of the Arduino (red wire). Please click here to view a larger version of this figure.

Figure 4: Swimming performance expressed in maximum swimming speed (Umax). a, b, and c indicate the statistical differences between the weekly swimming ability estimates within each group. *Indicates the difference between groups, comparison using two-way analysis of variance (Tukey posthoc). *p = 0.01; **p = 0.001; ***p = 0.0001. Please click here to view a larger version of this figure.

Figure 5: Morphological measurements. (A) Body condition indices (weight [g] / standard length [mm]2; BMI). (B) Representative image of the body measurement. (C) Body Condition Scoring (BCS). The error bar corresponds to a range of 0.026 to 0.045 for BMI and 0.4 to 0.96 for BCS. Please click here to view a larger version of this figure.
Supplementary Table 1: Materials and assembly guide for the zebrafish exercise apparatus. The table features letters that align with the assembly order illustrated in the figure. Please click here to download this File.
Supplementary File 1: Annotated Arduino sketch for measuring water speed. Comments for each line of code are incorporated after the double forward slashes. Please click here to download this File.
Supplementary File 2: Arduino file, including the setup details. Please click here to download this File.
Dyskusje
In this study, an innovative, cost-effective exercise system was developed inspired by the swim tunnel respirometer from Loligo Systems21 and the flume system22 for the comprehensive examination of zebrafish swimming performance. The Umax was determined by systematically increasing water flow in discrete stages, with speed increments occurring over short intervals (20-30 min) until the fish reached exhaustion, which was characterized by three consecutive fatigues or the inability to overcome the current in the swimming tunnel. These determinations were instrumental in designing two exercise protocols (MICT and HIIT). Unlike protocols involving relatively prolonged stage durations (ranging from one to several hours), the water flow speed at exhaustion is known as the Critical Swimming Speed (Ucri)12. The Umax values calculated using the exercise system fell within the range observed in other studies7, confirming the efficacy of this tunnel swimming apparatus for evaluating zebrafish physical performance. Furthermore, this compact apparatus possesses the versatility to cover the entire spectrum of water flow speeds, facilitating the customization of applied training protocols.
In this apparatus development, it is possible to create different protocols of exercise by controlling water flow velocity only. Accurately measuring water flow velocity posed challenges at low speeds, resulting in an approximate 30% error rate. Nonetheless, precision improved at intermediate and high speeds, nearing Umax, with a more reliable speed measurement and a reduced error rate of 3%-4%. Consequently, a limitation in device precision at low speeds was identified. Despite this limitation, the study found that even with a speed variation of 0.02 m/s, no significant impact on the physical capacity of the SED group during training was observed. This suggests that variations in low-intensity training may not exert significant effects on physical capacity, at least in the model presented in this study. Another limitation of the proposed apparatus is the absence of an oxygen sensor, rendering it impossible to measure oxygen consumption.
Previous studies have demonstrated a notable disparity in swimming speed between adult male and female zebrafish, with suggested attributions including morphological distinctions, such as the increased girth of gravid females, and physiological differences, such as reduced muscle power output in gravid fish5,23,24. This investigation exclusively subjected female zebrafish to the training regimen, acknowledging that these females underwent three breeding cycles before, during, and after training, consistently preceding body measurements. This strategic approach effectively mitigated inter-sex body differences. Moreover, this study revealed no discernible differences in body parameters among the training groups (Figure 5) or when comparing pre- and post-training conditions in females. While there was no control for age variation among individuals, it is well-documented that swim performance, and locomotion energy demands can markedly fluctuate throughout the life cycle due to ontogeny, reproduction, and senescence25,26,27. Despite the potential age differences within each group, all animals were meticulously grouped based on size, body weight, and a consistent sexually mature adult stage. Notably, no statistically significant differences were observed among the groups before commencing the exercise regimen. Consequently, it was postulated that the observed enhancement in swimming capacity can be confidently attributed solely to the exercise protocols employed.
This study aimed to assess the effectiveness of the exercise system by implementing two distinct exercise protocols. While MICT involves a sustained, continuous exercise regimen, HIIT incorporates short bursts of maximum-intensity exercise followed by brief, less intense recovery periods. Both protocols were meticulously adjusted to provide equivalent training loads throughout the training period without inducing exhaustion. The SED group underwent the same endurance test once a week for four weeks, like the other exercise groups, but exhibited minimal performance improvement. Endurance training is recognized for enhancing cardiac output, maximal oxygen consumption, and mitochondrial biogenesis28; however, the weekly exercise frequency proved insufficient to elicit significant changes in swimming performance. When zebrafish were subjected to MICT training, a noteworthy enhancement in swim performance was observed after the third week of training. However, there was no further improvement in the subsequent week. MICT involves individuals sustaining a submaximal workload for extended durations, necessitating a higher-than-average power output22. Most notably, the HIIT group demonstrated the most substantial improvement, with a significant increase in performance apparent after just two weeks of training, sustained until the fourth week. While it is well-documented that short, high-intensity exercise leads to endurance adaptation, the specific exercise type responsible for eliciting phenotypic muscle shifts remains a topic of ongoing investigation28.
Ujawnienia
It is essential to clarify that there are no competing financial interests associated with the research presented in this manuscript. No financial partnerships or affiliations with organizations or entities have been established that could potentially influence or bias the outcomes of this work. This statement serves as an assurance that the research process was straightforward and honest, with no financial conflicts influencing the results. The presentation of this work is motivated by a genuine passion for the subject matter, driven solely by a love for academia and the pursuit of scientific knowledge.
Podziękowania
Gratitude is extended to Dr. Omar Mertins for generously providing access to the laboratory for the maintenance of fish and execution of tests. Furthermore, acknowledgments are given to FAPESP (process numbers 2020/12084-8 and 2021/14313-7), CNPq (process number 151756/2022-8), and CAPES for awarding fellowships to support this research.
Materiały
| Name | Company | Catalog Number | Comments |
| CPVC Female 90-Degree Elbow for Plumbing | Tigre | 22150260 | 3/4-inch |
| 24AWG Wire | Sky Cablo Store | Connection between components in the Perforated Circuit Board (1m) | |
| Acrylic pipe | The Clear Plastic Shop | 41138408 | 3/4-inch |
| Aquarium Submersible Fish Tank | Aqua Tank | 300w | |
| CPVC Pipe | Tigre | 10121787 | 3/4-inch |
| Female Threaded Gate Water Valve | Tigre | 27950310 | 3/4-inch |
| Female Threaded Globe Water Valve | Tigre | 27940510 | 3/4-inch |
| hrough-hole resistor | BXV | 10 kΩ, 0.25W t | |
| Lab Support Stand With Clamp with 30 inch rod | Masiye Labs | RSC0001 | Support the horizontal pipes |
| LCD screen | Eichip | 16 x 2, model JHD162A | |
| Male x Male Dupont Jumpers | Chyan | Connection between arduino and flow sensor (30 cm) | |
| Perforated Circuit Board single sided | KY WIN ROBOT | 5 x 10 cm | |
| Potentiometer | LUSYA | DL-ALPSA01 | 10kΩ |
| Roll of Water Blocking Tape | One World | 5603131000 | To avoid leaks |
| Silicone hose | Tigre | 14211250 | 2 cm inner |
| Solder Station | QHTITEC | EU/US PLUG | Arduine system welding |
| Solder Wire Spool | BEEYIHF | I001-A001-Set | Arduine system welding |
| Threaded Male Socket and Unthreaded Female Socket CPVC Pipe Fitting | TIgre | 35447849 | 3/4-inch |
| Tricaine (MS-222) | Sigma-Aldrich | E10521 | Anesthetic |
| UNO-R3 board UNO R3 CH340G+MEGA328P Chip 16Mhz | FSXSEMI | For Arduino UNO R3 Development board | |
| Unthreaded CPVC Tee Pipe Fitting, Female | Tigre | 22200267 | 3/4-inch |
| Unthreaded Female CPVC Socket Pipe Fitting | Tigre | 22170260 | 3/4-inch |
| Water Flow Sensor model YF-B5 | Siqma Robotics | SQ8659 | 1-25 L/min |
| Water Pump | Sunsun | Model HJ-2041, 3000L/h, 65W | |
| Water reservoir | Custom | 30 L |
Odniesienia
- Seo, D. Y., et al. Humanized animal exercise model for clinical implication. Pflugers Arch Eur. J Physiol. 466 (9), 1673-1687 (2014).
- Nylén, E. S., Gandhi, S. M., Lakshman, R. Cardiorespiratory fitness, physical activity, and metabolic syndrome. Cardiorespiratory Fitness in Cardiometabolic Diseases: Prev. & Manag. in Clin. Pract. , Springer. 207-215 (2019).
- Cholewa, J., et al. Basic models modeling resistance training: an update for basic scientists interested in study skeletal muscle hypertrophy. J Cell Physiol. 229 (9), 1148-1156 (2014).
- Martin, B., Ji, S., Maudsley, S., Mattson, M. P. 34;Control" laboratory rodents are metabolically morbid: Why it matters. Proc Natl Acad Sci USA. 107 (14), 6127-6133 (2010).
- Palstra, A. P., et al. Swimming-induced exercise promotes hypertrophy and vascularization of fast skeletal muscle fibres and activation of myogenic and angiogenic transcriptional programs in adult zebrafish. BMC Genomics. 15 (1), 1-20 (2014).
- Blazina, A. R., Vianna, M. R., Lara, D. R. The spinning task: A new protocol to easily assess motor coordination and resistance in zebrafish. Zebrafish. 10 (4), 480-485 (2013).
- Gilbert, M. J. H., Zerulla, T. C., Tierney, K. B. Zebrafish (Danio rerio) as a model for the study of aging and exercise: Physical ability and trainability decrease with age. Exp Gerontol. 50, 106-113 (2014).
- Usui, T., et al. The French press: A repeatable and high-throughput approach to exercising zebrafish (Danio rerio). Peer J. 2018 (1), 1-12 (2018).
- Tierney, K. B. Swimming performance assessment in fishes. J. Vis. Exp. (51), e2572(2011).
- Palstra, A. P., et al. Establishing zebrafish as a novel exercise model: Swimming economy, swimming-enhanced growth and muscle growth marker gene expression. PLoS One. 5 (12), e0014483(2010).
- Arnold, G. P. Rheotropism in fishes. Biol Rev Camb Philos Soc. 49 (4), 515-576 (1974).
- Messerli, M., et al. Adaptation mechanism of the adult zebrafish respiratory organ to endurance training. PLoS One. 15 (2), 1-20 (2020).
- Bek, J. W., De Clercq, A., Coucke, P. J., Willaert, A. The ZE-tunnel: An affordable, easy-to-assemble, and user-friendly benchtop zebrafish swim tunnel. Zebrafish. 18 (1), 29-41 (2021).
- Lucon-Xiccato, T., et al. An automated low-cost swim tunnel for measuring swimming performance in fish. Zebrafish. 18 (3), 231-234 (2021).
- Blazina, A. R., Vianna, M. R., Lara, D. R. The spinning task: A new protocol to easily assess motor coordination and resistance in zebrafish. Zebrafish. 10 (4), 480-485 (2013).
- Depasquale, C., Leri, J. The influence of exercise on anxiety-like behavior in zebrafish (Danio rerio). Behav Processes. 157, 638-644 (2018).
- Karsenty, A. A comprehensive review of integrated hall effects in macro-, micro-, nanoscales, and quantum devices. Sensors. 20 (15), Basel, Switzerland. 4163(2020).
- Húngaro, T. G. R., et al. Physical exercise exacerbates acute kidney injury induced by LPS via toll-like receptor 4. Front Physiol. 11, 1-13 (2020).
- Marcinko, K., et al. High intensity interval training improves liver and adipose tissue insulin sensitivity. Mol Metab. 4 (12), 903-915 (2015).
- Chen, W., Ge, W. Gonad differentiation and puberty onset in the zebrafish: Evidence for the dependence of puberty onset on body growth but not age in females. Mol Reprod Develop. 80 (5), 384-392 (2013).
- Conradsen, C., Walker, J. A., Perna, C., McGuigan, K. Repeatability of locomotor performance and morphology-locomotor performance relationships. J Exp Biol. 219 (18), 2888-2897 (2016).
- Widrick, J. J., et al. An open source microcontroller based flume for evaluating swimming performance of larval, juvenile, and adult zebrafish. PLoS ONE. 13 (6), 1-14 (2018).
- Gilbert, M. J. H., Zerulla, T. C., Tierney, K. B. Zebrafish (Danio rerio) as a model for the study of aging and exercise: Physical ability and trainability decrease with age. Exp Gerontol. 50 (1), 106-113 (2013).
- Hammer, C. Fatigue and exercise tests with fish. Exp Gerontol. 112 (1), 1-20 (1995).
- Takahiro Hasumura, S. M. Exercise quantity-dependent muscle hypertrophy in adult zebrafish (Danio rerio). J Comp Physiol B. 186, 603-614 (2016).
- Wang, L., et al. Effect of aerobic exercise as a treatment on type 2 diabetes mellitus with depression-like behavior zebrafish. Life Sciences. 300, 120578(2022).
- Conradsen, C., McGuigan, K. Sexually dimorphic morphology and swimming performance relationships in wild-type zebrafish Danio rerio. J Fish Bio. 87 (5), 1219-1233 (2015).
- Hughes, D. C., Ellefsen, S., Baar, K. Adaptations to endurance and strength training. Cold Spring Harb Perspect Med. 8 (6), 1-18 (2018).
Przedruki i uprawnienia
Zapytaj o uprawnienia na użycie tekstu lub obrazów z tego artykułu JoVE
Zapytaj o uprawnieniaPrzeglądaj więcej artyków
This article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. Wszelkie prawa zastrzeżone