Method Article
Isolation and Culture of Primary Cochlear Hair Cells from Neonatal Mice
* Wspomniani autorzy wnieśli do projektu równy wkład.
W tym Artykule
Podsumowanie
Herein, we present a detailed protocol for isolating and culturing primary cochlear hair cells from mice. Initially, the organ of Corti was dissected from neonatal (aged 3-5 days) murine cochleae under a microscope. Subsequently, cells were enzymatically digested into a single-cell suspension and identified using immunofluorescence after several days in culture.
Streszczenie
Cochlear hair cells are the sensory receptors of the auditory system. These cells are located in the organ of Corti, the sensory organ responsible for hearing, within the osseous labyrinth of the inner ear. Cochlear hair cells consist of two anatomically and functionally distinct types: outer and inner hair cells. Damage to either of them results in hearing loss. Notably, as inner hair cells cannot regenerate, and damage to them is permanent. Hence, in vitro cultivation of primary hair cells is indispensable for investigating the protective or regenerative effects of cochlear hair cells. This study aimed to discover a method for isolating and cultivating mouse hair cells.
After manual removal of the cochlear lateral wall, the auditory epithelium was meticulously dissected from the cochlear modiolus under a microscope, incubated in a mixture consisting of 0.25% trypsin-EDTA for 10 min at 37 °C, and gently suspended in culture medium using a 200 µL pipette tip. The cell suspension was passed through a cell filter, the filtrate was centrifuged, and cells were cultured in 24-well plates. Hair cells were identified based on their capacity to express a mechanotransduction complex, myosin-VIIa, which is involved in motor tensions, and via selective labeling of F-actin using phalloidin. Cells reached >90% confluence after 4 d in culture. This method can enhance our understanding of the biological characteristics of in vitro cultured hair cells and demonstrate the efficiency of cochlear hair cell cultures, establishing a solid methodological foundation for further auditory research.
Wprowadzenie
Cochlear hair cells play important roles in sound detection and signal transmission to the auditory nerve. Hair cells are mechanistic cells that function as primary sensory receptors and convert sound vibrations into electrical signals in vertebrates. The sensory epithelium of the mammalian inner ear comprises a single row of inner hair cells and three rows of outer hair cells. In different basic membrane areas, hair cells perceive sounds at different frequencies (between 20 and 2,000 Hz)1. The function of outer hair cells is an active mechanical amplification process that helps fine-tune the mammalian inner ear, conferring high sensitivity to sound. Inner hair cells are responsible for detecting sounds. After graded depolarization, acoustic information is transmitted to the brain through the auditory nerve fibers2.
Hearing loss may be caused by genetic defects, aging, noise trauma, or the excessive use of ototoxic drugs, which constitute a major health concern worldwide3,4. Hearing loss mainly results from irreversible damage to hair cells5. Regarding noise-induced hearing loss, although researchers have reached a consensus on several details of its etiology, a comprehensive understanding of the numerous underlying mechanisms is lacking. Outer hair cells are particularly vulnerable to acoustic overexposure6. Mechanosensitive cochlear hair cells are involved in age-related hearing loss; however, the molecular and cellular mechanisms underlying hair cell degeneration remain unknown. Several changes in the molecular processes lead to hair cell aging, oxidative stress, DNA damage response, autophagy, and dysregulation of the expression and transcription of genes related to hair cell specialization7.
As the inner ear is encased in the temporal bone, deep in the hardest bone of the body, it is experimentally inaccessible, posing a challenge to investigations into the mechanisms of hair cell repair and regeneration. Hence, establishing in vitro cultures for investigating the function of hair cells has become an ideal method for research on the regeneration and injury mechanisms of the inner ear. The procedures for preparing cochlear organotypic cultures have been described in earlier studies8,9,10. Investigators worldwide have employed various cochlear microdissection and surface preparation techniques. Despite the persistent challenges, various primary hair cell culture systems have been successfully established in vitro. Cochlear organ cultures contain various cell types, including hair cells, Deiters cells, Hensen's cells, pillar cells, and auditory nerve fibers. An in-depth understanding of the changes in hair cells at the cellular and molecular levels after injury will enable the development of more powerful research tools. This study aimed to demonstrate the steps for isolating cochlear organs from neonatal mice and enzymatically detaching the abundant hair cells for in vitro studies. The nature of the cultured cells was confirmed using immunofluorescence staining.
Protokół
All animal experiments were approved (No. 2021-847) by the Xi'an Jiaotong University Committee on the Use and Care of Animals.
1. Sterilization and material preparation
- Sterilize the dissection tools using high-temperature and high-pressure steam disinfection and dry them in a 50 °C incubator overnight.
- Prepare 100 mL of the culture medium containing 10% fetal bovine serum (FBS) and 10 mg/mL penicillin/streptomycin (add 10 mL of FBS and 10 µL of penicillin stock solution to 90 mL of Dulbecco's Modified Eagle Medium (EMEM))in advance and store at 4 °C.
2. Dissection and removal of the temporal bone for collection of auditory epithelia
- Euthanize a total number of 10 newborn mice (aged between P3 and P5) by decapitation on ice. Use an alternative, ethically approved methodology, if required.
- Hold the head in place, open the scalp along the sagittal suture using micro-operating scissors, and separate and fix the scalp bilaterally with the fingers, as shown in Figure 1A.
- Remove the brain using a small periosteal elevator and bisect the basal skull. Cut the cranium with scissors and flip forward to open the skull; use the tip of the scissors to scrape the brain and expose the base of the skull.
- Observe the bilateral temporal bones at the base of the skull (Figure 1C). Use scissors to cut the base of the skull along the midline, scrape away the skin, and remove any unnecessary bone (Figure 1D,D').
- Retain and transfer the temporal bones into 35 mm sterile Petri dishes containing fresh Hank's balanced salt solution (HBSS) (Figure 1E).
- Use two #5-pointed forceps to remove the bulla and surrounding tissue from the petrous portion of the temporal bone (Figure 1E).
NOTE: At this stage, the bony labyrinth in the temporal bone of the mice would not be completely calcified and would be easily dissected using forceps. - Hold the forceps with one hand to fix the semicircular part of the temporal bone and stick the lower foot of the forceps into the round window niche with the other hand to separate the lateral bone of the cochlea from the scala vestibule. Carefully remove the petrous portion of the temporal bone without touching the OC epithelium. Subsequently, carefully separate and remove the bony labyrinth of the cochlea from the basal end to the apical end (Figure 1F).
- Carefully micro-isolate the organ of the Corti sensory epithelium from the modiolus using #5-pointed forceps (Figure 1G).
- Hold the spiral ligament, carefully separate it from the stria vascularis with micro-operating forceps, and transfer the clean auditory epithelium to a 3 mm sterile culture dish containing HBSS using a 200 µL pipette (Figure 1H).
- Collect 20 specimens from each animal and quickly transfer them to a 100 mm sterile Petri dish containing HBSS for the next preparation step (Figure 1).
3. Enzymatic disaggregation for obtaining auditory hair cells
- Transfer the auditory epithelium to 10 mL of fresh DMEM containing 0.25% trypsin and incubate at 37 °C for 12 min.
- Using a 200 µL pipette tip, gently separate the hair cells from the basal lamina and other cells under an operating microscope.
- Add another 10 mL of culture medium to inhibit the disaggregation.
- Filter the suspended cells in the culture medium through a 70 µm filter, collect the filtrate in a clean 50 mL tube, and centrifuge it at 300 × g for 5 min.
- Resuspend the hair cells in at least 5 mL of culture medium by gently pipetting them up and down using a 1,000 µL pipette tip. Avoid introducing bubbles.
- Place a coverslip at the bottom of a six-well plate in advance. Count the cells and culture them at a density of 106 cells/mL in six-well plates.
- Grow the adherent cells in 2 mL of DMEM (containing 10% of FBS, 100 units/mL of penicillin, and 100 µg/mL of streptomycin) at 37 °C and 5% CO2. Change the culture medium every day.
NOTE: It should be mentioned that using this protocol does not result in obtaining pure hair cells. Based on this primary cell culture method, we recommend using d2 or d3 cells for further studies, as hair cells might constitute approximately 70% of cells in culture and be in a good state.
4. Immunofluorescent staining
- Harvest the cultured cells from d1 to d6 (one well per day). Aspirate the culture medium and rinse cells 2x with phosphate-buffered saline (PBS).
- Fix cells with 4% paraformaldehyde for 15 min at room temperature (RT).
- Remove the fixative and rinse cells for 3 x 3 min with PBS.
- Permeabilize the cells with PBS containing 0.2% Triton X-100 for 10 min at RT.
- Incubate the permeabilized cells with a blocking solution consisting of 10% FBS in PBS for 20 min at RT.
- Stain the cells with anti-myosin monoclonal antibody (diluted at 1:200 in PBS) at 4 °C overnight.
- Wash the cells 3x with sterile PBS and incubate them with secondary antibody (Alexa Fluor 594 goat anti-rabbit MYO7A, diluted 1:500 in PBS) and fluorescein-labeled phalloidin (Alexa Fluor 488, to identify cell structure) for 2 h at RT.
- Rinse the cells 3x with PBS to remove the secondary antibodies.
- Add 1-2 drops of mounting medium with DAPI onto the slides, mount the coverslips, and place them under a laser scanning confocal microscope to capture photos of the cells.
5. Statistical analysis
- Perform two-way analysis of variance (ANOVA) followed by Tukey's post hoc tests to analyze the changes in the grey values of myosin-VIIa and phalloidin over time. Use the letter marking method to mark statistical differences.
- Perform additional one-way ANOVAs followed by Tukey's post-hoc tests to compare the phalloidin-positive cell ratio between cell samples from day 1 to day 6 and the myosin-VII-positive cell ratio on the same days.
Wyniki
Following this protocol, we seeded the isolated cells. Primary cochlear hair cell seeds were considered successful if the cells did not float in the culture medium and spread within 24 h. We determined the number of hair cells after they adhered and spread into flat aggregates at the bottom of the dish. After 1 day, live hair cells were tightly adhered to the bottom of the culture dish and non-adherent cells were removed by rinsing with PBS. Typically, the number of cells doubled after 3 d of culture (Figure 2).
Immunofluorescence (IF) revealed the expression of myosin-VIIa and phalloidin until d6 (Figure 3A). We observed that the myosin-VIIa grey value and positive cell ratio decreased with time, whereas the phalloidin grey value and positive cell ratio remained the same compared with those at d1 after culture (Figure 3B-D). We used an organic culture as a positive control to verify that the myosin-VIIa-positive cells were indeed hair cells. Supplemental Figure S1 shows the immunofluorescence staining of myosin-VIIa (green), phalloidin (green), and 4′6-diamidino-2-phenylindole (DAPI, blue) in the auditory epithelium after 2 days of culture.
Using IF, we confirmed that the obtained cells were hair cells. In particular, we observed that most cultured cells showed positive staining for the auditory cell marker myosin-VIIa (red) protein, also exhibiting an elongated appearance under a fluorescence microscope (Figure 3A). These results suggested that the cultured cells were mainly hair cells.
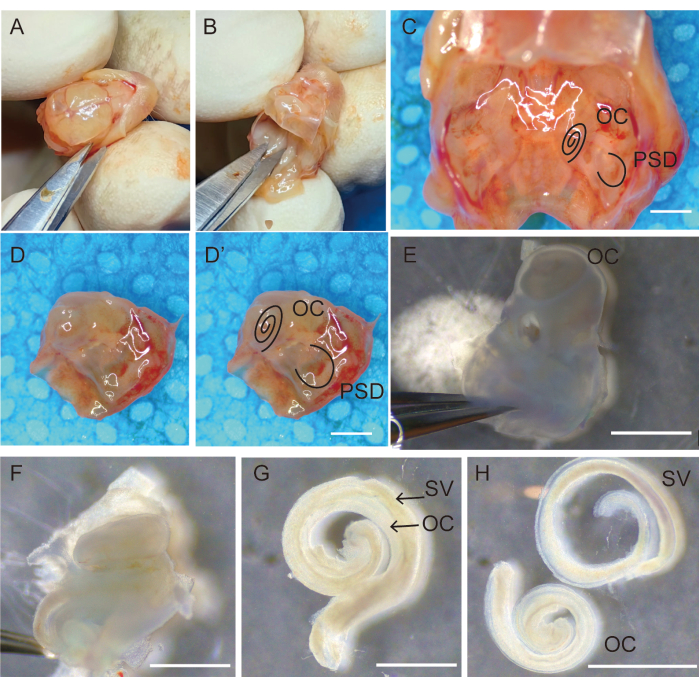
Figure 1: Dissection and removal of the temporal bone for collection of auditory epithelia. (A) Decapitating and opening the cranium along the sagittal suture; (B) removing the brain. (C) View of the bilateral temporal bones located in the skull base. The right temporal bones (D) after removing bones and tissures surrounding the temporal bone (D') marked with OC and PSD. (E) Transfer of temporal bones into fresh Hank's balanced salt solution (HBSS), remove the stapes and place with the oval window side up. (F) Carefully separate and remove the bony labyrinth of the cochlea from the basal to the apical end. The organ of Corti sensory epithelium (G) adhered to the stria vascularis, (H) separated from the stria vascularis. Scale bars = 1,000 µm (C-H). Abbreviations: OC = organ of Corti; PSD = posterior semicircular canal; SV = stria vascularis. Please click here to view a larger version of this figure.
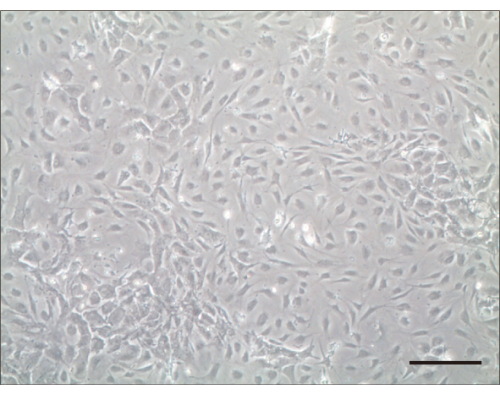
Figure 2: Hair cells 4 days after culturing. Light microscopic examination 4 days after culturing. Scale bar = 100 µm. Please click here to view a larger version of this figure.
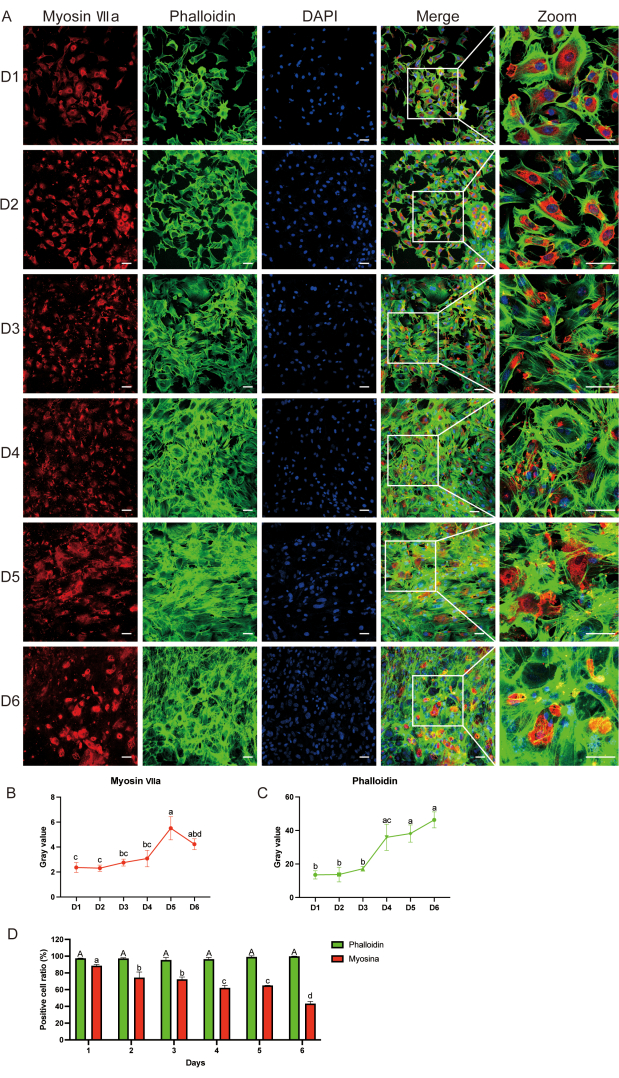
Figure 3: Detecting hair cell surface markers using immunofluorescence. (A) Myosin-VIIa (red), phalloidin (green), and DAPI (blue) immunofluorescence staining from day 1 to day 6 after cell culture. (B, C) Grey value analysis of myosin-VIIa and phalloidin; statistical differences are indicated by letters. (D) Comparison of phalloidin-positive cell ratio between cell samples from day 1 to day 6, and myosin-VII on the same days. Capital letters represent statistical results for phalloidin, whereas small letters represent statistical results for myosin-VIIa. Data are presented as the mean ± standard error of the mean. N = 5; scale bar = 50 µm. Two-way analysis of variance (ANOVA) followed by Tukey's post hoc test was performed for grey value analysis of myosin-VIIa and phalloidin. One-way ANOVA was performed for comparison of phalloidin and myosin-VII-positive cell ratio between cell samples from d 1 to d 6. Different letters indicate statistical differences in phalloidin staining, whereas small letters indicate statistical differences in myosin-VIIa staining. Please click here to view a larger version of this figure.
Supplemental Figure S1: Immunofluorescence detection of hair cell surface markers in organic culture. Immunofluorescence staining of myosin-VIIa (green), phalloidin (green), and DAPI (blue) was performed on d 2 of organic culture. Scale bar = 10 µm. Abbreviations: IHC, inner hair cell; OHC, outer hair cell; DAPI, 4′,6-diamidino-2-phenylindole. Please click here to download this File.
Supplemental Figure S2: Immunofluorescence detection of surface markers in passaged hair cells. (A) Immunofluorescence staining of myosin-VIIa (red), phalloidin (green), and DAPI (blue) was performed on organic cultures at passages 2 and 3. (B) Comparison of the numbers of phalloidin-positive cells, myosin-VIIa-positive cells, and total cells from primary cells to passage 3. N = 3; scale bar = 100 µm. Data are shown as the mean ± standard deviation. Please click here to download this File.
Dyskusje
Compared with the HEI-OC1 cell line, primary cultures of hair cells more accurately replicated the physiological state of cells in vivo. Therefore, the auditory primary culture method established by isolating living cells from cochlear organs and immediately culturing them appears to be a valuable tool for extensive research on auditory systems. Certain techniques are crucial for a successful culture. First, minimizing the duration of separation of the organ of Corti from the temporal bone enhances the likelihood of sustained activity of hair cells. Consequently, it is imperative for researchers to minimize the time interval between the dissection and immersion of organs in PBS. Based on our experimental observations, a duration of approximately 2-3 h suffices for dissecting 10 mice and obtaining the 20 inner ears at a time. During the enzymatic disaggregation process, it is advisable to restrict the duration to 12 min and transfer the cochlear organs to pipettes after an incubation period of 8 min. The organs were observed under a microscope after approximately 10 pipetting cycles, and the enzymatic disaggregation was stopped when almost all the cells had detached from the basilar membrane. Otherwise, the organs were returned to the incubator for another 4 min. The choice of antibiotic is also important. We recommend using 10 mg/mL penicillin/streptomycin to prevent potential contamination problems because some aminoglycoside antibiotics can be ototoxic and lead to hair cell death.
Although class VII myosins are widely expressed in animal tissues, myosin-VIIa is only expressed in thehair cells of the vestibular and cochlear organs of the inner ear alone. Class VIIa myosins play an important role in maintaining the stereocilia structure, as they help interconnect stereocilia at the ankle link region in hair cells11. Following an increase in the duration of the primary cell culture and number of cell passages,the degradation of myosin-VIIa also increased over time, whereas phalloidin remained stable, indicating that the function of the auditory cells decreased with time. The major limitation of this protocol is that auditory cells can only be used within 6-7 days and take longer to grow than HEI-OC1 cells12. Moreover, it is inevitable that primary cells eventually senesce and die, exhibiting limited growth potential. Furthermore, the time and economic costs of hair cell isolation and culturing are often high and require extensive expertise. The organ of Corti contains a variety of neurosensory cells, including inner hair, outer hair, and supporting cells. This protocol introduced a method for separating and culturing these cells in vitro. Primary cells can be distinguished between hair and supporting cells by staining for myosin-VIIa and Sox2, respectively13,14. Outer and inner hair cells perform different functions. Outer hair cells rapidly alter their length and stiffness in response to changes in membrane potential and are transduced into inner hair cells. Prestin is a motor protein of the outer hair cells, whereas Slc17a8 (vGlut3) is specifically expressed in IHCs and can be used for distinguishing inner hair cells in vitro15,16,17,18.
Compared with organic cultures, the morphology of hair cells changes significantly in primary hair cell cultures, resulting in different morphologies and mechanotransduction functions. Organic cultures are three-dimensional primary cell cultures that exhibit stereotypical cellular patterning, polarity, hair bundle formation, and neural connections within the organs of Corti. In contrast, primary HEI-OC1 cells have a typical polygonal shape and grow flat through static adherence in a two-dimensional culture environment19. Organic cultures and primary cells are directly isolated from tissues and exhibit normal cell morphology and important function-related markers. However, unlike HEI-OC1 cells, which are generally highly proliferative, easier to culture and transfect, and can be maintained for decades, these cells have a finite lifespan and limited expansion capacity.
Primary cells have many advantages in cell culture. As primary cells are obtained from body tissues and cultured under optimal growth conditions, they closely mimic the in vivo tissue environment. The use of primary cells avoids many ethical objections raised about animal experimentation and provides more relevant results than cell lines. Preselected primary cells are adequate models that closely represent molecular signaling in vivo. Cells from different donors respond differently to pro-inflammatory cytokines. The costs of isolation and culture are often high, although they are less expensive than those of animal models. The materials and animals used in the primary hair cell culture described here were simple, their cost was relatively low, and the system was easy to operate. If optimal culture conditions are not maintained, the characteristics of the primary cells may change with subsequent passages. As the number of passages increased, the cell state also weakened (Supplemental Figure S2). As they were derived directly from native body tissues without modification, the obtained auditory hair cells closely mimicked their in vivo state and physiology. Therefore, primary hair cells provide an excellent model system for studying the physiology and biochemistry of hair cells, including cell metabolism and drug toxicity. Although primary cells can only be maintained for a few days in vitro, they can be immortalized and divided indefinitely following genetic transformation to obtain secondary cell lines.
In conclusion, the current protocol described the isolation of auditory hair cells from the temporal bone using microdissection and enzymatic disaggregation. The entire protocol took approximately 3 h, from mouse dissection to cell plating. Cultured cells were maintained at high purity and remained healthy for 6-7 days. Despite the inherent limitations of primary hair cell cultures, which are confined to a mere two passages, this protocol employed straightforward methodologies and materials, thereby presenting a novel avenue for conducting highly efficient research on cochlear hair cells.
Ujawnienia
The authors have no conflicts of interest to disclose.
Podziękowania
This work was supported by the National Natural Science Foundation of China (NFSC 82101224 to YG). The authors sincerely thank Dr. Teru Kamogashira, MD, PhD (Department of Otolaryngology-Head and Neck Surgery, Faculty of Medicine, University of Tokyo, Japan) for his critical intellectual guidance and conceptual contributions to this study. The original research framework was developed under his mentorship, and his insights profoundly shaped the direction of this work. We are deeply grateful for his ongoing academic support.
Materiały
| Name | Company | Catalog Number | Comments |
| 100 mm BioLite cell culture dish | Thermo Fisher Scientific | 130182 | using for culture |
| 35 mm Nunc cell culture dish | Thermo Fisher Scientific | 150318 | using for culture |
| 6-well palate | Thermo Fisher Scientific | 310109005 | using for culture |
| 70 µm cell strainers | BD Company | 352350 | using for filter |
| Alexa Fluor 488 Phalloidin | Thermo Fisher Scientific | A12379 | immunofluorescent staining |
| Anti-rabbit IgG Alexa Fluor 488 | Thermo Fisher Scientifc | A11008 | immunofluorescent staining |
| day 3-5 neonatal murine | provided by Xi'an Jiaotong University | ||
| Dulbecco’s Modified Eagle Medium | Thermo Fisher Scientific | 11965092 | using for culture |
| Fetal Bovine Serum | Thermo Fisher Scientific | 12483020 | using for culture |
| Forceps | Dumont | 5# | using for dissection |
| Leica anatomy microscope | Germany | S9i | using for dissection |
| Penicillin/streptomycin | Thermo Fisher Scientific | 15140-122 | using for culture |
| Rabbit plyclonal to Myosin VIIa | Abcam company | ab92996 | immunofluorescent staining |
| Scissor | Belevor | 10cm/04.0524.10 | using for dissection |
| Triton X-100 | Sigma Aldrich | 9036-19-5 | immunofluorescent staining |
| Trypsin | Thermo Fisher Scientific | 25200072 | using for culture |
Odniesienia
- Tani, T., Koike-Tani, M., Tran, M. T., Shribak, M., Levic, S. Postnatal structural development of mammalian Basilar Membrane provides anatomical basis for the maturation of tonotopic maps and frequency tuning. Sci Rep. 11 (1), 7581 (2021).
- Goutman, J. D., Elgoyhen, A. B., Gomez-Casati, M. E. Cochlear hair cells: The sound-sensing machines. FEBS Lett. 589 (22), 3354-3361 (2015).
- Joo, Y., et al. The Contribution of Ototoxic Medications to Hearing Loss Among Older Adults. J Gerontol A Biol Sci Med Sci. 75 (3), 561-566 (2020).
- Nieman, C. L., Oh, E. S. Hearing Loss. Ann Intern Med. 173 (11), ITC81-ITC96 (2020).
- Mao, H., Chen, Y. Noise-Induced Hearing Loss: Updates on Molecular Targets and Potential Interventions. Neural Plast. 2021, 4784385 (2021).
- Morioka, S., et al. Hearing vulnerability after noise exposure in a mouse model of reactive oxygen species overproduction. J Neurochem. 146 (4), 459-473 (2018).
- Liu, H., et al. Molecular and cytological profiling of biological aging of mouse cochlear inner and outer hair cells. Cell Rep. 39 (2), 110665 (2022).
- Ogier, J. M., Burt, R. A., Drury, H. R., Lim, R., Nayagam, B. A. Organotypic Culture of Neonatal Murine Inner Ear Explants. Front Cell Neurosci. 13, 170 (2019).
- Ding, D., et al. Cisplatin ototoxicity in rat cochlear organotypic cultures. Hear Res. 282 (1-2), 196-203 (2011).
- Abitbol, J., et al. Cisplatin-induced ototoxicity in organotypic cochlear cultures occurs independent of gap junctional intercellular communication. Cell Death Dis. 11 (5), 342 (2020).
- Li, S., et al. Myosin-VIIa is expressed in multiple isoforms and essential for tensioning the hair cell mechanotransduction complex. Nat Commun. 11 (1), 2066 (2020).
- Kalinec, G. M., Park, C., Thein, P., Kalinec, F. Working with auditory HEI-OC1 cells. J Vis Exp. (115), (2016).
- Montgomery, S. C., Cox, B. C. Whole mount dissection and immunofluorescence of the adult mouse cochlea. J Vis Exp. (107), (2016).
- Xu, J., et al. Identification of mouse cochlear progenitors that develop hair and supporting cells in the organ of Corti. Nat Commun. 8, 15046 (2017).
- Zheng, J., et al. Prestin is the motor protein of cochlear outer hair cells. Nature. 405 (6783), 149-155 (2000).
- Ruel, J., et al. Impairment of SLC17A8 encoding vesicular glutamate transporter-3, VGLUT3, underlies nonsyndromic deafness DFNA25 and inner hair cell dysfunction in null mice. Am J Hum Genet. 83 (2), 278-292 (2008).
- Seal, R. P., et al. Sensorineural deafness and seizures in mice lacking vesicular glutamate transporter 3. Neuron. 57 (2), 263-275 (2008).
- Luo, Z., et al. Three distinct Atoh1 enhancers cooperate for sound receptor hair cell development. Proc Natl Acad Sci U S A. 119 (32), e2119850119 (2022).
- Kalinec, G., Thein, P., Park, C., Kalinec, F. HEI-OC1 cells as a model for investigating drug cytotoxicity. Hear Res. 335, 105-117 (2016).
Przedruki i uprawnienia
Zapytaj o uprawnienia na użycie tekstu lub obrazów z tego artykułu JoVE
Zapytaj o uprawnieniaThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. Wszelkie prawa zastrzeżone